Abstract
Life-threatening allergies to peanuts and tree nuts can be revealed by detecting antibodies (IgEs) to their allergens in patient serum. Herein, we compare several immunosensor-like methodologies for sensitive detection of antibodies to a peptide sequence from the major peanut allergen, Arachis hypogaea 2 (Ara h2). The sensors feature a synthetic peptide layer of the major IgE-binding epitope from Ara h2 attached to a dense gold nanoparticle (AuNP) film on a pyrolytic graphite (PG) electrode. The AuNP-peptide sensor was used to determine model chicken anti-peanut antibodies (IgY) in serum. Faradaic and non-Faradaic impedance strategies were compared to amperometric detection. Measurements employed goat anti-chicken secondary antibodies (Ab2) labeled with horseradish peroxidase (HRP) to bind to IgY on the sensor and provide amplified signals. The best impedimetric sensor configuration featured HPR-catalyzed precipitation of the enzyme product onto the sensor measured by non-Faradaic impedance. This sensor configuration had the best detection limit (DL) of 5 pg mL−1 and the best linear range of over 5 orders of magnitude (5 pg mL−1 to 1 µg mL−1) for IgY antibody in undiluted calf serum. This DL was 100-fold lower than label-free impedimetric immunosensors (0.5 ng mL−1), and 60-fold lower than when using HRP-Ab2 in amperometric immunosensors (0.3 ng mL−1).
INTRODUCTION
Allergies to peanuts and tree nuts are a critical health problem for millions of Americans. These allergies have occurred with such an increasing incidence in the U.S. that recently they have been called an epidemic.1 Estimates place the prevalence of peanut/tree nut allergy at approximately 1% of the US population.2 They are particular dangerous to young children. Allergy to nuts can result in severe anaphylactic shock, accounting for up to 30,000 emergency hospital visits per year in the US and approximately 150 deaths.3 The prevalence of foods that contain peanuts and peanut products makes avoidance difficult and risky for affected individuals.
Eight Arachis hypogaea proteins (Ara h1–8) are currently considered the most important peanut allergens.4 Amongst these, Ara h1, Ara h2, and Ara h3 elicit the majority of specific immunoglobulin E (IgE) antibodies in allergic individuals. Individual epitopes from these proteins can depend either on the three-dimensional structure of the protein (a conformational epitope) or simply on a given sequence of amino acids (an extended epitope). It has been suggested that extended epitopes are more immunogenic as evidenced by the persistence of an allergy in children with IgEs to extended epitopes, but a deterioration of allergy for those with IgEs to conformational epitopes.5,6
Specific IgE levels for individual peptide sequences of the Ara h1–3 proteins have been measured in serum from peanut-allergic patients. A key observation from these studies was that the severity of clinical allergy correlates with the diversity of specific IgEs in an allergic patient. That is, the greater the number of epitopes recognized by IgEs in an individual, the higher the likelihood for severe allergic reactions. Thus, IgE concentrations in serum can be used to assess the likelihood of patients having peanut allergies.7–9 Measurements of total allergen specific IgE antibodies from blood serum and an allergy skin prick test (SPT) are two available tests used to assist physicians in diagnosis of allergic patients,10,11 but they lack specificity.
Our long-term goal is to develop peptide-based bioelectronic sensor arrays to accurately measure multiple anti-peanut allergen IgEs in human serum. We hope to eventually develop a point-of-care diagnostic tool to evaluate risk factors associated with IgE-mediated peanut allergy. As a step toward this goal, here we evaluate several possible sensor formats for bioelectronic detection of IgE-like antibodies.
Faradaic and non-Faradaic impedance spectroscopies are effective methods to probe the interfacial electron transfer resistance at a functionalized electrode.12–14 In Faradaic impedance sensors, the interaction between a charged redox couple and the electrode surface is reflected by the charge transfer resistance (Rct), which increases as molecules bind to the electrode surface.15 For example, aptamers were attached to sensor surfaces in impedance assays16–19 to detect proteins and antibodies. Faradaic impedance spectroscopy has been used to detect insulating layers on electrodes. One highly sensitive strategy involves using enzyme labels to catalyze reactions that precipitate insoluble products, thus greatly increasing the impedance.20–22
Non-Faradaic impedance employs no redox probes, and impedance changes arise primarily from displacement of water and ions by molecules binding to the sensor surface. Careful method optimization for flow injection electrochemical impedance spectroscopy led to attomole detection of the protein interferon-γ using non-Faradaic impedance.23,24
An alternative to impedance sensors is to use amperometric sensing with nanostructured electrodes and multi-label nanoparticles in sandwich immunoassay formats. We employed this strategy to obtain sub-pg mL−1 detection limits for cancer biomarker proteins such as prostate specific antigen (PSA)25 and interleukin-6 (IL-6).26 The best analytical performance was obtained using electrodes coated with dense layers of 5 nm diameter glutathione-decorated gold nanoparticles AuNP (GSH-AuNP).27,28 These conductive, nanostructured films enlarge the electroactive surface area and provide carboxylate groups on the surface to covalently bind a dense layer of antibodies or proteins for detection of their binding partners.
To the best of our knowledge, impedance has not yet to be used with a peptide layer to detect IgE antibodies, although there are several reports of using epitopes to detect IgE in fluorescent immunoassays6,7 and of using aptamers to detect anti-IgE by impedance18, 29 and other methods.30,31 In this paper, we use a GSH-AuNP sensor platform to attach a film containing a peptide featuring the amino acid sequence from Ara h2 having specific affinity for the IgE antibody.5,6 Chicken anti-peanut antibody IgY was chosen as a model for IgE, since it has a similar structure to IgY and is easily obtained. The performance of Ara h2-based immunosensors for the detection of IgY using detection by single-label amperometry, Faradaic electrochemical impedance spectroscopy (EIS), and non-Faradaic impedance were compared. Among these methods, non-Faradaic impedance provided the best detection limit of 5 pg mL−1 and the best linear range of 5 pg mL−1 to 1 µg mL−1.
EXPERIMENTAL SECTION
Chemicals and Materials
Poly (diallydimethylammonium chloride) (PDDA, MW 20%), lyophilized 99% bovine serum albumin (BSA), casein, Tween-20, 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (NHSS), L-glutathione reduced (GSH, 99%), HAuCl4·3H2O (99.9%), sodium borohydride (99%), 3-mercaptopropanoic acid (MPA), 3,3′,5,5′-tetramethylbenzidine (TMB) and poly (ethylene glycol) (PEG, MW 1000) were all from Sigma-Aldrich. Sodium nitroferricyanide and hydrogen peroxide (H2O2, 30%) were from Fisher.
Affinity-purified chicken anti-peanut antibody (IgY) and horseradish peroxidase (HRP) conjugated goat anti-chicken IgY antibody (HRP-Ab2) were from Immunology Consultants Laboratory Inc. Peptide fragment of 28 amino acids Ara h 2-2 (H2N-QSPSYPDREYSDEDRQIKQMLHQECPRL-CONH2, 3450 Da, predicted isoelectric point 4.95) was synthesized by Anaspec Inc. Calf serum was from Sigma-Aldrich. Immunoreagents were dissolved in pH 7.2 phosphate saline (PBS) buffer (0.01 M in phosphate, 0.14 M NaCl, 2.7 mM KCl) unless otherwise noted. EDC and NHSS were dissolved in water immediately before use. GSH-AuNPs were prepared at as described previously,25,32 and were 5 ± 1 nm in diameter by transmission electron microscopy (TEM), showed in Supporting Information.
Instrumentation
A CHI 660 electrochemical workstation was used for cyclic voltammetry, impedance and amperometry at 22 ± 2 °C in a three-electrode cell. A basal plane pyrolytic graphite (PG) disk (A = 0.16 cm2) was the working electrode, with Pt counter electrode, and saturated calomel (SCE) reference. Faradaic impedance was done using 1 mM Fe(CN)6 4− and 1 mM Fe(CN)6 3− in pH 7.0 PBS at bias potential of 0.17 V, amplitude 5 mV and frequency range 0.1 to 100 kHz. Non-Faradaic impedance was done at 0 V vs. SCE at 5 mV amplitude in pH 7.0 buffer. EvolCRT software (Version 0.6, Wuhan University, China) was used to model the data with equivalent circuits.33 Amperometry was done at −0.3 V vs SCE with PG working electrode rotated at 3000 rpm.25 Quartz crystal microbalance (QCM) measurements employed a USI (Japan) with 9 MHz Au-QCM resonators (AT-cut, International Crystal, geometric area 0.16 cm2 per side) coated with mercaptopropanoic acid (MPA) to provide an underlayer for film formation resembling PG surfaces.34 A Reichert dual channel surface Plasma resonance (SPR) spectrometer was used to find optimized incubation times (see Supporting Information).
Preparation of Sensor Films
GSH-AuNPs electrodes were prepared by assembly of layers of poly (diallydimethylammonium chloride) (PDDA) and the AuNPs on a pyrolytic graphite (PG) electrode (0.16 cm2).25 Briefly, 20 µL of PDDA solution (2 mg mL−1 + 0.5 M NaCl) was placed on PG surface for 20 min, washed, covered with 20 µL of 2 mg mL−1 GSH-AuNPs for 40 min. After washing with water and drying in a nitrogen stream, GSH-AuNP electrodes were ready for derivitization (Scheme 1, I).
Scheme 1.
Comparative detection procedures for anti-peanut IgY using AuNP-Ara h2-2 sensors (I). System II: IgY antibodies (Ab1) are captured on the sensor surface by Ara h2-2 and measured by EIS. System III: HRP-conjugated secondary antibodies (HRP-Ab2) are added, and measured by EIS or by amperometry after adding hydrogen peroxide and mediator. System IV: the previous system was exposed to TMB/H2O2 for 20 min, and biocatalyzed formation of the insoluble product results electrical. Faradaic EIS was used to detect the change in charge transfer resistance (Rct), and compared to direct impedance.
For Ara h2-2 peptide attachment, 30 µL of freshly prepared 400 mM EDC and 100 mM NHSS in water was placed onto AuNP electrode surface for 10 min, washed with water, then reacted 1 h with 20 µL 0.5 mg mL−1 Ara h 2-2 in pH 7.0 PBS. Unbound peptides were removed by rinsing thoroughly with pH 7.2 PBS − 0.05% Tween-20 (PBST) and PBS buffer for 3 min each (Scheme 1, I).
Immunosensor procedure
Non-specific binding sites on AuNP-peptide electrodes were blocked by incubating the sensors with saturated casein (pH 7.2 PBST) for 1 h. The electrodes were washed with PBST and PBS for 3 min each, then 10 µL of IgY in calf serum was placed onto the sensor surface and incubated at room temperature for 1 h (Scheme 1, II). After several PBS washings, sensors were covered with 20 µL of 1:1000 diluted HRP-Ab2 (as received) in buffer containing 0.4% casein and 0.05% Tween-20 for 1 h. Sensors were rinsed thoroughly with PBST and PBS buffer, then placed in an electrochemical cell for measurement (Scheme 1, III). For amperometry, NSB blocking agent was 0.2 % BSA in PBST buffer and blocking was done after the Ab1 incubation step.
Preparation of TMB Substrate Solution
TMB 0.005% TMB + 0.5 mM H2O2 was freshly prepared in pH 3.3 0.01 M acetate buffer containing 0.05% sodium nitroferricyanide, a stabilizing agent for oxidized TMB to facilitate crystal formation.35,36 To amplify impedance, 20 µL of TMB peroxidase substrate solution was deposited onto each sensor after binding HRP-Ab2, and let stand 20 min. After washing with water for 3 min, impedance of sensors was monitored immediately or sensors were stored at 4 °C overnight before measurement.
RESULTS
Characterization of Immunosensor Fabrication
Ara h2 is the major peanut protein allergen.37 A fragment peptide, Ara h 2-2, was based on the known antigenic sequence QSPSYPDREYSDEDRQIKQMLHQECPRL of Ara h2 and was chosen for our sensors based on its high prevalence for binding serum IgEs of allergic patients according to epitope mapping studies.5,6,38,39 Amino groups at the N-terminus and in the lysine (K) side chain of Ara h2-2 sequence allowed peptide conjugation onto the GSH-AuNP electrodes via amide bond formation to GSH carboxylate groups.
Assembly of the PDDA/AuNP/peptide film and successive binding of antibodies was monitored at each step with a quartz crystal microbalance (QCM) while making films on gold coated QCM resonators (Figure 1). The resonance frequency (−ΔF) decreased reproducibly showing an increase in mass with each step suggesting that the PDDA/AuNP/peptide films were reproducibly and successfully assembled. QCM further shows that the sensors bind IgY, and that IgY on the sensors bind the anti-IgY antibody Ab2. The average frequency decreases for each fabrication step were used to estimate the mass of each layer according to the Sauerbrey equation,40 as well as the nominal thickness (Table S1, Supporting Information). A nominal thickness of 4.1 nm was observed for the AuNP layer, consistent with the particle size and nearly complete coverage of the surface by GSH-AuNP which is similar to previous results.25
Figure 1.
QCM frequency responses for each step in the fabrication and use of Ara h2-2 immunosensor. Au-QCM resonator was treated first with mercaptopropanoic acid (MPA). Details are described in Experimental Section.
The nominal thickness of the peptide layer was ~45 nm (Supporting Information, Table S1), while the calculated extended chain length of Ara h2-2 with 28 amino acid residues is about ~10 nm.41,42 However, on the rough AuNP surface, it is probable that the peptide film fills in spaces on the surface. This issue along with inherent uncertainties in QCM thickness estimates43 makes interpretation of the nominal thickness less than straightforward. However, results suggest that the peptide layer may be cross-linked. Even after washing, residual EDC/NHSS may remain to promote cross-linking between peptide molecules. Nevertheless, QCM shows that the Ara h2-2 film binds considerable IgY in a reproducible fashion (Figure 1, Table S1) appropriate for sensing.
Surface Plasmon Resonance (SPR) was used to further evaluate the interaction of the Ara h2-2 film with IgY. A gold SPR sensor chip with a self-assembled monolayer (SAM) terminating with carboxylate groups was treated first with EDC/NHSS to create a surface on which to link the peptide amino groups. The SPR curve increased immediately after Ara h2-2 was injected (See Supporting Information Figure S2), suggesting that the peptides were attached to the gold surface successfully. The signal leveled off after about 1 hr, then IgY was injected, and the SPR signal increased and reached a steady state within ~30 min, confirming IgY capture by the Ara h2-2 film. Injection of HRP-Ab2 gave a signal that reached a steady state after 1 hr. These data suggest that 1 hr is sufficient time for each binding event.
Fabrication of immunosensors was also monitored by electrochemical impedance spectroscopy (EIS) using Fe(CN)6 3−/4− couple as the electroactive redox probe at its formal potential of 0.17 V vs SCE.44 Figure 2A shows impedance spectra as Nyquist plots for successive fabrication steps. Bare PG electrodes and PG/PDDA films exhibited Warburg-type curves12 over a wide frequency range (not shown), characteristic of diffusion-controlled electrochemical processes. The impendence spectra evolved in shape as layers were added. When IgY was bound to the sensor, concentration dependent semicircles in the high frequency domain were observed indicating that the film behaved as a physical barrier limiting access of the redox probe to the electrode surface. The diameter of the semicircle is directly related to the electron-transfer resistance (Rct), which characterizes the electron-transfer kinetics of the redox probe at the electrode interface.12 Values of Rct were estimated by fitting the impedance data onto an equivalent circuit model (Scheme 3). 12,15,33 The increased Rct values (Figure 2B) were consistent with successful assembly of peptides and proteins onto PG/PDDA/AuNP sensor surface.
Figure 2.
Impedance results for fabrication and use of immunosensor (Scheme 1, System II): (A) Nyquist plot of Faradaic impedance spectra for using 1 mM Fe(CN)6 3−/4− in pH 7.0 PBS at its formal potential with amplitude: 5 mV and frequency range: 0.1 Hz – 100 kHz. Fabrication steps for (a) AuNP, (b) Ara h 2-2, (c) PEG (NSB), (d) serum (0 ng mL−1 IgY), (e) 10 ng mL−1 (f) 1000 ng mL−1 IgY in serum. (B) Resistance to charge transfer for sensor different fabrication steps using the equivalent circuit model in Scheme 3.
Scheme 3.
Equivalent circuit model for impedance data using redox probes: Rct = resistance to charge transfer; Zw = Warburg impedance; CPE = constant phase element13; Rs = solution resistance.
Amperometric immunosensors
Sensors were first evaluated for IgY detection by amperometry using optimized protocols. Goat anti-chicken IgY conjugated to HRP was used as HRP-Ab2 to detect a sandwich immunoassay according in Scheme 1. IgY standards were prepared in calf serum, which provides a good surrogate for human serum in immunoassays. 45The sensor was incubated for 1 hr with 10 µL calf serum containing IgY, followed by washing with PBST, PBS, and incubation with BSA for 1 hr to block non-specific binding (NSB). Next, the sensor was incubated with the HRP- Ab2, followed by washing with PBST and PBS buffer successively. This immunosensor was then placed in buffer containing hydroquinone as mediator and the amperometric signal detected after H2O2 was injected to activate HRP labels. Figure 3A shows that steady state current increased with concentration of IgY between 0.3 to 250 ng mL−1, with detection limit (DL) ~0.3 ng mL−1. The calibration curve was biphasic, with linear regions between 8 and 250 ng mL−1 and 0.3 to 2 ng mL−1 (Figure 3B).
Figure 3.
Amperometric results for immunosensors incubated with IgY in 10 µL calf serum for 1 h, then Ab2-HRP in 2% BSA and 0.05% Tween-20 PBS buffer for 1 h. (A) Current at −0.3 V vs SCE and 3000 rpm after placing electrodes in buffer containing 1 mM hydroquinone, then injecting H2O2 to 0.4 mM. (B) Influence of IgY concentration on steady-state current (corrected for background) for immunosensor using the Ab2-HRP bioconjugate. Error bars represent device-to-device standard deviation (n = 3). Inset shows the calibration from 0 to 8 ng mL−1.
Immunosensors Based on Faradaic Impedance
NSB is a more serious issue for impedance than for amperometric sensors that measure only the specific HRP label. Any biomolecule in the sample that binds to the sensor will contribute to the increase in Rct. Thus, we first used EIS to evaluate how effectively NSB was minimized by using 6 blocking agents, comparing signals from calf serum and 5 ng mL−1 IgY in calf serum (See Supporting Information, Figure S3). Results showed that saturated casein in PBST buffer gave the best Rct difference between the blank serum and 5 ng mL−1 IgY in serum, and so that solution was chosen as the blocking agent.
For EIS detection, AuNP/peptide immunosensors were first incubated for 1 h with 20 µL saturated casein in pH 7.2 PBST buffer. Then, 10 µL calf serum containing IgY was incubated on the inverted sensor surface, PBST and PBS buffer were used to wash, and the washed immunosensor was placed into an electrochemical cell containing 1 mM Fe(CN)6 3−/4− pH 7.0 buffer solution for signal development (System II in Scheme 1). The impedance spectra IgY in calf serum showed semicircles increasing with the concentration of IgY (Figure 2). Rct of each spectrum was estimated using the model in Scheme 3 and these values increased linearly with concentration of IgY (Figure 4) over a range of 0.5 to 1000 ng mL−1with a DL of 0.5 ng mL−1.
Figure 4.
Calibration curve of label-free immunosensor (System II) using EIS. 1 mM Fe(CN)63−/4− in pH 7.0 PBS buffer containing 0.1 M KCl, bias potential: 0.17 V, amplitude: 5 mV, frequency range: 0.1 Hz– 100 kHz.
Biocatalyzed Amplification with HRP-Ab2
HRP conjugated goat anti-chicken IgY was used as HRP-Ab2 to fabricate a sandwich immunoassay (Scheme 1) in which HRP produced an insoluble reaction product (see Scheme 2). After AuNP/peptide immunosensors were incubated with different concentrations of IgY calf serum for 1 h, the washed electrodes were then incubated with HRP-labeled secondary anti-IgY for another 1 h (System III in Scheme 1). After washing again, the sensors were transferred to pH 7.0 ferri/ferrocyanide solutions. Rct values estimated from EIS Nyquist curves for different concentrations gave sensitivity and reproducibility (See Supporting Information Figure S4) that were both worse than for the label-free immunosensors (Figures 4, and S4 in Supporting Information).
Scheme 2.
TMB is oxidized by peroxide-activated HRP to produce an insoluble oxidized product at pH 3.5 to amplifying the impedance of Ab2-HRP linked immunosensors.
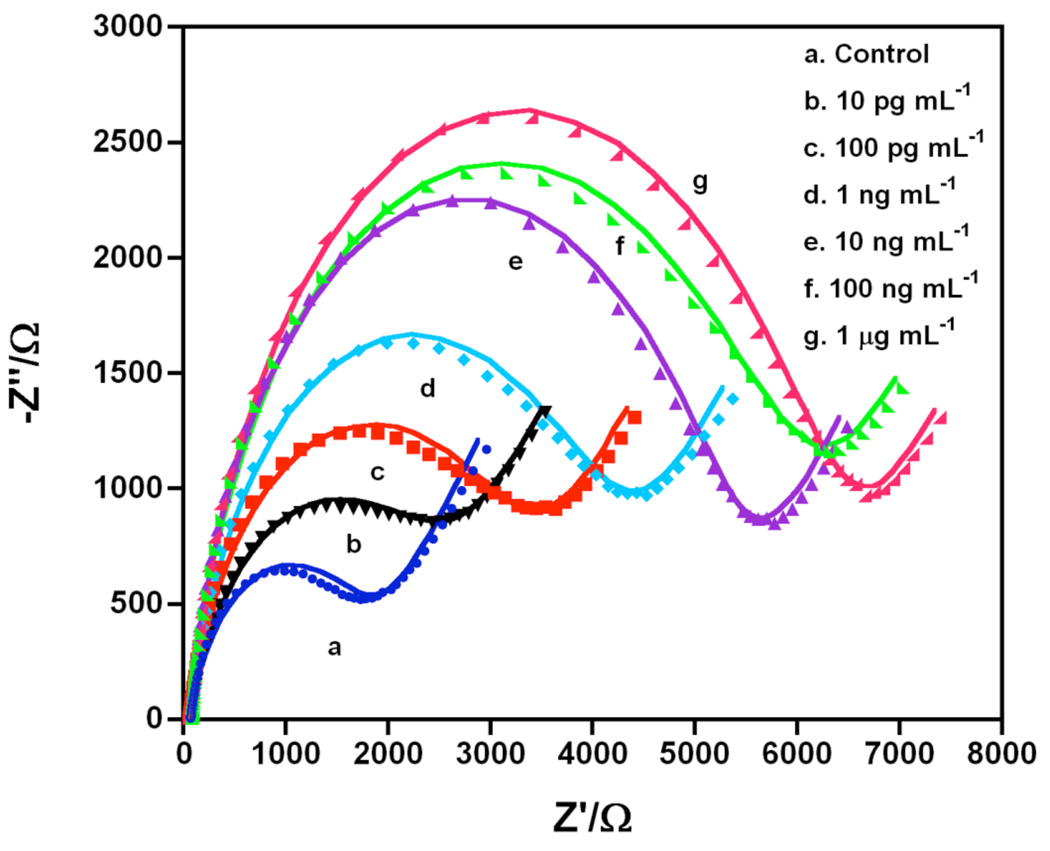
HRP was then used as a biocatalyst to oxidize 3,3′,5,5′-tetramethylbenzidine (TMB) to form an insoluble product on the sensor (Scheme 2).22,35,36 After the HRP-Ab2 incubation step, the immunosensors were dipped into TMB/H2O2 solution for 20 min, following by washing with water (System IV in Scheme 1). The washed immunosensors were then immersed into the redox probe buffer for EIS. Figure 5 shows Nyquist curves for different concentrations of IgY in serum. The semicircle of the curve increased with IgY concentration. Good fits of these data onto the model in Scheme 3 (Figure 5) provided Rct. This resulted in a linear calibration curve of Rct vs log CIgY from 5 pg mL−1 to 1 µg mL−1, extending over 5 decades, with a DL 5 pg mL−1 and sensitivity of 5.97 % increase per (ng mL−1) (Figure 6a).
Figure 5.
Faradaic Nyquist plots of immunosensor using Ab2-HRP after biocatalyzed precipitation for 20 min using TMB-H2O2 (System IV) in pH 3.3 buffer for IgY in pg mL−1at different concentrations. Points are raw data and lines presented fit to model in Scheme 3.
Figure 6.
Calibration curves for IgY in serum using System IV in pH 7.0 PBS buffer using: (a) Faradaic impedance in 1 mM Fe(CN)63−/4− at 0.17 V from 0.1 to 100 kHz, 5 mV amplitude, (b) Non-Faradic impedance in pH 7.0 buffer at 1 Hz and 0 V
The same immunosensors were adapted for non-Faradaic impedance. The typical impedance-time curve for the single immunosensor incubated with different concentrations of IgY in serum at 0 V vs SCE with 1 ± 0.1 Hz frequency was showed in Figure S5 in Supporting Information. The impedance values (Z) increased with the concentration of IgY. An excellent linear calibration curve was obtained from the Z value directly read from impedance-time curve, with the same linear range of 5 pg mL−1 to 1 µg mL−1 as in Faradaic EIS, but at 2-fold better sensitivity (Figures 5, 6 and Table 1).
Table 1.
Summary of analytical figures of merit for immunosensors for IgY using different detection schemes
| Methodologies | Linear range (ng mL−1) |
Detect. limit (pg mL−1) |
Sensitivity (%signal increase) ng mL−1 IgY cm−2 |
protocol |
|---|---|---|---|---|
| Amperometry | 15 – 250 | 300 | 8 | Scheme III |
| Faradaic EIS | 0.5 – 1000 | 500 | 119 | Scheme II |
| Faradaic EIS | 0.005 – 1000 a | 5 | 881b | Scheme IV |
| Direct Impedance | 0.005 – 1000 a | 5 | 1730b | Scheme IV |
The linear range was obtained from the linear calibration curve vs log CIgY from Figure 6.
The sensitivity was estimated from lower concentration of 0.005 to 0.1 ng mL−1 shown in Supporting Information Figure S6.
DISCUSSION
An Ara h2-2 peptide layer was successfully attached onto GSH-AuNP-coated sensors by amidation between amine groups on the peptide and carboxylic acid groups of GSH (Figures 1, 2 and Supporting Information S2). This layer provided an excellent bioaffinity interface for binding the Ara h2-2 specific antibody IgY, providing a good platform for fabrication of immunosensors. After IgY was bound from the serum, HRP-Ab2 binds to IgY on the sensor surface to provide amplification. Electrochemical impedance spectroscopy effectively detected the concentration of IgY in serum based on biorecognition by peptide Ara h2-2 at the sensor interface. Comparison of the analytical figures of merit for the different types of measurements show that non-Faradaic impedance gave the best combination of low detection limit, wide linear range and sensitivity (Table 1, Figures 3, 4 and 6). It provided the most useful and rapid method for specific detection of IgY in serum. The selectivity of the sensor is illustrated by its high sensitivity in the presence of the thousands of other proteins present in serum that could potentially interfere.46 Of course, the next step toward development of a viable anti-peanut antibody sensor is validation with real patient samples to demonstrate accuracy and reliability. We are currently pursuing this goal.
The advantages of non-Faradaic over Faradaic impedance include the elimination of the redox probe, and rapid direct measurement with no data fitting required. Simple direct readout at a single frequency suggests that non-Faradaic impedance immunosensors may be amenable to point-of-care applications. While the amperometric method is also direct readout, and sensitivity could be enhanced by adding multilabel strategies,25–27 this requires synthesis of multi-label particles. On the other hand, multi-label particles may also be useful to further improve sensitivity of the non-Faradaic impedance method as well.
Faradaic impedance using biocatalytic amplification (System VI) can be considered as the second most useful method (Figures 5 and 6). When the target antibody molecule binds to the recognition surface, changes in impedance can result solely from the presence of the target molecule. Thus, in principle the method can be label-free.15 However, this approach (Figure 4, Scheme II, Table 1) provided lower sensitivity than the labeled impedance methods and may not be sensitive enough for clinical applications.
One potential disadvantage of impedance sensing is the influence of non-specific binding. The measurement itself is non-specific, since any biomolecule in the sample that binds to the sensor will contribute to the increase in Rct. However, the relative influence of NSB is decreased when enzyme catalyzed precipitation is adopted, since the impedance change (Z in Figure 6) is generated mostly from precipitation on the sensor surface produced by the HRP label reaction.
CONCLUSIONS
We fabricated an allergen epitope peptide layer on a AuNP sensor surface to provide a sensitive biorecognition platform for the detection of an anti-peanut antibody. Non-Faradaic impedance with biocatalytic amplification gave the best analytical figures of merit along with an excellent DL of 5 pg mL−1 for IgY in serum. This DL compares very well with the best alternative assays for IgE, including DLs of 2 ng mL−1 by Surface Plasma resonance (SPR) immunosensors,47 75 ng mL−1 using a quartz crystal microbalance (QCM) sensor,48 15 ng mL−1 with other EIS sensors,18 0.5 ng mL−1 for electrochemical aptamer-based sensors,30 14 pg mL−1 with fluorescent aptamer-based sensors,31 38 ng mL−1 with carbon nanotube field-effect transistors,49 and 2 pg mL−1 total peanut specific IgE in diluted serum using a titration measured by fluorescence immunoassay.6
These impedance biosensors based on gold nanoparticle-peptide layers have the potential for simple, rapid, low-cost detection of specific IgEs to facilitate the diagnosis of peanut allergies, provide fundamental insight into the significance of individual epitopes, and drive the development of new therapies. This technology shows excellent promise for future development of bioelectronic arrays for multiple antibody detection, and this work is currently in progress.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful for financial support from U.S. PHS Grant ES013557 from National Institute of Environmental Health Sciences (NIEHS), NIH and a grant from the University of Connecticut Research Foundation. The authors thank Dr. Challa V. Kumar and Sadagopan Krishnan for assistance with SPR experiments, and Shenmin Pan for TEM experiments.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Full experimental details and one table of QCM results and five additional figures of SPR and impedance results are presented. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.de Leon MP, Rolland JM, O'Hehir RE. Expert Rev. Mol. Med. 2007;9:1–18. doi: 10.1017/S1462399407000208. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA. J. Allergy Clin. Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. J. Allergy Clin. Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 4.a) Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker W-M. Int. Arch. Allergy Immunol. 1999;119:265–274. doi: 10.1159/000024203. [DOI] [PubMed] [Google Scholar]; b) Bannon GA, Besler M, Hefle SL, Hourihane JO’B, Sicherer SH. Internet Symposium on Food Allergens. 2000;2:87–123. [Google Scholar]
- 5.Shreffler WG, Beyer K, Chu T-HT, Burks AW, Sampson HA. J. Allergy Clin. Immunol. 2004;113:776–782. doi: 10.1016/j.jaci.2003.12.588. [DOI] [PubMed] [Google Scholar]
- 6.Shreffler WG, Lencer DA, Bardina L, Sampson HA. J. Allergy Clin. Immunol. 2005;116:893–899. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Perry TT, Matsui EC, Conover-Walker MK, Wood RA. J. Allergy Clin. Immunol. 2004;114:144–149. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Sampson HA, Ho DG. J. Allergy Clin. Immunol. 1997;100:444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HA. J. Allergy Clin. Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch S, Fourdrilis S, Dobson R, Scippo M-L, Maghuin-Rogister Guy, De Pauw E. Anal. Bioanal. Chem. 2009;395:57–67. doi: 10.1007/s00216-009-2869-7. [DOI] [PubMed] [Google Scholar]
- 11.Cox L, Williams B, Sicherer S, Oppenheimer J, Sher L, Hamilton R, Golden D. Annals Allergy, Asthma & Immunol. 2008;101:580–592. [PubMed] [Google Scholar]
- 12.Bard AJ, Faulkner LR. Electrochemical Methods. 2nd ed. New York: Wiley; 2001. [Google Scholar]
- 13.Katz E, Willner I. Electroanal. 2003;15:913–947. [Google Scholar]
- 14.Davis JJ, Tkac J. In: Engineering the Bioelectronic Interface. Davis JJ, editor. UK: Royal Soc. Chem.; 2009. pp. 193–224. [Google Scholar]
- 15.Daniels JS, Pourmand N. Electroanalysis. 2007;19:1239–1257. doi: 10.1002/elan.200603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez MC, Kawde A-N, Wang J. Chem. Commun. 2005:4267–4269. doi: 10.1039/b506571b. [DOI] [PubMed] [Google Scholar]
- 17.Radi A-E, Acero Sanchez JL, Baldrich E, O'Sullivan CK. Anal. Chem. 2005;77:6320–6323. doi: 10.1021/ac0505775. [DOI] [PubMed] [Google Scholar]
- 18.Xu D, Xu D, Yu X, Liu Z, He W, Ma Z. Anal. Chem. 2005;77:5107–5113. doi: 10.1021/ac050192m. [DOI] [PubMed] [Google Scholar]
- 19.Davis JJ, Tkac J, Humphreys R, Buxton AT, Lee TA, Ferrigno PK. Anal. Chem. 2009;81:3314–3320. doi: 10.1021/ac802513n. [DOI] [PubMed] [Google Scholar]
- 20.Patolsky F, Zayats M, Katz E, Willner I. Anal. Chem. 1999;71:3171–3180. doi: 10.1021/ac9901541. [DOI] [PubMed] [Google Scholar]
- 21.Pei R, Cheng Z, Wang E, Yang X. Biosens. Bioelectron. 2001;16:355–361. doi: 10.1016/s0956-5663(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 22.Alfonta L, Katz E, Willner I. Anal. Chem. 2000;72:927–935. doi: 10.1021/ac990439d. [DOI] [PubMed] [Google Scholar]
- 23.Dijksma M, Kamp B, Hoogvliet JC, van Bennekom WP. Anal. Chem. 2001;73:901–906. doi: 10.1021/ac001051h. [DOI] [PubMed] [Google Scholar]
- 24.Bart M, Stigter ECA, Stapert HR, de Jong GJ, van Bennekom WP. Biosens. Bioelectron. 2005;21:49–59. doi: 10.1016/j.bios.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF. ACS Nano. 2009;3:585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra R, Patel V, Vaque JP, Gutkind JS, Rusling JF. Anal. Chem. 2010;82:3118–3123. doi: 10.1021/ac902802b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munge BS, Krause CE, Malhotra R, Patel V, Gutkind JS, Rusling JF. Electrochem. Comm. 2009;11:1009–1012. doi: 10.1016/j.elecom.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikkaveeraiah BV, Liu H, Mani V, Papadimitrakopoulos F, Rusling JF. Electrochem. Comm. 2009;11:819–822. doi: 10.1016/j.elecom.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, Han H, He W, Liu Z, Xu D, Liu X. Electroanalysis. 2006;18:1815–1820. [Google Scholar]
- 30.Wang J, Munir A, Li Z, Zhou H. Talanta. 2010;81:63–67. doi: 10.1016/j.talanta.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Hu P, Zhou H, Shen G, Yu R. Biomaterials. 2010;31:1918–1924. doi: 10.1016/j.biomaterials.2009.11.054. [DOI] [PubMed] [Google Scholar]
- 32.Huang X. J. Am. Chem. Soc. 2004;126:12047–12054. doi: 10.1021/ja047029d. [DOI] [PubMed] [Google Scholar]
- 33.Pan J, Yang Q. Anal. Bioanal. Chem. 2007;388:279–286. doi: 10.1007/s00216-007-1224-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Yang J, Estavillo C, Stuart JD, Schenkman JB, Rusling JF. J. Am. Chem. Soc. 2003;125:1431–1436. doi: 10.1021/ja0290274. [DOI] [PubMed] [Google Scholar]
- 35.(a) Mesulam M. J. Histochem. Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]; b) Olucha F, Martinez-Garcia F, Lopez-Garcia C. J, Neurosci. Methods. 1985;12:131–138. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- 36.Bardea A, Baram A, Tatikonda AK, Naaman R. J. Am. Chem. Soc. 2009;131:18260–18262. doi: 10.1021/ja908675c. [DOI] [PubMed] [Google Scholar]
- 37.Burks AW, Williams LW, Connaughton C, Cockrell G, O'Brien TJ, Helm RM. J. Allergy Clin. Immunol. 1992;90:962–969. doi: 10.1016/0091-6749(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 38.Beyer K, Ellman-Grunther L, Järvinen K-M, Wood RA, Hourihane J, Sampson HA. J. Allergy Clin. Immunol. 2003;112:202–207. doi: 10.1067/mai.2003.1621. [DOI] [PubMed] [Google Scholar]
- 39.Shin DS, Compadre CM, Maleki SJ, Kopper RA, Sampson H, Huang SK, Burks AW, Bannon GA. J. Biol. Chem. 1998;273:13753–13759. doi: 10.1074/jbc.273.22.13753. [DOI] [PubMed] [Google Scholar]
- 40.Sauerbrey GZ. Phys. 1959;155:206–222. [Google Scholar]
- 41.Wang Y, Chang YC. Macromolecules. 2003;36:6511–6518. [Google Scholar]
- 42.Bhagavan NV. Medical Biochemistry. Boston/London: Jones & Barelett; 1992. [Google Scholar]
- 43.Lvov Y. In: Handbook Of Surfaces And Interfaces Of Materials. Nalwa RW, editor. Vol. 3. San Diego: Academic Press; 2001. pp. 170–189. [Google Scholar]
- 44.Lu H, Hu N. J. Phys. Chem. B. 2007;111:1984–1993. doi: 10.1021/jp065551b. [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Munge B, Patel V, Jensen G, Bhirde A, Gong JD, Kim SN, Gillespie J, Gutkind JS, Papadimitrakopoulos F. Rusling. J. Am. Chem. Soc. 2006;128:11199–11205. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanash SM, Pitteri SJ, Faca VM. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 47.Kim YH, Kim JP, Han SJ, Sim SJ. Sens. Actuators B: Chem. 2009;139:471–475. [Google Scholar]
- 48.Liss M, Petersen B, Wolf H, Prohaska E. Anal. Chem. 2002;74:4488–4495. doi: 10.1021/ac011294p. [DOI] [PubMed] [Google Scholar]
- 49.Maehashi K, Katsura T, Takamura Y, Matsumoto K, Tamiya E. Anal. Chem. 2007;79:782–787. doi: 10.1021/ac060830g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.