Abstract
Purpose
To estimate the 4-year incidence and progression of diabetic retinopathy macular edema (ME), and clinically significant macular edema (CSME) among adult Latinos with diabetes mellitus.
Design
A population-based, longitudinal study of 4658 self-identified Latinos (primarily Mexican-Americans), residing in Los Angeles, examined at baseline (2000-2003) and at 4 years (2004-2008).
Methods
Participants underwent a standardized ophthalmic examination. Diabetic retinopathy (DR) and CSME were detected by grading of stereoscopic fundus photographs using the Modified Airlie House classification scheme. Chi-square and trend tests were used to assess differences in incidence when stratifying by age and duration of diabetes.
Results
The 4-year incidence of DR, ME and CSME was 34.0% (182/535) and 5.4% (38/699) and 7.2% (50/699) respectively. Younger persons and those with longer duration of diabetes mellitus had a higher incidence on DR compared to those who were older and had shorter duration of diabetes mellitus. A higher incidence of ME was associated with longer duration of diabetes mellitus (P=0.004). Worsening/Progression of any DR was found in 38.9% (126/324) and improvement occurred in 14.0% (37/265) of participants. Progression from non-proliferative (NPDR) to proliferative DR (PDR) and from NPDR to PDR with high-risk characteristics occurred in 5.3% and 1.9% of participants.
Conclusions
The 4-year incidence and progression of DR and the incidence of ME and CSME among Latinos are high compared to non-Hispanic Whites. These findings support the need to identify and modify risk factors associated with these long-term complications.
Latinos are the largest US minority and the fastest growing segment of the US population. In the 2000 US Census, 12.5% of residents in this country, or 35 million people, were reported as Latino.1 This figure is expected to double by the year 2025.2 Latinos have unique demographic, socioeconomic, and ocular disease characteristics, and bear a disproportionate amount of eye disease burden compared to other racial and ethnic groups;3 With the aging of the US population and growing numbers of US Latinos, this burden is expected to increase over the next several decades.
While there are few population-based epidemiologic studies focusing on the incidence and progression of diabetic retinopathy 4 and macular edema (ME) in Latinos many previous studies have consistently reported that Latinos have a high prevalence of diabetes.4-7 Among those with diabetes, the prevalence of retinopathy ranges from 30%- 50% for DR and from 10%-15% for ME. To date, only one other study has examined incidence of DR in a population-based sample of Latinos in the U.S. The San Luis Valley study, 1984-1992, obtained estimates of the 4-year incidence of DR in Latinos.8 However, the study was limited by the small number of young Latinos included in its cohort.
The current investigation provided us with an opportunity to study incidence estimates of DR, ME and clinically significant macular edema (CSME) among Latinos in the LALES cohort and to compare them to other population-based studies in non-Hispanic Whites and Afro-Caribbean persons with diabetes mellitus. In addition to incidence and progression of DR and ME in the first eye, data from LALES have previously shown that bilateral visual impairment has more of an impact on a person’s health-related quality of life than does unilateral visual impairment.9 For this reason, obtaining incidence estimates of bilateral retinopathy and macular edema in a group of those who already have unilateral disease is important from a quality of life perspective. No previous epidemiological studies on incidence and progression of DR or ME have reported the development of retinopathy and macular edema in the second eye.8, 10-14
METHODS
Study Population and Design
The Los Angeles Latino Eye Study (LALES) is a population-based cohort study of eye disease in self-identified Latinos aged 40 years and older living in six census tracts in the city of La Puente, Los Angeles County, California. The baseline clinical examination was performed from 2000 to 2003, and the 4-year follow-up examination was performed from 2004 to 2008. At baseline, 6357 participants completed an in-home questionnaire and a clinical ophthalmic examination. Details of the study design, methods, and baseline data have been reported elsewhere.15 The study protocol for this investigation was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and adhered to the recommendations of the Declaration of Helsinki. Written informed consent was obtained from all participants.
All living eligible participants (n=6100) from the LALES baseline examination were invited to participate in a home interview and a clinical examination for this 4-year follow-up study. Similar questionnaire and examination procedures were used for both baseline and follow-up studies. Trained ophthalmologists and technicians performed a comprehensive ocular examination using standardized protocols.
Determination of Diabetes Mellitus
At baseline examination all participants were asked if they had previously received a diagnosis of diabetes mellitus and if they were following any treatment regimen, including oral hypoglycemic medications, insulin, or diet only. All participants were assessed for levels of random blood glucose and glycosylated hemoglobin (HbA1c) using the Hemocue B-Glucose Analyzer (Hemacue Inc., Lake Forest, California, USA) and the DCA 2000+ System (Bayer Corp., Tarrytown, New York, USA), respectively. Upon interview and clinical assessment, definite diabetes mellitus was diagnosed for participants who (i) had a history of diabetes and were following a treatment regimen, (ii) had an HbA1c level ≥7.0%, or (iii) had a random blood glucose level ≥200mg%. Duration of diabetes mellitus was calculated as the difference between the year of diagnosis (self-reported by participant) and the year of the LALES baseline examination.
Grading of Photographs for Diabetic Retinopathy and Macular Edema
At both baseline and follow-up, participants with definite diabetes underwent 30° color stereoscopic fundus photography of 7 standard Early Treatment Diabetic Retinopathy Study (ETDRS) fields for each eye after maximal dilation. Masked graders at the Wisconsin Ocular Epidemiology Grading Center graded each fundus photograph for retinopathy and individual lesion severity levels using modifications of the ETDRS adaptation of the modified Airlie House classification of DR. Detailed description of all grading procedures and definitions has been previously presented.12 In brief, all 7 photographic fields were graded. Each eye was graded independent of the contralateral eye. Any discrepancies between the two initial graders were adjudicated by a senior grader using standardized edit rules. All data from the detailed grading were checked for progression or regression of DR lesions using a custom program. For eyes that showed changes in lesion severity by two or more steps between the baseline and 4-year follow-up examinations, a longitudinal review was conducted through side-by-side comparison of photographs from both examination periods. Graders were masked to the year the photographs were taken. Eyes with incident or progressed DR including ME were reviewed by a trained ophthalmologist (RK) for final confirmation.
Definitions of Diabetic Retinopathy
Diabetic retinopathy was classified by standards set forth in the ETDRS.16-18 Diabetic retinopathy was classified into severity levels, each corresponding to a specific clinical characteristic seen on a per-eye basis while grading the fundus photographs (refer to supplemental table 1 for details). The retinopathy level for each person was derived by concatenating the levels for the two eyes, giving the eye with the higher level greater weight (refer to supplemental table 2 for details). For concatenation purposes, levels 12 and 13 are grouped as level 10 and levels 14 and 15 are collapsed with level 20. No DR corresponds to step 1, minimal retinopathy corresponds to steps 2-3, mild non-proliferative DR (NPDR) corresponds to steps 4-7, moderate NPDR corresponds to steps 8-9, severe NPDR corresponds to steps 10-13, and proliferative DR (PDR) corresponds to steps 14-15.
Macular Edema
The definitions of ME and CSME used by LALES were modeled after the definitions proposed in the WESDR and ETDRS.8
Macular edema
An individual was considered to have ME (non-CSME) if at least one of two conditions were met: 1) there was thickening of the retina with or without partial loss of transparency within 1 disc diameter from the center of the macula, or 2) fundus photographs show focal photocoagulation scarring in the macula.
Clinically significant macular edema
Individuals were considered to have CSME if at least one of three conditions were met: 1) there is retinal thickening at or within 500 μm from the center of the macula, or there are hard exudates within 500 μm of the center of the macula associated with thickening of the adjacent retina; 2) there is a zone or zones of retinal thickening, at least 1 disc area in size, at least part of which was 1 disc diameter from the center of the macula, or 3) there are signs of past focal photocoagulation treatment.
Describing Incidence and Progression
Incidence estimates for this study were calculated using three different approaches (refer to supplemental table 3 for details): 1) Incidence in the first eye and required that both eyes be free of disease at baseline; 2) incidence of disease in the second eye and required that, at baseline, one eye be free of disease while the contralateral eye have disease; 3) combined incidence in the first eye with incidence in the second eye to obtain incidence in either eye.
Incidence of diabetic retinopathy
Individuals were considered at risk for incidence of DR if participants were at step 1 (level 10/10) at baseline. Individuals were considered to have DR if they had no retinopathy at baseline (step1: level 10/10) and developed a retinopathy level of 20/<20 (step 2) or higher at the time of the follow-up examination. Individuals were considered to have DR in the first eye if one or both eyes developed retinopathy at follow-up. Individuals were considered to have DR in the second eye if one eye developed retinopathy at follow-up and the contralateral eye had evidence of DR at baseline.
Incidence of macular edema
Individuals were at risk for incidence of ME (non-CSME) if they were free of any ME at baseline and had no evidence of past focal photocoagulation treatment. Individuals were considered to have ME if evidence of ME (as defined above) was present at follow-up.
Incidence of clinically significant macular edema
Individuals at risk for CSME were free of ME and showed no evidence of focal photocoagulation at baseline.
Progression of any diabetic retinopathy (2-step increase)
Progression of DR required that individuals have a step score of at least 20/<20 (step 2) at baseline and that they have progressed two steps or more at the follow-up examination (refer to supplemental table 4 for details). Likewise, individuals who were at 60+/<60 and 60+/60+ (steps 14 and 15, respectively) at baseline were not at risk for progression, since they were incapable of worsening by two steps or more. Similarly, persons classified as step 1 (10/10) with no DR at baseline, were not at risk for progression of disease.
Progression of non-proliferative diabetic retinopathy (NPDR) to proliferative diabetic retinopathy(PDR)
Progression from NPDR to PDR required that individuals be grouped into steps 2-13 (20/<20, 20/20, 31/<31, 31/31, 37/<37, 37/37, 43/<43, 43/43, 47/<47, 47/47, 53/<53, and 53/53) at baseline. If persons progressed to steps 14 (≥ 60/< 60) or 15 (≥ 60/≥ 60) at follow-up, they were classified as having progressed from NPDR to PDR.
Progression of NPDR toPDR with high-risk characteristics (HRC)
Progression from NPDR to PDR with HRC required that individuals with NPDR (levels 53/53 or less; steps 2-13) at baseline progress to levels 71 or worse (presence of high risk characteristics) at follow-up.
Improvement of diabetic retinopathy
Improvement of diabetic retinopathy required that individuals who were at steps 3 to 15 (20/20 to 60+/60+) at baseline improve by two or more steps at follow-up.
Data and Statistical Analysis
All clinical and grading data were entered into a central database with internal automated quality control checks. Incidence and progression of disease were dichotomized into yes/no categories. Comparisons were made between gender, age and duration of diabetes groups. Age at baseline examination was categorized into four groups (40-49 years, 50-59 years, 60-69 years, 70-79 years) for all analysis. Duration of diabetes was defined at baseline and categorized into five groups (0 years, 1-4 years, 5-9 years, 10-14 years, 15+ years). The at-risk cohort for incidence excluded participants who were not diagnosed with the particular outcome at baseline examination. Conversely, the at-risk cohort for progression included those who were diagnosed with disease at baseline examination. Chi-square tests were used to detect gender differences, and tests for trend were used to detect linear trends in age and duration of diabetes across the predefined strata. Incidence data from LALES was compared with other population-based incidence studies using annualized percentages. The Statistical Analysis System (version 9)19 was used for tabulations and statistical analysis. All tests were done at a significance level of 0.05.
RESULTS
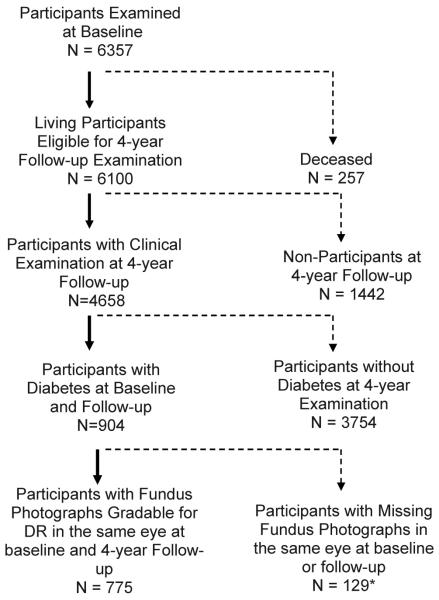
Of the 6,357 participants examined at baseline, 6,100 living eligible participants were identified for the 4-year follow-up study, and 4,658 (76%) completed the follow-up examination. Of these, 904 had definite diabetes at baseline (of which 69/904, 7.6% were newly diagnosed, and 835/904, 92.4% were previously diagnosed), and 775 had gradable fundus photographs in the same eye at baseline and at follow-up, hence this is the cohort used for the analyses in this paper. Compared to those not included in this analysis, (n=129) participants included in this analysis (n=775) were slightly older (58 [±9.7] vs. 56 [±10.9] years, p<0.05), more likely to report having a good-excellent visual health status (39% vs. 28%, p<0.05), and were more likely to have health insurance (72% vs. 59%, p<0.05). There were no other significant differences in baseline characteristics between the analysis cohort (n=775) and those who did not have gradable photographs at baseline and follow-up (129). A flow-chart for assessing the analytical cohort is presented in Figure 1. Of the 775 diabetic participants with gradable photos at baseline and at follow-up, 404 diabetics were at risk of developing any retinopathy, 647 were at risk of developing macular edema, and 324 diabetics with DR at baseline were at risk for progression of DR.
Figure 1.
Participation flowchart for assessing 4-year incidence and progression of diabetic retinopathy 4 in the Los Angeles Latino Eye Study.
*Photographs were not taken due to participant refusal or poor dilation.
†Photographs were not gradable for DR due to media opacities, poor camera focus, or other conditions (e.g. diabetic macular edema).
4-Year Incidence of Diabetic Retinopathy and Macular Edema
Incidence in the first eye
Diabetic retinopathy in the first eye (Table 1) was observed in 28.2% of the diabetic participants. Age-specific incidence ranged from 37.5% in the 40-49 year age group to 23.5% in the 70 or more year age group. There was an overall inverse relationship between age and DR incidence (P= 0.01), with age group 40-49 having the highest incidence. There was a significant increase in incidence of DR with increasing duration of diabetes (P=0.001), increasing from 17.3% in the newly diagnosed to 41.9% in diabetics with 15 or more years duration.
Table 1.
Estimated Four-year Incidence of Any Diabetic Retinopathy Stratified by Age and Duration of Diabetes at Baseline
| Baseline Characteristics |
Incidence in 1st eye* |
Incidence in 2nd eye† |
Incidence in either eye‡ |
|||
|---|---|---|---|---|---|---|
| n | % 22 | N | % 22 | n | % 22 | |
| Age (years) | ||||||
| 40-49 | 96 | 37.5 (27.8, 47.2) | 25 | 76.0 (59.3, 92.7) | 121 | 45.5 (36.6, 54.3) |
| 50-59 | 146 | 30.1 (22.7, 37.6) | 53 | 64.2 (51.2, 77.1) | 199 | 39.2 (32.4, 46.0) |
| 60-69 | 111 | 19.8 (12.4, 27.2) | 30 | 30.0 (13.6, 46.4) | 141 | 22.0 (15.2, 28.8) |
| 70+ | 51 | 23.5 (11.9, 35.2) | 23 | 26.1 (8.1, 44.0) | 74 | 24.3 (14.6, 34.1) |
| P = 0.01 | P < 0.001 | P < 0.001 | ||||
| Overall | 404 | 28.2 (23.8, 32.6) | 131 | 51.9 (43.4, 60.5) | 535 | 34.0 (30.0, 38.0) |
| Age-standardized§ | 30.0 (24.5, 35.6) | 57.4 (43.8, 71.1) | 36.4 (31.1, 41.7) | |||
|
| ||||||
| Duration of Diabetes (years) | ||||||
| New€ | 139 | 17.3 (11.0, 23.6) | 28 | 35.7 (18.0, 53.5) | 167 | 20.4 (14.3, 26.5) |
| 1-4 | 124 | 27.4 (19.6, 35.3) | 34 | 58.8 (42.3, 75.4) | 158 | 34.2 (26.8, 41.6) |
| 5-9 | 67 | 31.3 (20.2, 42.5) | 31 | 58.1 (40.7, 75.4) | 98 | 39.8 (30.1, 49.5) |
| 10-14 | 43 | 51.2 (36.2, 66.1) | 26 | 61.5 (42.8, 80.2) | 69 | 55.1 (43.3, 66.8) |
| ≥ 15 | 31 | 41.9 (24.6, 59.3) | 12 | 33.3 (6.7, 60.0) | 43 | 39.5 (24.9, 54.2) |
| P < 0.001 | P = 0.51 | P < 0.001 | ||||
| Overall | 404 | 28.2 (23.8, 32.6) | 131 | 51.9 (43.4, 60.5) | 535 | 34.0 (30.0, 38.0) |
n = number at risk at baseline; persons with gradable fundus photographs at baseline and at follow-up, and have diabetes.. P = Test of trend. Incidence data presented as percent (95% confidence interval),
At-risk cohort for incidence in the 1st eye: Both eyes did not have evidence of any diabetic retinopathy 4 at baseline (severity level 10/10) and were at risk of developing DR in either or both eyes at follow-up (severity level >20/≤ 20 or>20/>20).
At -risk cohort for incidence in 2nd eye: Only one eye which did not have evidence of any DR at baseline (severity level 10) was at risk of developing DR at follow-up. The contralateral eye had some evidence of DR at baseline (severity level >20), or was ungradable.
The-risk cohort for incidence in either eye (combines columns 1 (both eyes at risk) and 2 (one eye at risk) to determine incidenceat of any DR in either eye.
Age-standardized to the LALES population.
Refers to persons who were newly diagnosed with diabetes at the time of baseline examination.
Incidence for ME (without CSME) in the first eye was 5.0% (Table 2). No age-related association was observed (P=0.34). However, there was a significant increasing trend in incidence with increasing duration of diabetes (P=0.004). Duration-specific incidence ranged from 3.0% for new cases of diabetes to 11.9% for persons with 15 or more years of diabetes. Incidence in the first eye for CSME was 6.3% (Table 3). Incidence did not significantly increase with age (P = 0.35), but there was a significant positive association between duration of diabetes and CSME (P=0.01) (Table 3). Duration-specific incidence of CSME ranged from 1.8% for newly diagnosed cases of diabetes to 6.0% for persons who had had diabetes for 15 or more years.
Table 2.
Estimated Four-year Incidence of Macular Edema (without Clinically Significant Macular Edema) Stratified by Age and Duration of Diabetes at Baseline
| Baseline Characteristics |
Incidence in 1st eye* |
Incidence in 2nd eye† |
Incidence in either eye‡ |
|||
|---|---|---|---|---|---|---|
| n | % 22 | n | % 22 | n | % 22 | |
| Age (years) | ||||||
| 40-49 | 151 | 6.6 (2.7, 10.6) | 8 | 37.5 (4.0, 71.1) | 159 | 8.2 (3.9, 12.4) |
| 50-59 | 242 | 5.4 (2.5, 8.2) | 21 | 9.5 (0.0, 22.1) | 263 | 5.7 (2.9, 8.5) |
| 60-69 | 174 | 2.3 (0.1, 4.5) | 10 | μ | 184 | 2.2 (0.1, 4.3) |
| 70+ | 80 | 6.3 (1.0, 11.6) | 13 | 7.7 (0.0, 22.2) | 93 | 6.5 (1.5, 11.4) |
| P = 0.34 | P = 0.07 | P = 0.14 | ||||
| Overall | 647 | 5.0 (3.3, 6.6) | 52 | 11.5 (2.9, 20.2) | 699 | 5.4 (3.8, 7.1) |
| Age-standardized§ | 5.3 (3.5, 7.1) | 17.8 (3.6, 32.0) | 6.0 (4.1, 7.9) | |||
|
| ||||||
| Duration of Diabetes (years) | ||||||
| New€ | 166 | 3.0 (0.4, 5.6) | 11 | μ | 177 | 2.8 (0.4, 5.3) |
| 1-4 | 168 | 4.2 (1.1, 7.2) | 8 | 25.0 (0.0, 55.0) | 176 | 5.1 (1.9, 8.4) |
| 5-9 | 130 | 2.3 (0.0, 4.9) | 10 | 10.0 (0.0, 28.6) | 140 | 2.9 (0.1, 5.6) |
| 10-14 | 99 | 7.1 (2.0, 12.1) | 8 | 25.0 (0.0, 55.0) | 107 | 8.4 (3.2, 13.7) |
| ≥ 15 | 84 | 11.9 (5.0, 18.8) | 15 | 6.7 (0.0, 19.3) | 99 | 11.1 (4.9, 17.3) |
| P = 0.004 | P = 0.76 | P = 0.003 | ||||
| Overall | 647 | 5.0 (3.3, 6.6) | 52 | 11.5 (2.9, 20.2) | 699 | 5.4 (3.8, 7.1) |
n = number at risk at baseline; persons with gradable fundus photographs at baseline and at follow-up, and have diabetes.
P = Test of trend. Incidence data presented as percent (95% confidence interval),
At-risk cohort for incidence in the 1st eye: Both eyes did not have evidence of any macular edema (ME) and had not been previously treated with focal photocoagulation at baseline and were at risk of developing lesions characteristic of ME but not clinically significant macular edema (CSME) in either or both eyes at follow-up.
At-risk cohort for incidence in the 2nd eye: Only one eye which did not have any evidence of ME and had not been previously treated with focal photocoagulation at baseline was at risk of developing lesions characteristic of ME but not CSME at follow-up. The contralateral eye had some evidence of ME, or had been previously treated with focal photocoagulation at baseline, or was ungradable.
The-risk cohort for incidence in either eye (combines columns 1 (no ME in both eyes) and 2 (no ME in one eye) to determineat incidence of ME without CSME in either eye.§Age-standardized to the LALES population.
Refers to persons who were newly diagnosed with diabetes at the time of baseline examination.
No incidence cases.
Table 3.
Estimated Four-year Incidence of Clinically Significant Macular Edema Stratified by Age and Duration of Diabetes at Baseline
| Baseline Characteristics |
Incidence in 1st eye* |
Incidence in 2nd eye† |
Incidence in either eye‡ |
|||
|---|---|---|---|---|---|---|
| n | % 22 | n | % 22 | n | % 22 | |
| Age (years) | ||||||
| 40-49 | 151 | 6.6 (2.7, 10.6) | 8 | 25.0 (0.0, 55.0) | 159 | 7.6 (3.4, 11.7) |
| 50-59 | 242 | 7.9 (4.5, 11.2) | 21 | 14.3 (0.0, 29.3) | 263 | 8.4 (5.0, 11.7) |
| 60-69 | 174 | 4.6 (1.5, 7.7) | 10 | 10.0 (0.0, 28.6) | 184 | 4.9 (1.8, 8.0) |
| 70-79 | 80 | 5.0 (0.2, 9.8) | 13 | 23.1 (0.1, 46.0) | 93 | 7.5 (2.2, 12.9) |
| P = 0.35 | P = 0.96 | P = 0.53 | ||||
| Overall | 647 | 6.3 (4.5, 8.2) | 52 | 17.3 (7.0, 27.6) | 699 | 7.2 (5.2, 9.1) |
| Age-standardized§ | 6.4 (4.5, 8.4) | 18.3 (6.4, 30.3) | 7.3 (5.2, 9.3) | |||
|
| ||||||
| Duration of Diabetes (years) | ||||||
| New€ | 166 | 1.8 (0.0, 3.8) | 11 | 9.1 (0.0, 26.1) | 177 | 2.3 (0.1, 4.5) |
| 1-4 | 168 | 5.4 (2.0, 8.8) | 8 | μ | 176 | 5.1 (1.9, 8.4) |
| 5-9 | 130 | 10.0 (4.8, 15.2) | 10 | 30.0 (1.6, 58.4) | 140 | 11.4 (6.2, 16.7) |
| 10-14 | 99 | 11.1 (4.9, 17.3) | 8 | 12.5 (0.0, 35.4) | 107 | 11.2 (5.2, 17.2) |
| ≥ 15 | 84 | 6.0 (0.9, 11.0) | 15 | 26.7 (4.3, 49.1) | 99 | 9.1 (3.4, 14.8) |
| P = 0.01 | P = 0.17 | P = 0.002 | ||||
| Overall | 647 | 6.3 (4.5, 8.2) | 52 | 17.3 (7.0, 27.6) | 699 | 7.2 (5.2, 9.1) |
n = number at risk at baseline; persons with gradable fundus photographs at baseline and at follow-up, and have definite diabetes. P = Test of trend. Incidence data presented as percent (95% confidence interval),
At-risk cohort for incidence in the 1st eye: Both eyes did not have evidence of any macular edema (ME) and had not been previously treated with photocoagulation at baseline, and were at risk of developing lesions characteristic of clinically significant macular edema (CSME) or be treated with focal and/or grid photocoagulation in either or both eyes at follow-up.
At-risk cohort for incidence in the 2nd eye: Only one eye which did not have evidence of any ME and had not been previously treated with photocoagulation at baseline was at risk of developing lesions characteristic of CSME or be treated with focal and/or grid photocoagulation at follow-up. The contralateral eye had some evidence of CSME, had been previously treated with photocoagulation at baseline, or was ungradable.
The-risk cohort at for incidence in either eye combines columns 1 (no ME or photocoagulation signs in both eyes) and 2 (no ME or photocoagulation signs in one eye) to determine an incidence of CSME in either eye.
Age-standardized to the LALES population.
Refers to persons who were newly diagnosed with diabetes at the time of baseline examination.
No incidence cases.
Incidence in the second eye
Incidence of DR in the second eye was higher than in the first eye (51.9% vs 28.2%, Table 1). Incidence of DR decreased with increasing age, from 76.0% (40-49 years) to 26.1% (70+ years), (P<0.001). No pattern was observed with duration of diabetes and incidence of DR (P=0.51). Incidence of ME in the second eye was 11.5%, double that observed in the first eye (5.0%, Table 2). Increasing age was marginally associated with ME (P=0.07), but longer duration of diabetes was not associated with ME (P=0.76). Clinically significant macular edema in the second eye was observed in 17.3% participants (Table 3). Age-specific estimates ranged from 25.0% to 23.1% for 40-49 years and 70+ years, respectively, with no significant pattern (P=0.96). Duration of diabetes was not associated with incidence in the second eye (P=0.17).
Incidence in either eye
The overall incidence of DR in either eye was 34.0% (Table 1). Age-specific incidence of DR in this group decreased significantly with age (P<0.001). Incidence ranged from 45.5% in participants 40-49 years old to 24.3% in those 70 or more years old (P<0.001). Duration-specific incidence ranged from 20.4% (0 years) to 55.1% (10-14 years), then dropped to 39.5% in those who had had diabetes for 15 or more years (P<0.001). The overall incidence of ME in either eye was 5.4% (Table 2). There was no trend in incidence across age groups (P=0.14). Incidence of ME increased with duration of diabetes from 2.8% (0 years) to 11.1% (15+ years) (P=0.003). Clinically significant macular edema in either eye was observed in 7.2% (Table 3). Age-specific estimates were not significantly associated with CSME (P=0.53). Duration-specific incidence ranged from 2.3% (0 years) to 9.1% (15+ years) (P=0.002).
Incidence of DR and Treatment
When we examined the association of DR with treatment for diabetes (results not shown), we found a significantly higher incidence of DR in people who received no treatment for diabetes (33.3%, 92/276) compared to those who received treatment (17.1%, 22/128)(P=0.008). Any treatment included insulin, pills, diet, natural remedy and other treatments.
4-Year Progression and Improvement of Diabetic Retinopathy
Progression of any DR was noted in 38.9% (Table 4) of diabetics with DR at baseline. When results were stratified by age group, higher incidence of DR was observed in younger age groups. Incidence of DR ranged from 58.2% for those 40-49 years of age to 25.0% to those 70+ years of age, (P<0.001). Duration of diabetes was not significantly associated with progression (P=0.17). Among diabetics with NPDR at baseline, 5.3% (Table 4) had developed PDR at follow-up. Age-specific estimates ranged from 4.5% for those aged 40-49 to 4.2% for participants 70+ years. No significant pattern was found (P=0.61). Duration-specific estimates ranged from 2.8% for those with 0 years to 8.3% for those with 15+ years of diabetes (P=0.08). Progression of NPDR to PDR with high-risk characteristics occurred in 1.9% (Table 4) of diabetics. Age-specific and duration-specific estimates were too small to test for differences since some strata reported no progression.
Table 4.
Estimated Four-year Progression and Improvement of Diabetic Retinopathy Stratified by Age at Baseline
| Baseline Characteristics |
Progression of any DR* |
Progression from NPDR to PDR† |
Progression from NPDR to PDR with HRC‡ |
Improvement§ |
||||
|---|---|---|---|---|---|---|---|---|
| n | % 22 | n | % 22 | n | % 22 | n | % 22 | |
| Age (years) | ||||||||
| 40-49 | 67 | 58.2 (46.4, 70.0) | 67 | 4.5 (0.0, 9.4) | 67 | 1.5 (0.0, 4.4) | 52 | 5.8 (0.0, 12.1) |
| 50-59 | 124 | 39.5 (30.9, 48.1) | 124 | 7.3 (2.7, 11.8) | 124 | 4.0 (0.6, 7.5) | 99 | 15.2 (8.1, 22.2) |
| 60-69 | 85 | 30.6 (20.8, 40.4) | 85 | 3.5 (0.0, 7.5) | 85 | μ | 77 | 15.6 (7.5, 23.7) |
| 70-79 | 48 | 25.0 (12.8, 37.3) | 48 | 4.2 (0.0, 9.8) | 48 | μ | 37 | 18.9 (6.3, 31.5) |
| P < 0.001 | P = 0.61 | P = 0.19 | P = 0.09 | |||||
| Overall | 324 | 38.9 (33.6, 44.2) | 324 | 5.3 (2.8, 7.7) | 324 | 1.9 (0.4, 3.3) | 265 | 14.0 (9.8, 18.1) |
| Age-standardized∥ | 43.1 | (35.6, 50.6) | 5.1 (2.7, 7.6) | 1.8 (0.4, 3.3) | 12.2 (8.2, 16.1) | |||
|
| ||||||||
| Duration of Diabetes (years) | ||||||||
| New€ | 36 | 36.1 (20.4, 51.8) | 36 | 2.8 (0.0, 8.2) | 36 | μ | 25 | 20.0 (4.3, 35.7) |
| 1-4 | 55 | 40.0 (27.1, 53.0) | 55 | 1.8 (0.0, 5.4) | 55 | 1.8 (0.0, 5.4) | 32 | 6.3 (0.0, 14.6) |
| 5-9 | 80 | 48.8 (37.8, 59.7) | 80 | 5.0 (0.2, 9.8) | 80 | 6.3 (1.0, 11.6) | 56 | 10.7 (2.6, 18.8) |
| 10-14 | 69 | 42.0 (30.4, 53.7) | 69 | 5.8 (0.3, 11.3) | 69 | μ | 59 | 13.6 (4.8, 22.3) |
| ≥ 15 | 84 | 27.4 (17.8, 36.9) | 84 | 8.3 (2.4, 14.2) | 84 | μ | 93 | 17.2 (9.5, 24.9) |
| P = 0.17 | P = 0.08 | P = 0.34 | P = 0.48 | |||||
| Overall | 324 | 38.9 (33.6, 44.2) | 324 | 5.3 (2.8, 7.7) | 324 | 1.9 (0.4, 3.3) | 265 | 14.0 (9.8, 18.1) |
n = number at risk at baseline. P = Test of trend. DR = diabetic retinopathy; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy; HRC = high-risk characteristics for visual loss. Data presented as percent (95% confidence interval).
Progression of any DR: Baseline = Presence of some retinopathy in either eye (severity level>20/≤ or >20/>20).Follow-up: A two-step or more increase in severity of retinopathy.
Progression from NPDR to PDR: Baseline = No PDR in both eyes,but with some retinopathy in either eye (severity level >20/≤20,or >20/>20, up through <60/<60). Follow-up: Presence of PDR (severity level ≥ 60/<60, or ≥ 60/≥ 60) in at least one eye, or signs of panretinal photocoagulation treatment.
Progression from NPDR to PDR with HRC: Baseline = No PDR in both eyes, but with some retinopathy in either eye (severity level >20/≤20 or >20/>20, up through <60/<60). Follow-up: Presence of PDR with high-risk characteristics for visual loss (severity level ≥ 71/<71, or ≥71/≥ 71) as defined in the Diabetic Retinopathy Study, in at least one eye.
Improvement: Baseline = Presence of some retinopathy in either eye (severity level >20/≤ 20 or >20/>20). Follow-up: A two-step or more decrease in severity of retinopathy.
Age-standardized to the LALES population.
Refers to persons who were newly diagnosed with diabetes at the time of baseline examination.
No progression
Improvement of DR was seen in 14.0% (Table 4) of diabetics with some level of retinopathy at baseline. Age-specific estimates ranged from 5.8% for those 40-49 years to 18.9% for those 70+ years of age. The results revealed a marginally significant pattern (P=0.09). Duration of diabetes showed no significant pattern (P=0.48).
DISCUSSION
In LALES, we found that incidence of DR (28% in first eye) and ME (5% in first eye) in Latinos was high compared to incidence previously reported for US non-Hispanic whites. A higher incidence of DR, ME or CSME was associated with longer duration of diabetes, despite the fact that incidence of these ocular diseases decreased with age. For example, incidence of DR( in the first eye, second eye, or both) at the 4- year follow-up examination was highest among the youngest age groups (40–49 years and 50-59 years). One explanation for the higher incidence in the youngest age groups may be that the younger adults had a lower prevalence of existing retinopathy at baseline; 74% of the 40-49 year olds at baseline reported living with diabetes for a maximum of 5 years. We found that more (40%) diabetics progressed to any DR (2-step increase), and only a few (<1%) diabetics progressed from NPDR to PDR with HRC. There was no clear association between age, duration of diabetes, and progression and of DR.
Improvement in retinopathy occurred in approximately 14% of adults with DR at baseline and occurred more often in those who were older or who had diabetes for a longer duration. Of the 37 participants who improved, none reported having undergone panretinal photocoagulation treatment, indicating their improvement was related to some factor other than surgery.
At the 4-year follow-up examination, incidence of DR in the second eye (among those with DR in one eye at baseline) was nearly twice as high as compared to incidence in the first eye (among those with no DR at baseline). This finding is significant from a health-related quality of life perspective because individuals with disease in only one eye tend to rely heavily on their contralateral eye for daily tasks. When the healthy eye also develops disease, the ability of people to complete vision related tasks and normal activities become severely impaired.
Comparison with Other Studies
Compared to other studies (Table 9) the incidence of DR in the first eye in the LALES was similar to that reported in persons with type 2 diabetes mellitus in the predominantly white WESDR cohort and the black BISED cohort but higher than reported in the non-Hispanic white BMES cohort with type 2 diabetes mellitus. The estimated annualized incidence was 7.1% for LALES, 8.6% for WESDR, 7.5% for BISED, and 4.4% for BMES. The estimated annual incidence in the LALES is also higher than the estimated annualized incidence in non-Hispanic Whites from AusDiab study20 (5 year cumulative incidence 13.9 %; annualized 2.78%) as well as in Liverpool eye study (5 year cumulative incidence 3.9 %; annualized 0.8 %)21. The WESDR study was designed to assess complications associated with diabetes e.g., retinopathy, ME, nephropathy. It identified persons with diabetes defined as having a fasting blood sugar or 140 mg/dL or higher or a random blood sugar of 200 mg/dL or higher living in and receiving their care in an eleven county area in southern Wisconsin in 1978-80 different from LALES which included only participants with diabetes in the general population who had either been previously diagnosed to have diabetes or who were newly defined as having diabetes if the glycosylated hemoglobin was higher than 7.0% or random blood sugar >200 mg/dL. The higher incidence of DR in the LALES than in other white cohorts is consistent with data from previous studies showing higher prevalence in Latinos compared to whites even when risk factors e.g., glycosylated hemoglobin, blood pressure, was controlled for.
Findings from the San Luis Valley Study8 report lower rates of DR than our study. The San Luis Valley Study reported that Hispanics had a lower incidence of DR than non-Hispanic whites. However, the cohort for the San Luis Valley Study was younger and the study had fewer adults at risk for retinopathy (n=169).
Strengths and Limitations
LALES has several strengths. First, it is a population-based study with a large sample size and a high participation rate (76% for LALES II). Second, the use of standard methods of diagnosis and assessment of severity levels of DR allows comparison with other incidence studies, such as BDES, BISED, and WESDR. Third, the strict quality control procedures implemented in LALES minimize measurement error. Fourth, it is the largest study to evaluate incidence and progression of DR and ME in a Latino population.
Our study also has some limitations. The sample includes all eligible members in a household, which may result in the overestimation of incidence if DR or ME are more likely to occur within families. However, we have previously shown that such a cluster effect does not impact the prevalence estimates of visual impairment in this study. A second potential limitation is that these results are not generalizable to all Latino subgroups because the study population is primarily comprised of Mexican-Americans. Third, since we did not study institutionalized Latinos, the study may under estimate the incidence of DR as Klein and associates have noted that those living in nursing homes have a much higher rate of vision loss compared to non-institutionalized persons. However, since Latinos are less likely to utilize nursing homes (compared to non-Hispanic Whites) for family members, we expect the small number of Latinos actually residing in such facilities would not significantly impact the incidence estimates.
In summary, we found the 4-yr. incidence and progression of DR and ME in adult Latinos was high compared to frequencies reported in other racial/ethnic groups. These results emphasize the importance of timely dilated ophthalmologic examinations for Latinos who have diabetes and are at risk for vision-threatening DR. In addition, it confirms the importance of managing diabetes in diabetic persons to help detect and reduce the risk of proliferative diabetic retinopathy.
Supplementary Material
Table 5.
Estimated Annual Incidence of Diabetic Retinopathy in Population-Based Studies
| Study population and location |
Year of study | Age range (years) |
No. of photo- graphic fields graded |
Crude Annual Incidence of DR* |
Crude Annual Incidence of DR by Duration of Diabetes (years) % (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Base- line |
Follow -up |
n | % (95% CI) | <5† | 5-9 | 10-14 | ≥15 | |||
| Latinos | ||||||||||
| Los Angeles, USA |
2000 2003 |
2004- 2007 |
≥40 | 7 | 421 | 7.1 (4.6, 9.6) | 5.6 (2.9, 8.3) | 7.7 (1.4, 14.1) | 12.5 (2.9, 22.1) | 10.5 (0.0, 21.3) |
|
| ||||||||||
| San Luis Valley, USA |
1984 1988 |
1988- 1992 |
20-74 | 3 | 116 | 5.2 (1.2, 9.2) | Did not stratify results by duration of diabetes | |||
| African-ancestry | ||||||||||
| Barbados | 1988 1992 |
1992- 1997 |
40-84 | 2 | 306 | 7.5 (5.0, 10.0) | 5.7 (2.5, 8.9) | 12.7 (4.3, 21.1) | 10.0 (0.0, 21.8) | 7.2 (0.0, 18.2) |
| Non-Hispanic white | ||||||||||
| Wisconsin, USA |
1980- 1982 |
1984- 1986 |
≥30 | 7 | 320 | 8.6 (5.5, 11.7) | 7.8 (3.5, 12.0) | 8.1 (2.7, 13.4) | 9.5 (0.0, 20.1) | 12.9 (2.1, 23.6) |
|
| ||||||||||
| San Luis Valley, USA |
1984- 1988 |
1988- 1992 |
20-74 | 3 | 53 | 6.6 (0.0, 13.2) | Did not stratify results by duration of diabetes | |||
|
| ||||||||||
| Blue Mountains, Australia |
1992- 1994 |
1997- 1999 |
≥49 | 5 | 90 | 4.4 (0.2, 8.7) | Did not stratify results by duration of diabetes | |||
|
| ||||||||||
| Melbourne, Australia |
1992- 1994 |
1997- 1999 |
≥40 | 2 | 73 | 2.2 (0.0, 5.6) | Did not stratify results by duration of diabetes | |||
DR = diabetic retinopathy.%=incidence CI = confidence interval, n = number at risk for incidence of DR with definite diabetes at baseline.
Incidence of DR defined as absence of retinopathy in both eyes for persons with definite diabetes at baseline, and presence of any retinopathy in either eye at follow-up. Crude annual incidence estimated from 4-year incidence for studies in Los Angeles, San Luis Valley, Barbados and Wisconsin, and 5-year incidence for studies in Melbourne and Blue Mountains.
Include persons who were newly diagnosed with diabetes at
ACKNOWLEDGEMENTS
A. Support: National Institutes of Health Grants NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
D. Statement about Conformity: The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
E. Other Acknowledgements: The Los Angeles Latino Eye Study Group (LALES I)
University of Southern California, Los Angeles, CA.-Rohit Varma, MD, MPH; Sylvia H. Paz, MS; Fernando Pena, MD; Stanley P. Azen, PhD; Jaime Barrera; Lupe Cisneros, COA; Elizabeth Corona; Carolina Cuestas, OD; Jeanne Dzekov, BS; Ana Evans, OD; Denise R. Globe, PhD; Carlos Lastra, MD; Mei-Ying Lai, MS; George Martinez; Ronald E. Smith, MD; LaVina Tetrow, Mina Torres, MS; Natalia Uribe, OD; Joanne Wu, MPH; Myrna Zuniga.
Battelle Survey Research Center, St. Louis, MO, Lisa John, PhD; Candace Kwong MS; Karen Tucker, MA; Natasha L. Walker; Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI Ronald Klein, MD, MPH.
The Los Angeles Latino Eye Study Group (LALES II)
University of Southern California, Los Angeles, CA: Rohit Varma, MD, MPH (Principal Investigator); Stanley P. Azen, PhD (Co–Principal Investigator); Mina Torres, MS (Project Director); Jaime Barrera; Farzana Choudhury, MBBS, MPH; Lupe Cisneros, COA; Jessica Chung, MPH, PhD; Elizabeth Corona; Carolina Cuestas, OD; Anne DiLauro, MPH (2005-2007); Jeanne Dzekov; Ana Evans (2004-2007); Athena W.P. Foong; Carlos Lastra, MD; Mei-Ying Lai, MS; George Martinez; Roberta McKean-Cowdin, PhD; Carlos Moya; Sylvia H. Paz, MS (2004-2005); Fernando Pena, MD (2004-2005); Corina Shtir, MS; Ronald E. Smith, MD; LaVina Tetrow (2004-2005); Heather Volk, PhD; Ying Wang, MS (2006-2007); Joanne Wu, MPH (2004-2006). Battelle Survey Research Center, St. Louis, MO: Lisa John, MSW; Karen Tucker, MA; Natasha Van Leeuwen.
Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI: Ronald Klein, MD, MPH; Tiffany Jan; Michael D. Knudtson, MA; Stacy E. Meuer; Michael Neider.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
B. Financial Disclosure: The authors have no proprietary or commercial interest.
Supplemental Materials available at AJO.com
REFERENCES
- 1.US Census Bureau United States Census 2000. 2000
- 2.US Census Bureau U.S. Census Bureau projections of the resident population by race, Hispanic origin, and nativity: middle series 2050 to 2070, NP-T5-G ed
- 3.Wilson MR, Eezzuduemhoi DR. Ophthalmologic disorders in minority populations. Med Clin North Am. 2005;89:795–804. doi: 10.1016/j.mcna.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.West SK, Klein R, Rodriguez J, et al. Diabetes and diabetic retinopathy in a Mexican-American population: Proyecto VER. Diabetes Care. 2001;24:1204–9. doi: 10.2337/diacare.24.7.1204. [DOI] [PubMed] [Google Scholar]
- 5.Varma R, Torres M, Pena F, Klein R, Azen SP. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1298–306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Hamman RF, Mayer EJ, Moo-Young GA, Hildebrandt W, Marshall JA, Baxter J. Prevalence and risk factors of diabetic retinopathy in non-Hispanic whites and Hispanics with NIDDM. San Luis Valley Diabetes Study. Diabetes. 1989;38:1231–7. doi: 10.2337/diab.38.10.1231. [DOI] [PubMed] [Google Scholar]
- 7.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, hispanics, and asians. Diabetes Care. 2004;27:2317–24. doi: 10.2337/diacare.27.10.2317. [DOI] [PubMed] [Google Scholar]
- 8.Tudor SM, Hamman RF, Baron A, Johnson DW, Shetterly SM. Incidence and progression of diabetic retinopathy in Hispanics and non-Hispanic whites with type 2 diabetes. San Luis Valley Diabetes Study, Colorado. Diabetes Care. 1998;21:53–61. doi: 10.2337/diacare.21.1.53. [DOI] [PubMed] [Google Scholar]
- 9.Varma R, Wu J, Chong K, Azen SP, Hays RD. Impact of severity and bilaterality of visual impairment on health-related quality of life. Ophthalmology. 2006;113:1846–53. doi: 10.1016/j.ophtha.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217–28. doi: 10.1001/archopht.1994.01090210105023. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107:244–9. doi: 10.1001/archopht.1989.01070010250031. [DOI] [PubMed] [Google Scholar]
- 13.Leske MC, Wu SY, Hyman L, Nemesure B, Hennis A, Schachat AP. Four-year incidence of visual impairment: Barbados Incidence Study of Eye Diseases. Ophthalmology. 2004;111:118–24. doi: 10.1016/j.ophtha.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Leske MC, Wu SY, Nemesure B, Yang L, Hennis A. Nine-year incidence of lens opacities in the Barbados Eye Studies. Ophthalmology. 2004;111:483–90. doi: 10.1016/j.ophtha.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–31. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–7. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 17.Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 18.ETDRS Manual of Operations. Maryland Medical Research Institute; Baltimore: 1980. Early Treatment Diabetic Retinopathy Study Research Group: Patient eligibility and exclusion criteria; pp. 1–10. [Google Scholar]
- 19.SAS Institute SAS. Cary, NC.
- 20.Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008;31:1349–54. doi: 10.2337/dc07-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younis N, Broadbent DM, Vora JP, Harding SP. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. 2003;361:195–200. doi: 10.1016/s0140-6736(03)12267-2. [DOI] [PubMed] [Google Scholar]
- 22.Varma R, Macias GL, Torres M, Klein R, Pena FY, Azen SP. Biologic risk factors associated with diabetic retinopathy: the Los Angeles Latino Eye Study. Ophthalmology. 2007;114:1332–40. doi: 10.1016/j.ophtha.2006.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.