Abstract
T cell receptor (TCR) dependent regulatory T cell (Treg) activity controls effector T cell (Teff) function and is inhibited by the inflammatory cytokine tumor necrosis factor (TNF)-α. Protein kinase C-θ (PKC-θ) recruitment to the immunological synapse is required for full Teff activation. In contrast, PKC-θ was sequestered away from the Treg immunological synapse. Furthermore, PKC-θ blockade enhanced Treg function, demonstrating PKC-θ inhibits Treg-mediated suppression. Inhibition of PKC-θ protected Treg from inactivation by TNF-α, restored activity of defective Treg from rheumatoid arthritis patients, and enhanced protection of mice from inflammatory colitis. Treg freed of PKC-θ mediated inhibition can function in the presence of inflammatory cytokines and thus have therapeutic potential in control of inflammatory diseases.
CD4+ CD25+ regulatory T cells (Treg) suppress the function of CD4+ and CD8+ effector T cells (Teff) through a T cell receptor (TCR) engagement and cell contact dependent mechanism (1–3). Inflammatory signals delivered by cytokines like TNF-α decrease Treg activity (4, 5), perhaps as a mechanism to reduce interference by Tregs in immune responses to pathogens. In rheumatoid arthritis, Tregs circulate in normal numbers, but they have decreased activity ex vivo (5, 6). Besides the negative signals initiated by TNF-α, Treg also receive inhibitory signals via the TCR. Akt activation by the TCR can reduce Treg function and thus appears to be tightly regulated (7). This suggests TCR signaling in Treg can negatively feed back to inhibit Treg-mediated suppression. Moreover, TCR signaling leads to formation of the immunological synapse within seconds of T cell activation. Thus, the differences in TCR signaling in Treg may emerge at the level of the immunological synapse (IS), a structured interface between T cells and antigen presenting cells where TCR signalosomes are assembled (8). Whereas Treg can form stable contacts with APCs with functional consequences both in vitro and in vivo (9–11), signaling events in the Treg IS have not been investigated.
To study signaling in human Treg IS we developed a model system on supported planar bilayers containing the mobile fluorescently labeled adhesion molecule ICAM-1 and antigen surrogate anti-CD3 (the signaling subunit of the TCR) antibodies, and CD4+ CD25− Teff or CD4+ CD25+ Treg freshly isolated from peripheral blood (fig. S1, A–C). Teff and Treg both formed IS, defined by a symmetric pattern consisting of a central cluster of anti-CD3 surrounded by a ring of ICAM-1 (12, 13) (Fig. 1A). Treg IS were more stable than Teff IS (fig. S2, A and B), which displayed symmetry breaking within 20 minutes as previously described (14). Ex vivo expanded human umbilical cord blood Treg (15), displayed similar behavior to adult peripheral blood Treg (fig. S2, C–E). We measured recruitment of TCR proximal signaling molecules to IS by staining with phospho-Src kinase activation loop and ZAP-70 kinase interdomain A tyrosine 319 antibodies and imaging with total internal reflection fluorescence microscopy (TIRFM) (16). Signals were quantified based on unbiased measurement of IS proximal fluorescence intensity. Teff IS displayed significantly higher amounts of phospho-Src than Treg (fig. S3A); however, we observed a similar intensity of phosphorylation of the downstream kinase ZAP-70 (fig. S3B). We next explored the protein kinase C-θ (PKC-θ) pathway, which is downstream of Src family kinases (17) and mediates IS breaking (14), because ZAP-70 phosphorylation appeared normal in Treg.
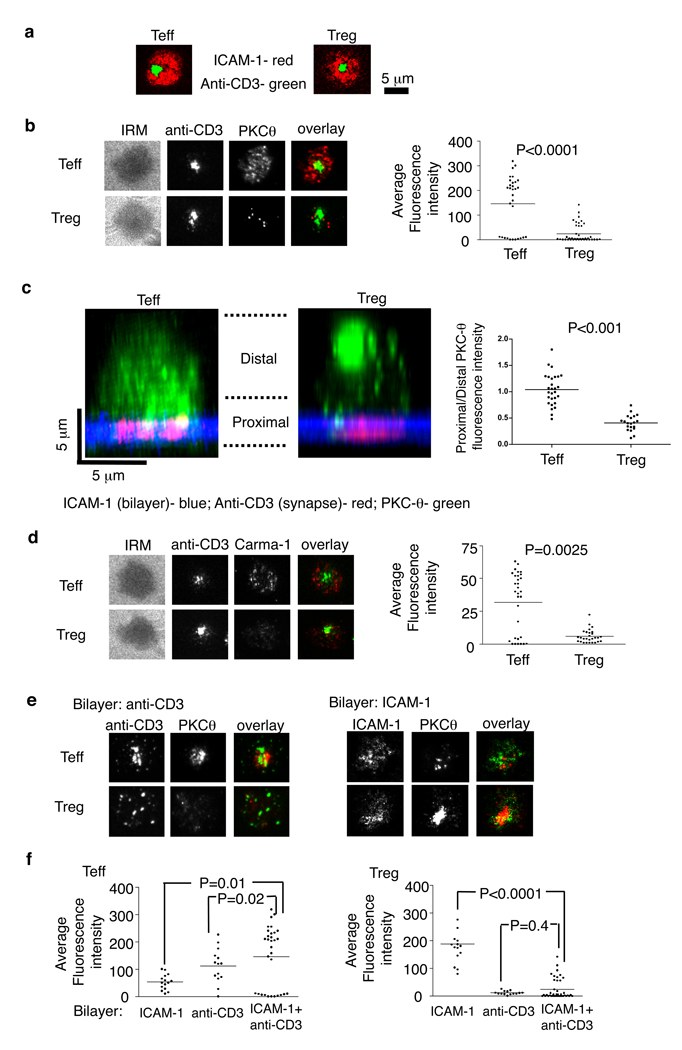
Fig. 1.
Human Treg form altered IS. Human Teff and Treg were introduced into bilayers containing anti-CD3 (5 µg/ml) and ICAM-1 at 250 molecules/mm2 (A–D), fixed at 6 min and permeabilized, stained and imaged by TIRFM (A, B, D, E) or by confocal microscopy (C). (A) Localization of ICAM-1 (red) and anti-CD3 (green) in the IS. (B and D) Staining and average fluorescence intensity of PKC-θ (B) and Carma-1 (D). (C). Distribution of endogenous PKC-θ in cells. Histogram shows average of (bottom planes “proximal”/ upper planes “distal”) ratio of anti-PKCθ intensity per cell. The panels show representative images. (E and F) PKC-θ staining (E) and average fluorescence intensity (F) in Teff or Treg on bilayers containing only anti-CD3 (left) or ICAM-1 (right). Data are representative of three (A–D) or five (E–F) different experiments. P values were calculated by Mann-Whitney test.
PKC-θ recruitment to the IS leads to recruitment of Carma-1, a MAGUK (membrane-associated guanylate kinase) protein, that enables the assembly of a Carma-1-Bcl10-Malt1 complex necessary for NF-κB activation and subsequent Teff activation (18). We quantified PKC-θ and Carma-1 recruitment in IS on planar bilayers by TIRFM. Teff IS recruited PKC-θ in a broad pattern overlapping with early TCR signaling, as previously reported (14) (Fig. 1B). Treg displayed six-fold lower PKC-θ recruitment to IS than Teff and this recruitment was focused in a limited area defined by a small number of bright puncta (Fig. 1B). The same difference in the ability to recruit PKC-θ was found with ex vivo expanded human umbilical cord blood Treg and expanded CD4+ CD25− Teff cells (fig. S4). CD28 co-stimulation plays an important role in PKC-θ recruitment to IS (13, 17). Thus, we compared the ability of Treg and Teff to recruit PKC-θ in the presence of a CD28 ligand, CD80, in the bilayer. The co-stimulatory signal increased the amounts of PKC-θ recruited to IS in both Treg and Teff (fig. S5). Even in the presence of CD80 in the bilayer, the Treg still have significantly less PKC-θ recruited to the IS than Teff (P < 0.001). Interestingly, total PKC-θ expression was even higher in Treg compared to Teff (fig. S6). Moreover, the confocal imaging revealed that PKC-θ was sequestered away from the Treg IS, whereas PKC-θ in Teff accessed the IS and formed cytoplasmic puncta (Fig. 1C and fig. S7). Treg IS also displayed significantly less Carma-1 recruitment than Teff IS (Fig. 1D). Thus, recruitment of PKC-θ and its down-stream target to the IS are reduced in Treg.
We further dissected the TCR and LFA-1 integrin-dependent components of PKC-θ recruitment using bilayers containing anti-CD3 or ICAM-1 only, respectively. TCR engagement in Teff was necessary and sufficient for increased PKC-θ recruitment based on a two-fold increase in PKC-θ fluorescence intensity on anti-CD3 only bilayers compared to ICAM-1 alone bilayers (Fig. 1, E and F). TCR engagement alone in Treg recruited 11-fold less PKC-θ than in Teff, whereas LFA-1 engagement alone recruited 3.5-fold more PKC-θ than in Teff (Fig. 1, E and F). Thus, TCR triggering in Treg actually down-regulates PKC-θ recruitment to the IS by 7.7-fold compared to basal recruitment by LFA-1 engagement alone (Fig. 1F).
We hypothesized that PKC-θ activation may be part of a negative feedback loop controlling Treg function because TCR signals are essential for Treg function, but suppress PKC-θ recruitment. We investigated whether inhibition of PKC-θ may affect the suppressive function of human CD4+ CD25+ Treg cells. Teff function was measured as cytokine secretion and cell proliferation. C20 treatment of only the Tregs significantly up-regulated their suppressive ability, even in the presence of CD28-mediated costimulation, but did not induce suppressive activity in treated Teff (Fig. 2, A and B and fig. S8A). Consistent with imaging data showing that co-stimulation up-regulates the recruitment of PKC-θ to IS in Treg (fig. S5), untreated Treg demonstrated reduced ability to suppress Teff function in the presence of CD28 antibodies (40% and 25% respectively; fig. S8A), and this difference was abrogated by treatment with C20. Inhibition of PKC-θ seemed to increase Treg suppressive function in general, without preference for specific helper T cell type (fig. S8B). C20 also significantly increased Treg function in an antigen presenting cell-dependent assay (fig. S8C).
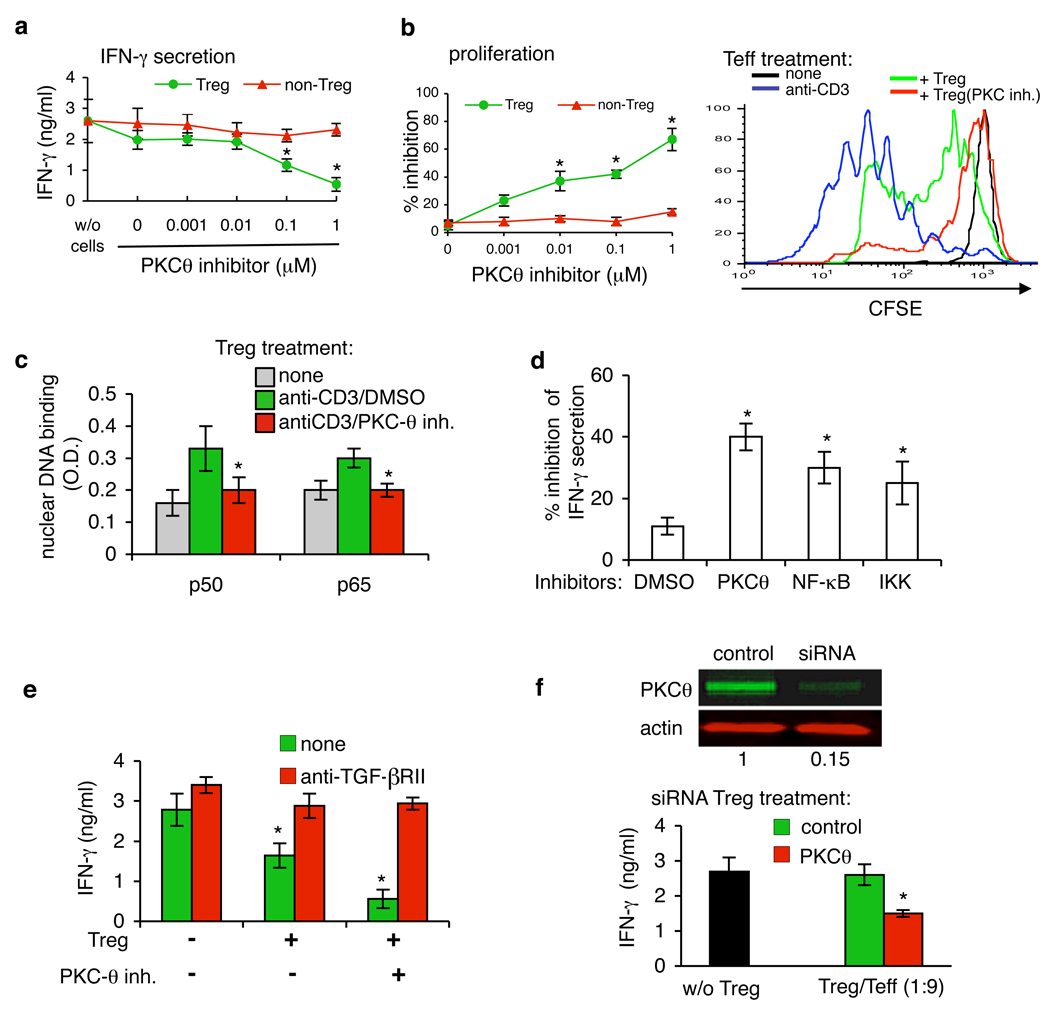
Fig. 2.
PKC-θ inhibition up-regulates the suppressive function of Treg in vitro. (A and B) Treg and Teff were treated with PKC-θ inhibitor C20 at 0.001–1 µM for 30 min and washed three times. Treated cells were then mixed with CD4+ CD25− T (Teff) cells at a 1:9 ratio and plated on immobilized anti-CD3 mAb. (A) The supernatants were analyzed for IFN-γ after 24 hours by ELISA. (B)Cell proliferation was determined after 96 hours by Alamar Blue (left panel) or Treg were treated with 1 µM C20 for 30 min, washed, mixed with Teff and proliferation was assayed by CFSE (right panel) (C) Treg were treated with 1 µM of C20 for 30 min, washed, stimulated by anti-CD3 and p65/p50 specific binding to NF-κB consensus sequence was determined by ELISA. (D) Tregs were treated with 1 µM of NF-κB and IKK inhibitors, mixed with Teff and IFN-γ secretion was analyzed as in (A) after 48 hours. (E) Treg were treated with 1 µM of C20, mixed with Teff and cultured on anti-CD3 mAb with or without of neutralizing anti-TGF-β RII antibodies (20 µg/ml). IFN-γ secretion was analyzed as in (A) after 48 hours. (F) Treg were transfected with small interfering RNA (siRNA) targeting PKC-θ, or with control siRNA and plated on anti-CD3. After 48 hours PKC-θ expression was measured by Western blot analysis (top panel). siRNA-transfected Treg were mixed with CD4+ CD25− T (Teff) cells at 1:9 ratio and plated on immobilized anti-CD3 mAb. The supernatants were analyzed for IFN-γ after 48 hours. Average of four different experiments are shown for all panels. P values were calculated by t-test.
It has been reported that the CD4+ CD25high Tregs are the most potent suppressors in vitro (20); we therefore sorted CD4+ T cells according to their CD25 expression (fig. S1D), pretreated the sorted Treg with C20 and co-cultured the CD4+ CD25int or CD4+ CD25high T cells with target CD4+ CD25− T cells. Although the CD4+ CD25high T cells manifested a greater suppressive activity, pretreatment with C20 significantly enhanced their suppression of IFN-γ secretion (fig. S8D). Finally, the effect of C20 on Treg function was time-dependent; we observed the peak of suppressive function after 30 min of treatment (fig. S8E).
TCR-induced DNA binding of NF-κB p65 and p50 subunits, which indicates NF-κB activation, and the ability to proliferate were greatly reduced by C20 in both Treg and Teff (Fig. 2C and fig. S9). To test whether activation of NF-κB was a critical PKC-θ target in control of Treg activity we inhibited NF-κB activation using two different types of inhibitors and both significantly increased Treg activity (Fig. 2D). Moreover, treatment of Treg with analogs of C20 with different IC50 values demonstrated that of the analogs tested, only PKC-θ inhibitors with IC50 ≤ 1nM significantly up-regulated Treg suppressive function (fig. S10). Finally, neutralizing antibodies against TGF-β receptor II completely blocked the suppressive function of Treg induced by inhibition of PKC-θ either in APC-free or in presence of APC in the co-culture (Fig. 2E and fig. S8C), suggesting the possible involvement of TGF-β presented by Treg as a suppressive mechanism. To confirm the conclusion that inhibition of PKC-θ up-regulates the suppressive activity of human Treg, we specifically silenced PKC-θ gene expression using RNA interference (21). The specific PKC-θ siRNA reduced PKC-θ expression by 80% (Fig. 2F). Moreover, silencing of PKC-θ significantly increased Treg-mediated suppression of IFN-γ secretion by Teff (Fig. 2F). PKC-θ silencing in Teff resulted in the expected down-regulation of IFN-γ secretion and cell proliferation (fig. S11). In summary, we concluded that PKC-θ activity induced by TCR signaling mediates a negative feedback loop that reduces the activity of human CD4+ CD25high Treg to suppress cytokine secretion and proliferation of Teff in vitro.
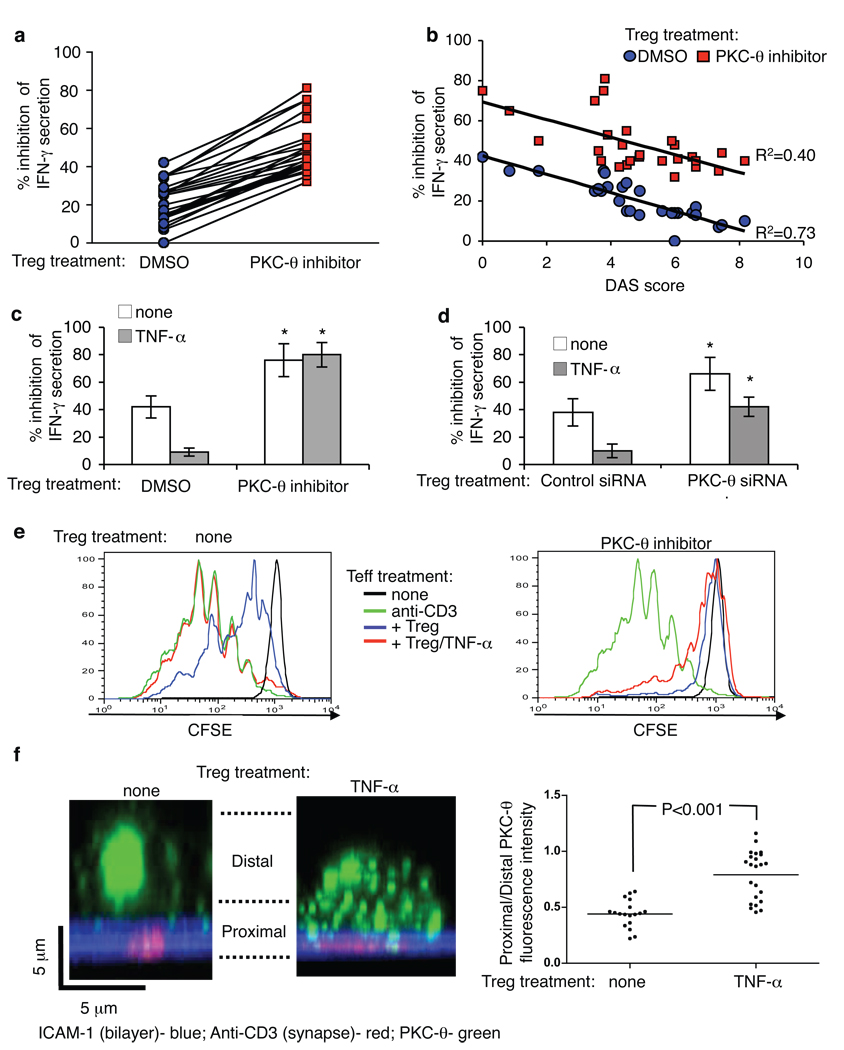
Rheumatoid arthritis (RA) is a chronic autoimmune disorder that results in the destruction of joint architecture (22). Recent studies in RA patients demonstrated that the function of CD4+ CD25high Treg is impaired (5, 6). We used Treg purified from peripheral blood of 25 RA patients with different severities of disease (fig. S12) and found despite anergic state and comparable Treg numbers to healthy donors (fig. S13A), RA Treg demonstrated significantly reduced suppression of IFN-γ from autologous CD4+ CD25− Teff (fig. S13B). The loss of function was due to defective intrinsic function of Treg from RA patients and not due to increased resistance of Teff (fig. S13C), consistent with previously published results (5). Treatment with C20 significantly increased the suppressive function of Treg purified from all 25 RA patients (Fig. 3A) to levels comparable with healthy donor-derived Treg (30–50% inhibition at a Treg/Teff of 1:3, fig. S13B). Moreover, the defective Treg function in RA patients was inversely correlated with the Disease Active Score (DAS score) and the shift in IFN-γ secretion was similar across the disease score spectrum (Fig. 3B). Thus, inhibition of PKC-θ boosts the suppressive function of Treg isolated from RA patients independent of the severity of disease.
Fig. 3.
Inhibition of PKC-θ rescues and protects Treg function. (A and B) Freshly purified Treg from healthy donors and RA patients were treated or not with PKC-θ inhibitor C20 for 30 min at 1µM, washed three times, mixed with CD4+CD25− T cells at ratio 1:3, and plated on immobilized anti-CD3. The supernatants were analyzed for IFN-γ after 24–48 hours by ELISA. (C–E) Treg from healthy donors were treated with PKC-θ inhibitor C20 (C, E) or with PKC-θ siRNA (D) as described above, and co-cultured with CD4+ CD25− T cells with or without TNF-α (50 ng/ml). IFN-γ secretion was determined after 48 hours by ELISA (C and D) and proliferation was determined after 96 hours (E). (F) Untreated or TNF-α-treated (50 ng/ml, for 24 hr) Treg were introduced to bilayers with anti-CD3 and ICAM-1, fixed, and imaged by confocal microscopy. The panels show representative images with ICAM-1 (blue), anti-CD3 (red) and PKC-θ (green). Plots show the distribution of endogenous PKC-θ in cells. Each bar shows average of (bottom planes “synapse proximal”/ upper planes “synapse distal”) ratio of anti-PKCθ intensity per cell. Data points from three independent experiments are included in the analysis.
Treg treatment with TNF-α inhibits their activity and down-regulates expression of the Treg master regulator transcription factor FoxP3 (5). We investigated the possibility that elimination of the PKC-θ mediated negative feedback on Treg function may render Treg resistant to inhibition by TNF-α. As expected, in the presence of TNF-α, Treg displayed significantly reduced inhibition of IFN-γ secretion and proliferation in Teff, and this effect was largely reversed by C20 or PKC-θ-specific siRNA (Fig. 3, C–E; fig. S14A). Moreover, C20 prevented TNF-α-induced down-regulation of Foxp3 in Treg (fig. S14B). Strikingly, TNF-α treatment induced increased PKC-θ recruitment to IS in Treg by decreasing sequestration at the distal pole (Fig. 3F), consistent with the idea that TCR activated PKC-θ mediates negative feedback on Treg function that is further enhanced by TNF-α.
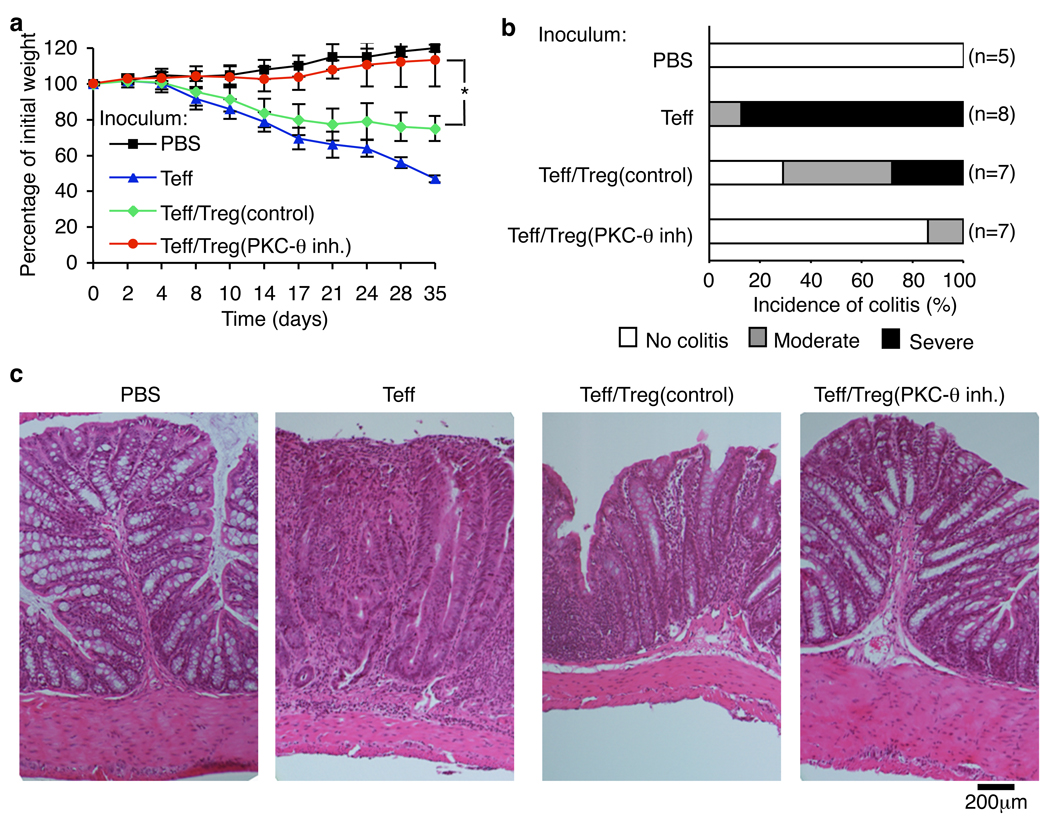
Finally, we determined the ability of C20 to increase Treg function in vivo using a colitis model in TCRα−/− β−/− mice induced by transfer of the CD4+ CD25− CD45RBhigh Teff cells (23, 24). Treatment of murine Treg with C20 up-regulated their suppressive function in vitro (fig. S15, A and B). Moreover, C20 treated- CD4+ CD25+ Treg cells provided significant protection from colitis, as demonstrated by normal weight gain and normal histology of the distal colon in 7 of 8 mice (Fig. 4, A–C). C20 treatment increased the number of Treg recovered from mesenteric lymph nodes and the spleen (fig. S15C). This protection was significantly greater than afforded by Treg left untreated (Fig. 4, A–C). Treatment of CD4+ CD25− Teff with C20 prior to transfer with untreated Teff did not protect mice from colitis (fig. S16). Thus, PKC-θ̣inhibition significantly increased the suppressive effect of Treg in vivo.
Fig. 4.
Treatment with PKC-θ inhibitor up-regulates Treg function in vivo. Colitis was induced in C57BL/10.PL TCRα−/− β−/− mice (31). Disease progression was monitored by (A) body weight loss, (B) colitis score and (C) histology. Numbers in parentheses indicate number of mice. Combined data of three independent experiments are presented. P values were calculated by t-test.
Treg function is crucial to prevent autoimmunity in mice and humans. Although Treg numbers in patients suffering from autoimmune diseases are similar to healthy controls, Treg function is defective, probably due to negative regulation of Treg by the inflammatory milieu (20). In the present study we demonstrated that PKC-θ is sequestered in the distal pole of Treg cells in a manner that reduces its recruitment to the IS. Moreover, inhibition of PKC-θ protects both mouse and human Treg against negative effects of TNF-α, which appears to act in Treg by unleashing PKC-θ from the distal pole. In Teff, PKC-θ is part of a strong positive circuit with free access to TCR signalosomes in the IS, NF-κB activation, increased expression of IL-2, proliferation and survival (17, 25, 26). PKC-θ is not sequestered in the distal pole of Teff, although the negative regulators like Csk binding protein are (27). In contrast, we noted that Treg display increased IS stability, consistent with decreased PKC-θ activity in the IS and attenuated NF-κB activation. The proposed role of NF-κB in inhibition of Treg function is consistent with the system wide role of this family of transcription factors in promoting inflammation (28). IS stabilization may enhance Treg function based on recent evidence for an important role of IS in Treg effects mediated through DC (1, 29).
In conclusion, we have demonstrated that formation of IS induces altered signaling pathways in Treg, characterized by reduced recruitment of Src kinases, PKC-θ and Carma-1 to the IS. Moreover, in Treg, PKC-θ acts as a pro-inflammatory mediator and this effect is enhanced by TNF-α. Indeed, Treg treated with C20 displayed enhanced ability to prevent autoimmune colitis and restore function of Treg from RA patients. Thus, targeting the PKC-θ mediated negative feedback loop enhances the activity of Treg and makes them resistant to cytokines associated with the inflammatory milieu found in some autoimmune diseases. Thus inhibition of PKC-θ in Treg may be a valuable component in Treg adoptive immunotherapy to treat autoimmunity and graft versus host disease (30).
Supplementary Material
References and Notes
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Cell. 2008 May 30;133:775. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM, et al. Immunol Rev. 2006 Aug;212:60. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Rudensky AY. Nat Immunol. 2007 May;8:457. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 4.Flores-Borja F, Mauri C, Ehrenstein MR. Eur J Immunol. 2008 Apr;38:934. doi: 10.1002/eji.200738107. [DOI] [PubMed] [Google Scholar]
- 5.Valencia X, et al. Blood. 2006 Jul 1;108:253. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrenstein MR, et al. J Exp Med. 2004 Aug 2;200:277. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crellin NK, Garcia RV, Levings MK. Blood. 2007 Mar 1;109:2014. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 8.Dustin ML, Tseng SY, Varma R, Campi G. Curr Opin Immunol. 2006 Aug;18:512. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, et al. Nat Immunol. 2006 Jan;7:83. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran DQ, et al. J Immunol. 2009 Mar 1;182:2929. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing K, et al. Science. 2008 Oct 10;322:271. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 12.Grakoui A, et al. Science. 1999 Jul 9;285:221. [Google Scholar]
- 13.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Nature. 1998 Sep 3;395:82. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 14.Sims TN, et al. Cell. 2007 May 18;129:773. [Google Scholar]
- 15.Godfrey WR, et al. Blood. 2005 Jan 15;105:750. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 16.Campi G, Varma R, Dustin ML. J Exp Med. 2005 Oct 17;202:1031. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi K, Altman A. Pharmacol Res. 2007 Jun;55:537. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawlings DJ, Sommer K, Moreno-Garcia ME. Nat Rev Immunol. 2006 Nov;6:799. doi: 10.1038/nri1944. [DOI] [PubMed] [Google Scholar]
- 19.Cywin CL, et al. Bioorg Med Chem Lett. 2007 Jan 1;17:225. doi: 10.1016/j.bmcl.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 20.Shevach EM. Immunity. 2009 May;30:636. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava KK, et al. J Biol Chem. 2004 Jul 16;279:29911. doi: 10.1074/jbc.M401997200. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann M. Nat Rev Immunol. 2002 May;2:364. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 23.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. J Exp Med. 1996 Jun 1;183:2669. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Nat Med. 2008 Feb;14:162. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 25.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Nature. 1997 Jan 2;385:83. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, et al. Nature. 2000 Mar 23;404:402. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer MH, et al. J Immunol. 2009 Jan 15;182:1021. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes PJ, Karin M. N Engl J Med. 1997 Apr 10;336:1066. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 29.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Immunity. 2008 Mar;28:402. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley JL, June CH, Blazar BR. Immunity. 2009 May;30:656. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go to Science.com for Supplemental on-line methods.
- 32.This work was funded by the National Institutes of Health through the NIH Roadmap for Medical Research PN2 EY016586 and R01 AI43542 to MLD; a Leukemia and Lymphoma Translational Research grant 6098-09 to BB. SK was supported by the Osaka University Immunology Frontier Research Center. B. Blazer is an ad hoc advisor to Becton Dickinson. Becton Dickinson has purchased licensing rights for the use of Tregs to prevent alloresponses and autoimmunity. An MTA agreement is required for use of Compound 20. M. Dustin and A. Zanin-Zhorov have applied for patents for the use of Compound 20 and RNAi against PKCθ in adoptive immunotherapy (patent application #61173237 and 61286871)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.