Abstract
Ion channels are classically understood to regulate the flux of ions across the plasma membrane in response to a variety of environmental and intracellular cues. Ion channels serve a number of functions in intracellular membranes (IMs) as well. These channels may be temporarily localized to IMs as a function of their biosynthetic or secretory pathways, i.e., en route to their destination location. IM ion channels may also be located in the endocytic pathways, either being recycled back to the plasma membrane (PM) or targeted to the lysosome for degradation. Several channels do participate in intracellular signal transduction; the most well known example is the inositol 3’ phosphatase (IP3) receptor in the endoplasmic reticulum (ER). Some organellar IM channels are required for the ionic homeostasis of their residing organelles. Several newly-discovered IM Ca2+ channels actually play active roles in membrane trafficking. Transient Receptor Potential (TRP) proteins are a superfamily (28 members in mammal) of Ca2+-permeable channels with diverse tissue distribution, subcellular localization, and physiological functions. Almost all mammalian TRP channels studied thus far, like their ancestor yeast TRP channel (TRPY1) that localizes to the vacuole compartment, are also (in addition to their PM localization) found to be localized to intracellular membranes. Accumulated evidence suggests that intracellularly-localized TRP channels actively participate in regulating membrane traffic, signal transduction, and vesicular ion homeostasis. This review aims to provide a summary of these recent works. The discussion will also be extended to the basic membrane and electrical properties of the TRP-residing compartments.
Keywords: transient receptor potential (TRP) channel, endosomes, lysosomes, TRPML, membrane traffic, intracellular channel
I: TRP Channels and Organelles
Overview of TRP channels
Transient receptor potential (TRP) is a cation channel superfamily with diverse physiological functions including thermosensation and mechanosensation (excellently reviewed in Refs. (Clapham 2003, Montell 2005, Nilius et al. 2007)). Mammalian TRPs can be divided into 6 subfamilies: TRPC(1–7), TRPV(1–6), TRPM(1–8), TRPA(1), TRPP(1–3), and TRPML(1–3) (Fig. 1). In past years, extensive efforts have been made to elucidate three basic aspects of TRP channels: the channel pore properties, the activation (gating) mechanisms, and the channels’ subcellular localization. Of these, the biophysical properties of the TRP channel pore have been best characterized. When activated, most TRPs conduct Ca2+, as well as Na+ and K+ ions. The activation/gating mechanisms, however, have not been described in detail for most TRPs. In sensory cells such as somatosensory neurons, a subset of TRP channels (so-called “sensory” TRPs) are activated by a variety of environmental cues such as temperature, mechanical force, and plant-derived volatiles (reviewed in Refs. (Clapham 2003, Montell 2005, Nilius et al. 2007)). Many of the sensory TRPs, along with the non-sensory TRPs, are also expressed in non-sensory tissues. Here the endogenous cellular cues activating the TRPs are largely unknown, although ligands of the GPCR (G Protein-Coupled Receptor) and RTK (Receptor Tyrosine Kinase) families reportedly activate or modulate TRPs in heterologous systems (reviewed in Ref. (Ramsey et al. 2006)). Initially thought to be solely plasma membrane (PM) channels mediating Ca2+ entry, almost all mammalian TRPs studied thus far have also been found to be localized to intracellular vesicular membranes (Bezzerides et al. 2004, Dong et al. 2008, Krapivinsky et al. 2006, Lange et al. 2009, Turner et al. 2003). Despite the fact that TRP channel activity can be recorded in the plasma membrane using functional assays, and that TRP proteins can be detected in the plasma membrane using surface labeling techniques, the majority of heterologously-expressed and many of the endogenous TRP proteins are located intracellularly (for example see Refs (Turner et al. 2003, Bezzerides et al. 2004, Krapivinsky et al. 2006, Dong et al. 2008, Lange et al. 2009, Oancea et al. 2009)). The molecular identities of these intracellular TRP-residing compartments are poorly defined. It remains unclear whether TRPs appear in these compartments as intermediates of biosynthetic pathways being shuttled to their ultimate destinations, or if they are active participants in signal transduction and/or membrane trafficking. If the latter is true, the larger question arises as to how TRPs function in vesicles and are activated and/or modulated by cellular cues. TRPs localized to the plasma membrane (PM TRPs) are modulated by G protein signaling, lipid signaling, and protein phosphorylation and dephosphorylation events (Clapham 2003, Ramsey et al. 2006). It is not known if vesicular IM TRPs are modulated by these same signaling molecules. It is also not known whether IM TRPs are modulated by luminal factors. Given that the chemicophysical properties of intracellular membranes differ significantly from those of the plasma membrane (Watson & Pessin 2001), it is of interest whether vesicular TRPs function in the secretory/endocytic pathways in a similar fashion to their plasma membrane counterparts. In this review we will mainly discuss TRP channels whose residing compartments have been reasonably defined, especially those for whom channel activities have been measured in their residing compartments.
Figure 1. Intracellular location and putative activation mechanisms of TRP channels.
TRPs can be divided into 6 groups (TRPC, TRPV, TRPM, TRPA, TRPML, and TRPP). TRPML1–3, TRPV2, and TRPY1 (yeast TRP yvc1), and TRPM7 (in red) are likely to play active roles in membrane traffic and exocytosis. TRPM2, TRPM8, TRPV1, TRPP1, TRPA1, and TRPV4 (in green) have been shown to be active in intracellular membranes and may play roles in intracellular signal transduction. TRPC3–6, TRPMV5/6, TRPM1, TRPM7, and TRPML2/3 (in blue) have been shown to undergo regulated exocytosis. Intracellular localization of other TRPs (in black) has not been well documented.
Overview of intracellular organelles
In most mammalian cells there are at least eight different types of intracellular organelles, which can be arbitrarily divided into two groups (Michelangeli et al. 2005). The first endocytic, secretory and autophagic group (group I) includes the endoplasmic reticulum (ER), the Golgi apparatus, secretory vesicles/granules, endosomes, autophagosomes, and lysosomes (see Fig. 2). These group I organelles exchange materials with each other, either directly by complete or partial (“kiss and run”) membrane fusion, or with the aid of intermediate transport vesicles that bud off (membrane fission) from the donor membrane and migrate to the target membrane (Piper & Luzio 2004, Roth 2004, Luzio et al. 2007b, Stenmark 2009). In specialized cell types, there are also cell-type-specific vesicular compartments derived from endocytic and secretory pathways. For example melanosomes, which release melanin in response to light, are specialized lysosome-related organelles (LROs) in melanocytes (Blott & Griffiths 2002). In neurons, synaptic vesicles that release neurotransmitters upon electrochemical stimulation are derived from endosomes (Suudhof 2008). The second group of intracellular organelles includes mitochondria, peroxisomes, and the nucleus. Both group I and group II organelles function as intracellular Ca2+ stores with the luminal Ca2+ concentration ([Ca2+]lumen) ranging from micromolar (µM) to millimolar (mM) values, 10- to 10,000-fold higher than the level of resting cytosolic Ca2+ ([Ca2+]cyt, ~ 100 nM) (Michelangeli et al. 2005). The Ca2+ gradient in each of these organelles is actively established and maintained by various Ca2+ pumps and/or secondary Ca2+ transporters (Fig. 2; see reviews (Camello et al. 2002, Michelangeli et al. 2005)).
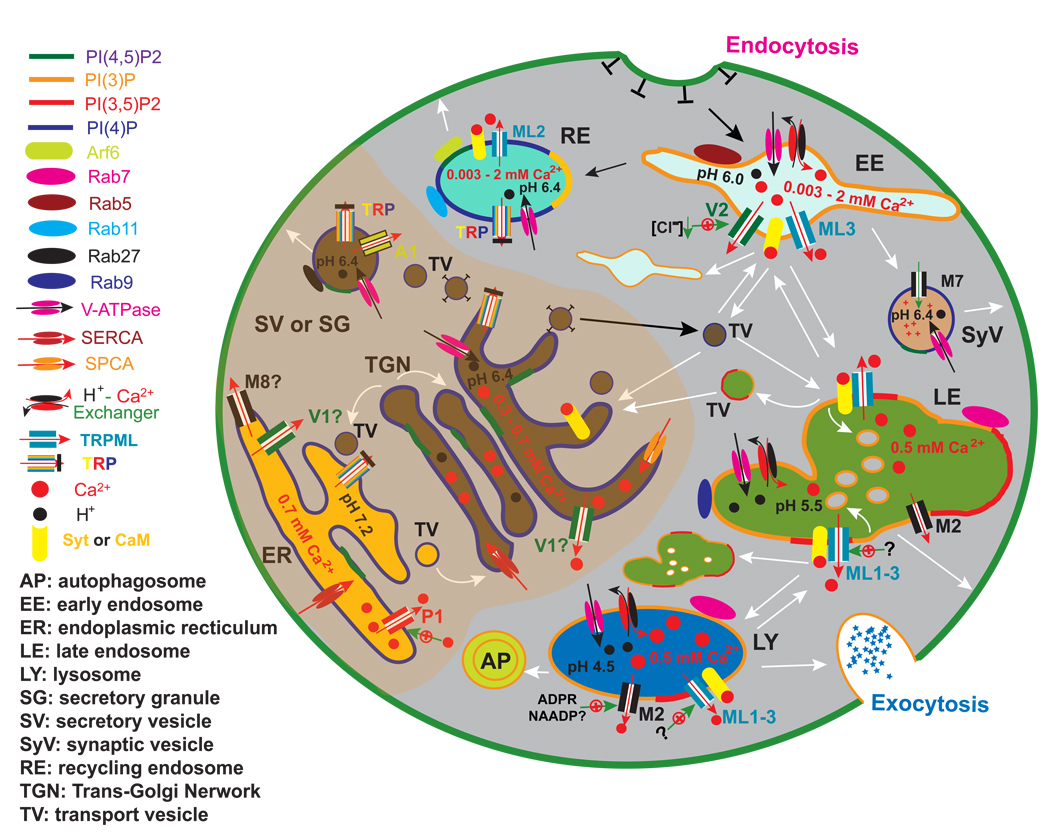
Figure 2. Intracellular TRP channels in the secretory and endocytic pathways.
Intracellular compartments undergo membrane fusion and fission/budding. There are two kinds of membrane fusions: “kiss and run” and complete fusion. In some steps, transport vesicles fission off from the source membranes and fuse with the target membranes to delivery cargos. Ca2+-sensitive membrane fusion and fission steps are indicated with white arrows. The molecular identities of intracellular compartments are defined by specific recruitment of small G proteins (Rab and Arf GTPases) and the composition of phosphoinositides (PIPs). The luminal ionic (H+ and Ca2+) composition is indicated for each organelle. (A) The Biosynthetic Pathway essentially all TRPs (labeled in mixed color) in the biosynthetic pathway may be present in the ER (pH 7.2; [Ca2+]ER ~ 0. 7 mM; PI(4)P + PI(4,5)P2) and the Golgi apparatus (Trans Golgi Network; TGN; pH 6.4; [Ca2+]Golgi ~ 0. 3–0.7 mM; PI(4)P + PI(4,5)P2). The Ca2+ gradients are established by the thapsigargin (TG) -sensitive SERCA (Sarco/Endoplasmic Reticulum Ca2+) pump in the ER, and by both SERCA and TG-insensitive SPCA (Secretory Pathway Ca2+) pumps in the Golgi. TRPV1 is reportedly functional in the ER or Golgi. There are intermediate transport vesicles derived from the ER and the Golgi apparatus. These transport vesicles may deliver cargos to early endosomes (EE; pH 6.0; [Ca2+]EE ~ 0. 003–2 mM; PI(3)P; Rab5), late endosomes (LE; pH 5.5; [Ca2+]LE ~ 0.5 mM; PI(3)P+PI(3,5)P2; Rab7 and Rab9), or the plasma membrane (PM) through secretory vesicles (SV; pH6.4)) and/or secretory granules (SG; pH 6.4). [Ca2+]EE changes significantly during the maturation of early EE, dropping from 2 mM in the primary endocytic vesicles to ~ 0.003 mM 20 min after endocytosis (Gerasimenko et al. 1998). SVs and SGs deliver the newly synthesized TRP channels to the PM. (B) The Endocytic Pathway EEs are derived from the primary endocytic vesicles after endocytosis. In addition to the late endocytic pathway, contents in the EE can also be sorted into recycling endosomes (RE; pH 6.4; [Ca2+]RE ~ 0. 003 – 2 mM; PI(3)P + PI(4)P + PI(4,5)P2; Rab11/Rab4), which are subsequently recycled back to the PM. TRP channels may be detected in the EE and RE as cargos during this cycle of endocytosis and recycling. In addition, TRPV2, TRPML2, and TRPML3 may play active roles in the early endocytic pathways. TRPV2 is activated by low pH and a reduction of intra-endosomal [Cl−]. The channel activity of TRPML2 (in RE) may regulate the activation of small GTPase Arf6, an important regulator of the recycling pathway. The activation mechanism of TRPML3 (in EE) is still not known. In sympathetic neurons, TRPM7 is localized in synaptic vesicles (SyV; pH 6.4) that are derived from EEs. The channel activity of TRPM7 plays a role in controlling neurotransmitter release. In EEs, intra-endosomal Ca2+ release may activate Ca2+ sensor proteins such as Synaptotagmin (Syt) and calmodulin (CaM). Subsequently, homotypic and heterotypic fusion events occur. In the late endocytic pathways, late endosomes (LEs) may “kiss and run” or completely fuse with other LE or lysosomes (LY; pH 4.5; [Ca2+]LY ~ 0.5 mM; PI(3)P+PI(3,5)P2; Rab7). TRPML1–3 channels are predominantly localized in LEs and LYs. Activation of TRPML channels by unidentified cellular cues may induce intralysosomal Ca2+ release. LEs, LYs, or hybrids of LEs and LYs, will then undergo calmodulin- (CaM) or synaptotagmin- (Syt) dependent membrane fusion or fission/budding. Membrane proteins enter the degradation pathway following membrane invagination to form multi-vesicular bodies (MVB) in LEs. The inward budding of internal vesicles into MVBs is a Ca2+-dependent process. In addition, MVBs may also undergo Ca2+-dependent exocytosis to release internal vesicles (exosomes) (Savina et al. 2003). Retrograde (retromer) transport vesicles (TVs), derived from EEs, LEs, or LYs upon membrane fission, transport lipids and proteins retrogradely to the TGN. In addition to fusion with LEs, LYs can also undergo fusion with autophagosomes (APs) to form autolysosomes (ALs), or with the PM, i.e., lysosomal exocytosis. TRPM2 in LEL compartments is activated by ADPR, and likely by NAADP as well.
Role of Ca2+ in signal transduction and organellar homeostasis
Intra-organellar Ca2+ release (efflux) has been shown to have an important role in signal transduction. For example, the IP3 receptor (IP3R) and the ryanodine receptor (RyR) in the ER and SR (sarcoplasmic reticulum), respectively, couple numerous extracellular signals to intracellular events such as hormone secretion and muscle contraction (Berridge et al. 2003). Alternatively other second messengers, such as nicotinic acid adenine dinucleotide phosphate (NAADP) and sphingolipid-derived messengers, may induce Ca2+ release from intracellular stores via novel mechanisms (Berridge et al. 2003, Calcraft et al. 2009, Zhang & Li 2007). Intra-organellar Ca2+ release, effecting a reduction of [Ca2+]lumen, may also modulate the function of organelles. For example, a reduction of [Ca2+]lumen may affect the protein folding and lipid synthesis in the ER (Corbett & Michalak 2000).
Ca2+-dependence of membrane traffic
Numerous in vitro and in vivo studies suggest that Ca2+ release (efflux) from group I organelles (ER, Golgi, endosomes, and lysosomes) is essential for membrane trafficking, fusion and fission (Burgoyne & Clague 2003, Hay 2007, Luzio et al. 2007b). The basic steps of fusion (tethering, docking, priming, and bilayer fusion) and the fusion machinery (SNAREs, phosphoinositides, and Rabs) involved are similar for fusion between intracellular membranes and for plasma membrane exocytosis (Martens & McMahon 2008, Suudhof 2008), suggesting that a similar mechanism may regulate both processes. Ca2+-dependence has been well documented for regulated exocytotic events, i.e. fusion of intracellular organelles such as synaptic vesicles, secretory vesicles/granules, or lysosomes with the plasma membrane (Reddy et al. 2001, Blott & Griffiths 2002, Suudhof 2008). On the other hand, intracellular membrane traffic has been traditionally classified to be “constitutive” (Roth 2004, Hay 2007). Evidence now exists, however, suggesting that intracellular traffic is also highly regulated. The final trigger of intracellular membrane fusion is also likely to be a rise of [Ca2+]cyt, whose amplitude and duration might be in a scale different from fusion at plasma membrane (Burgoyne & Clague 2003, Hay 2007). Both in vitro and in vivo studies suggest that [Ca2+]cyt in the vicinity of organelles plays a critical role in most, but not all, fusion events during the biosynthetic, secretory and endocytic pathways (Burgoyne & Clague 2003, Chen et al. 2002, Hay 2007, Holroyd et al. 1999, Luzio et al. 2007b, Luzio et al. 2000, Pryor et al. 2000). Using cell extracts from yeast or mammalian cells, in vitro fusion (content mixing) has been successfully reconstituted between various endosomal compartments: early endosome-early endosome, late endosome - late endosome, late endosome - lysosome, and lysosome-lysosome (Peters & Mayer 1998, Holroyd et al. 1999, Pryor et al. 2000). In all cases, fusion is inhibited by BAPTA, but not EGTA. Although both are strong chelators for Ca2+, BAPTA binds Ca2+ ions at least one hundred times faster than EGTA (Chen et al. 2002, Hay 2007). The increased sensitivity of endosomal fusion to BAPTA versus EGTA has been widely interpreted as evidence to support that intraluminal Ca2+ release is essential for triggering intracellular membrane fusion, and that the putative action site is extremely close (estimated to be < 20 nm) to the Ca2+ release site (Chen et al. 2002, Hay 2007, Pryor et al. 2000). Using membrane permeable forms of chelators, i.e., BAPTA-AM and EGTA-AM, it was shown that, in intact mammalian cells, intraluminal Ca2+ release is required for many steps of intracellular transport. For example, anterograde intra-Golgi transport and retrograde endosome-to-trans Golgi network (TGN) transport require intraluminal Ca2+ release (Chen et al. 2002, Burgoyne & Clague 2003, Hay 2007). The Ca2+-sensitive transport steps in the secretory and endocytotic pathways are summarized in Figure 2 (white arrows). Upon localized juxta-organellar Ca2+ elevation, two kinds of Ca2+ sensor proteins are activated to initiate membrane fusion. Synaptotagmins (Syts), a family of proteins with C2-type Ca2+ binding sites, are involved in the exocytosis of secretory vesicles, secretory granules, synaptic vesicles and lysosomes (Hay 2007, Luzio et al. 2007b, Reddy et al. 2001, Suudhof 2008). Calmodulin (CaM), an EF-hand cytosolic protein, has been shown to play important roles in multiple transport steps of secretory (for example, intragolgi transport) and endocytic pathways (for example, early and late endosomal fusions) (Peters & Mayer 1998, Burgoyne & Clague 2003, Hay 2007). How CaM is recruited to various intracellular vesicles is still not clear.
Juxta-organellar luminal Ca2+ release also regulates membrane fission/budding events (Hay 2007, Luzio et al. 2007a, Luzio et al. 2007b). Fission events share many common mechanisms with fusion events, although the outcome of fission or budding is to extract, rather than add, membrane. For example, Rab proteins and phosphoinositides (PIPs) can regulate both membrane fission and fusion (Roth 2004). Membrane fission is also dependent upon Ca2+ (Hay 2007, Luzio et al. 2007a, Luzio et al. 2007b). For instance BAPTA-AM, but not EGTA-AM, inhibited vesicle budding in vitro (Ahluwalia et al. 2001). The Ca2+ effector protein involved in the fission process is not known. In the TGN, both neuronal calcium sensor 1 (NCS-1) and Arf proteins are implicated (Hay 2007).
Although the importance of organellar Ca2+ release in signal transduction, organelle homeostasis, and membrane traffic has been established, the molecular identities of the Ca2+ release proteins resident in these compartments have remained elusive. Furthermore, the mechanisms that activate the release channels are largely unknown. Since many TRP proteins are Ca2+-permeable channels localized in the membranes of vesicles involved in the secretory and endocytic pathways, they are natural candidates to mediate organellar Ca2+ release. A list of TRP channels with their reported intracellular locations and potential functions is provided in Figure 1 and will be discussed in detail below.
II: TRP Channels in the Endocytic and Autophagic Pathways
Biogenesis of endosomes and lysosomes
The primary functions of endosomes and lysosomes (endo-lysosomes, collectively) are degradation, membrane trafficking, protein transport and signal transduction (for review, see Refs. (Berridge et al. 2003, Luzio et al. 2007b)). Endosomes are endocytotic vesicles derived from the plasma membrane (Fig. 2). Primary endocytic vesicles undergo maturation and trafficking to become early endosomes initially and late endosomes or multi-vesicular bodies later with a time scale of minutes or tens of minutes (Maxfield & McGraw 2004). Derived from late endosomes, lysosomes are membrane-enclosed compartments containing hydrolases for the intracellular digestion of macro-molecules (Luzio et al. 2007b). Lysosomes may fuse with late endosomes or multi-vesicular bodies to degrade macro-molecules (for example, the ligand-bound EGF receptor) that are taken up from the plasma membrane via endocytosis (Luzio et al. 2007b). Alternatively, lysosomes may fuse with obsolete parts of the cell via autophagy (Luzio et al. 2007b). In both cases, ingested materials are degraded to obtain energy and to recycle building materials which are then released into the cytosol or delivered to the TGN via a retrograde route. Internalized membrane proteins that are not targeted for degradation, for example, transferrin receptor, are recycled back to the plasma membrane. In this case, the protein cargo is sorted into the recycling endosome (Fig. 2).
Structural and regulatory mechanisms of membrane traffic
To control the direction and specificity of transport, a collection of structural and signaling proteins are recruited during various stages of the endocytic pathway. The fusion of two distinct vesicular compartments requires the contact of one v-SNARE (Soluble NSF Attachment Protein Receptors) and two or three t-SNARE proteins (Martens & McMahon 2008). SNAREs and their accessory proteins are regulated by both G proteins and lipid signaling (Roth 2004, Stenmark 2009). Rabs are small GTPases that are involved in mediating the identification and transport of organelles (review see Ref. (Stenmark 2009)). There are more than sixty Rabs in the secretory and endocytic pathways. The mechanisms for recruiting specific Rabs are not clear, although the cargos involved are known to play a crucial role (Stenmark 2009). The GTP-GDP cycle controls the recruitment of Rabs and their effector proteins to vesicles (Stenmark 2009). To control the transport specificity, various lipid kinases and phosphatases are often recruited to generate a set of organelle-specific phosphoinositides (PIPs) (Roth 2004, Poccia & Larijani 2009). Rabs and PIPs collaboratively determine the vesicular identities (see Fig. 2). For example, Rab5 and PI (3)P define early endosomes; Rab7 and PI(3,5)P2 define late endosomes (Roth 2004, Poccia & Larijani 2009). Dysregulated Rab signaling or PIP levels result in defective membrane trafficking (Roth 2004, Poccia & Larijani 2009, Stenmark 2009). While Rabs and PIPs can determine the direction of membrane traffic, the final step(s), i.e., the fusion of two vesicular compartments, depends on a brief increase in juxta-organellar [Ca2+](Luzio et al. 2007a, Luzio et al. 2007b).
Ionic composition and electrical properties of endolysosomes
The properties of endo-lysosomes (see Fig. 3) fit well with their primary functions. For example, the degradative functions of hydrolases are dependent on an acidic environment. A salient feature of endosomes and lysosomes is a low luminal pH (pH ~5–6 for endosomes and ~4–5 for lysosomes), which is established by a vacuolar (V)- type H+-ATPase (Luzio et al. 2007b). As mentioned previously, endo-lysosomes are also intracellular Ca2+ stores with the luminal Ca2+ concentration ([Ca2+]lumen) estimated to be ~0.5 mM for lysosomes (Christensen et al. 2002), and varying from 0.003 mM to 2 mM for early endosomes (from 2 mM in the primary endocytotic vesicles to ~ 0.003 mM after 20 min post- endocytosis) (Gerasimenko et al. 1998) (Fig. 2). The proton (H+) gradient helps in establishing the Ca2+ gradient in the endo-lysosome, presumably through a H+-Ca2+ exchanger (Christensen et al. 2002, Michelangeli et al. 2005, Luzio et al. 2007b). Inhibition of the V-ATPase by bafilomycin A1 leads to depletion of endo-lysosomal Ca2+ stores. The electrical potentials across the membranes of endosomes and lysosome are not precisely known. Based on studies of synaptic vesicles (Van der Kloot 2003) and phagosomes (Steinberg et al. 2007), transmembrane potentials are presumed to be positive in the lumen (relative to the cytosol) and likely to be between + 30 and +110 mV (see Fig. 3). The positive potential of endo-lysosomes provides a driving force for Ca2+ release into the cytosol. Keeping the basic properties and primary functions of endosomes and lysosomes in mind, we now will discuss the function of TRP channels found in the endo-lysosomal membrane.
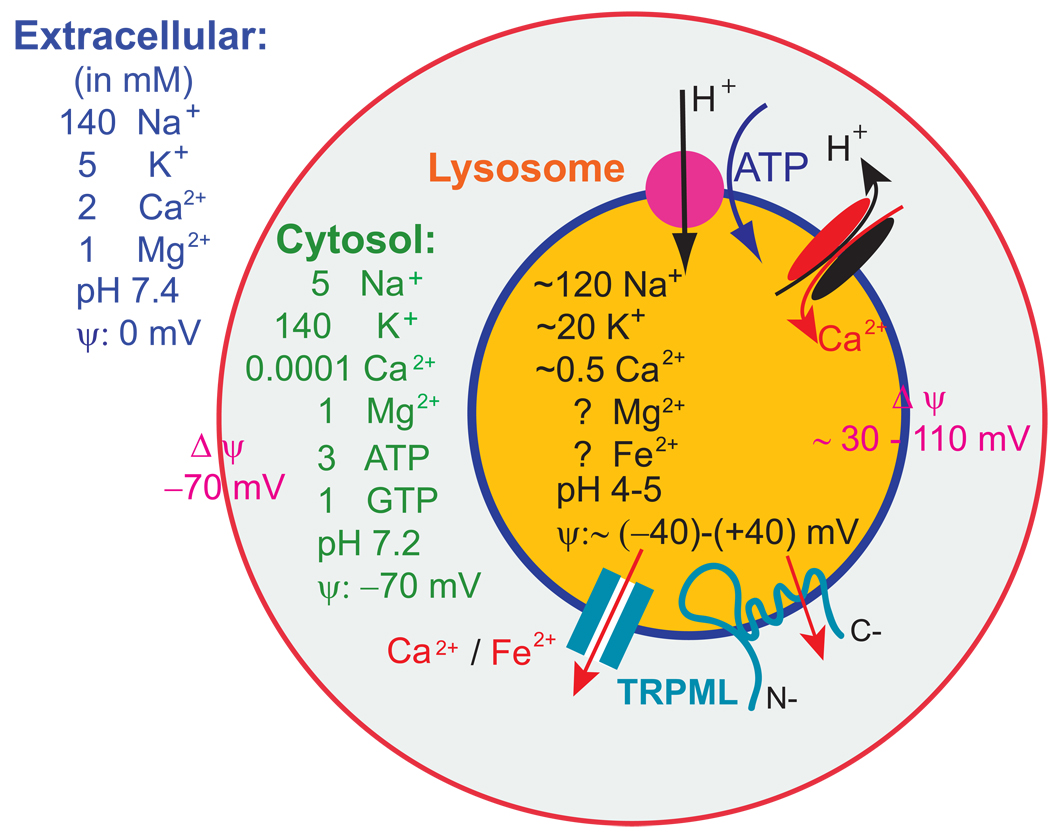
Figure 3. Electrical properties of lysosomes.
While the ionic compositions of extracellular space and cytosol have been well established, ion concentrations in the lumen of lysosome are not clear. The luminal pH is between 4 and 5 and is established by a V-type ATPase. The [Ca2+]LY is ~ 0.5 mM, which is maintained by an unidentified H+-Ca2+ exchanger. The resting membrane potential (Δφ cytosol relative to lumen or extracellular) of the cell is ~ −70 mV (cytoplasmic-side negative). Based on studies on synaptic vesicles and phagosomes, the membrane potential across the lysosomal membrane is estimated to be ~ + 30 to + 110 mV (luminal-side positive).
TRPV2 in early endosomes
Initially reported to be a temperature-activated channel in somatosensory neurons (Caterina et al. 1999), TRPV2 is also localized to intracellular vesicles (Kanzaki et al. 1999). A proteomic study revealing the presence of TRPV2 in the early endosome led Saito et al. (Saito et al. 2007) to test the hypothesis that TRPV2 is an endosomal Ca2+ channel. Using an elegantly modified patch-clamp technique, Saito et al. successfully measured endogenous ionic currents (IEE) in the isolated enlarged early endosome, which was made possible after endosomal fusion was genetically promoted (Saito et al. 2007). IEE is activated by reductions in the luminal pH and Cl− concentration, which usually occur within 20 min post-endocytosis (Gerasimenko et al. 1998). The pharmacological properties of IEE resemble those of ITRPV2 studied in heterologous systems. For example 2-APB, a known activator and RuR, a known blocker of ITRPV2, enhanced and inhibited IEE, respectively. Saito et al. proposed that activation of TRPV2 leads to Ca2+-dependent fusion between endosomal membranes. To ultimately prove the hypothesis, more experiments are necessary. For example, if TRPV2 indeed is an endosomal Ca2+ channel important for endosomal fusion, IEE is expected to be augmented by overexpression of TRPV2, but reduced by a decrease or depletion of endogenous TRPV2 expression. In addition, inactivation of TRPV2 is expected to alter the size and morphology of early endosomes.
TRPY1 in the yeast vacuole
The only TRP-like protein in yeast is the TRPY1(also called yvc1) protein (Palmer et al. 2001, Denis & Cyert 2002). TRPY1 is localized in the yeast vacuole, which is equivalent to the mammalian lysosome. In a series of elegant studies, patch-clamp recordings were performed directly on isolated yeast vacuoles. It was found that mechanical force or cytoplasmic Ca2+ could directly activate TRPY1 (Palmer et al. 2001, Zhou et al. 2003). ITRPY1 is a large-conductance inwardly rectifying Ca2+-permeable current. Using the aequorin Ca2+ reporter, Denis and Cyert have demonstrated that TRPY1 mediates the vacuolar Ca2+ release upon hyperosmotic shock (Denis & Cyert 2002). Collectively, these works have provided the most convincing evidence that TRPY1 is a physiologically important channel for intracellular Ca2+ release.
TRPML channels in late endosomal, lysosomal and other endocytic compartments
Tissue distribution and subcellular localization
Unlike other TRP channels, TRPML proteins are ideal candidate endo-lysosomal Ca2+ channels, since cells which lack TRPML1 exhibit enlarged endo-lysosomes (Chen et al. 1998, Piper & Luzio 2004). There are three TRPML proteins in mammals (TRPML1–3, also called MCOLN1–3) (Puertollano & Kiselyov 2009). TRPML1 is expressed in most tissues (Slaugenhaupt 2002, Cheng 2010) and co-localizes exclusively with late endosomal and lysosomal (LEL) markers (Dong et al. 2009, Puertollano & Kiselyov 2009, Cheng 2010) (see Fig. 1). The LEL localization of TRPML1 is also supported by gradient fractionation studies on both endogenous and heterologously-expressed proteins (Kim et al. 2009, Zeevi et al. 2009). In addition, TRPML2 and TRPML3 exhibit more restrictive tissue distribution patterns, but are also located in the LEL compartment (Cuajungco & Samie 2008, Puertollano & Kiselyov 2009, Zeevi et al. 2009). TRPML2 and TRPML3 are also found in recycling endosomes (Arf6-positive pathway) and early endosomes, respectively (Karacsonyi et al. 2007, Kim et al. 2009, Martina et al. 2009). TRPML distribution and localization are reviewed in detail recently (Puertollano & Kiselyov 2009, Cheng 2010).
Pore properties of TRPML channels
All three TRPMLs have been shown to be Ca2+-permeable (LaPlante et al. 2002, Xu et al. 2007, Dong et al. 2008, Kim et al. 2008, Kim et al. 2009), making them candidates to be lysosomal Ca2+ release channels. The first electrophysiologically characterized TRPML channel was TRPML3 (Kim et al. 2007, Xu et al. 2007, Kim et al. 2008). Although the majority of heterologously-expressed TRPML3 proteins are vesicular, a small portion of them are able to traffic to the plasma membrane and give rise to whole-cell currents (ITRPML3) (Grimm et al. 2007, Xu et al. 2007, Cuajungco & Samie 2008, Nagata et al. 2008, Kim et al. 2009, Martina et al. 2009, Puertollano & Kiselyov 2009). ITRPML3 is an inwardly-rectifying Ca2+-permeable current that is inhibited by low extracellular (analogous to the luminal side) pH but increased by Na+ manipulation (removal followed by re-addition) (Kim et al. 2007, Kim et al. 2008). Mutations in the mouse TRPML3 (A419P) result in the varitint-waddler (Va) phenotype (Cuajungco & Samie 2008, Puertollano & Kiselyov 2009). Va mice are deaf, exhibit circling behavior and have pigmentation defects. Compared with wild-type TRPML3, much larger TRPML3-mediated currents are seen in cells expressing TRPML3A419P (TRPML3Va). The TRPML3Va channel exhibits similar pore properties as wild-type TRPML3, but with altered gating behavior, suggesting that Va is a channel gain-of-function mutation (Grimm et al. 2007, Kim et al. 2007, Xu et al. 2007, Cuajungco & Samie 2008, Kim et al. 2008, Nagata et al. 2008, Puertollano & Kiselyov 2009). By artificially introducing a Va-like mutation in the analogous position of TRPML1 (V432P), Xu et al were able to characterize the pore properties of TRPML1 (Xu et al. 2007). Like TRPML3, TRPML1Va is an inwardly rectifying Ca2+-permeable but proton-impermeable channel. But, unlike ITRPML3, ITRPML1-Va is potentiated by low pH. Similarly, ITRPML2-Va is also a proton-potentiated inwardly rectifying Ca2+-permeable current (Dong et al. 2008). It remains a possibility that Va-like mutations change the pore properties of TRPML1 and TRPML2, although this is least likely. Recently, Dong et al developed a patch-clamp method to record currents directly from isolated endo-lysosomes which were pharmacologically enlarged (from 0.1–0.5 to 2–3 µm) using the small molecule compound vacuolin-1 (Dong et al. 2008). With this method, Dong et al were able to record ITRPML1 in enlarged endo-lysosomes. Lysosomal ITRPML1, although much smaller in amplitude than ITRPML1-Va, largely resembles ITRPML1-Va, suggesting that although TRPML1 is likely to be gated by other, unidentified, cellular cues, the activating mutation is still a valid approach for characterizing the pore properties of TRPML1 and TRPML2. In summary, TRPMLs constitute a family of inwardly rectifying, Ca2+-permeant but proton-impermeant cation channels. TRPML channel properties are reviewed in detail recently (Puertollano & Kiselyov 2009, Cheng 2010).
Dual roles of TRPMLs in membrane trafficking: fusion and fission
The role of the TRPMLs in postendocytic membrane trafficking and organelle dynamics in the late endocytic pathway has been extensively studied both in vitro and in vivo (reviewed in Refs. (Puertollano & Kiselyov 2009, Cheng 2010)). Mutations in human TRPML1 cause type IV mucolipidosis (ML4), a devastating neurodegenerative disease in young children (Slaugenhaupt 2002). ML4 patients exhibit motor defects, mental retardation, retinal degeneration, and iron-deficiency anemia. Loss-of-function studies revealed a role of TRPML1 in membrane fission from the LEL compartment. Fibroblasts derived from ML4 patients (ML4 cells), expressing loss-of-function TRPML1 mutations, exhibit enlarged, swollen lysosome-like vacuoles (Chen et al. 1998, Slaugenhaupt 2002). Endocytic delivery to lysosomes involves direct fusion between late endosomes and lysosomes to produce late endosome–lysosome hybrid organelles, from which the lysosomes are re-formed (Luzio et al. 2000, Pryor et al. 2000, Luzio et al. 2007b). Genetic studies of the cup-5 mutant, a C elegans orthologue of mammalian TRPMLs, reveal that the enlarged vacuoles of cup-5 cells contain markers for both late endosomes and lysosomes and are likely to be hybrid organelles (Treusch et al. 2004). Mechanistically, enlarged endo-lysosomes could result from uncontrolled and excessive fusion, defective membrane fission, or impaired organellar osmoregulation (Luzio et al. 2007a). In most cases, enlarged endosomes and lysosomes are suggestive of defective trafficking. For example, dysregulation of Rabs and PIPs, two essential regulators of membrane trafficking, often leads to similar phenotypes, i.e., enlarged vacuoles containing hybrid markers (Stenmark 2009, Roth 2004, Poccia & Larijani 2009). While overactive Rab5 causes enlarged endosomes, loss-of-function mutations of PIKfyve/Fab1, a LEL-specific lipid kinase synthesizing PI(3,5)P2 from PI(3)P, causes enlarged LEL compartments. ML4 cells also accumulate a variety of undigested lipids and water-soluble substances in the LEL compartment (Chen et al. 1998). Lipid accumulation may result from the defective degradation of cellular components and/or disruptions in membrane trafficking. Defective degradation could in turn be due to either a lack of specific hydrolase enzymes, as revealed in most lysosome storage diseases (Puertollano & Kiselyov 2009), or an impaired ion homeostasis (especially H+) of organelles. However, in TRPML1-deficient (TRPML1−/−) cells, accumulated lipids and storage materials are of heterogeneous origins (Chen et al. 1998, Treusch et al. 2004, Venugopal et al. 2007, Venkatachalam et al. 2008), suggesting that the defects might be related to the homeostasis of LEL compartments and/or disruption of broad spectrum lipid metabolism. The hydrolase-catalyzed lysosomal degradation of storage materials, however, is largely unaffected by the TRPML1 defect in ML4 cells (Chen et al. 1998). Thus the most likely defect of ML4 cells is membrane trafficking. Consistent with this, the use of labeled lipids such as fluorescent lactosylceramide in pulse-chase experiments demonstrated a delay in the retrograde transport of lipids from lysosomes to the TGN, consistent with the role of TRPML1 in late endocytic pathway trafficking (Chen et al. 1998, Pryor et al. 2006, Thompson et al. 2007). Although lipid accumulation phenotype may be caused by secondary effects due to the chronic accumulation of undigested lipids (Miedel et al. 2008), the lipid accumulation in ML4 cells, however, can be acutely rescued by introduction of the wild-type TRPML1 gene (Pryor et al. 2006). Thus the defective lipid exit from the LEL compartment may be a direct consequence of TRPML1-deficiency.
The simplest model to reconcile most results in the literature is that the formation of transport vesicles from the LEL compartment to the TGN, and the reformation of lysosomes from the late endosome-lysosome hybrid organelles, are blocked in ML4 or cup-5 cells (Piper & Luzio 2004, Treusch et al. 2004, Thompson et al. 2007). In other words, TRPML1 may be required for the biogenesis of lysosomes and LEL-derived transport vesicles. Consistent with this idea, TRPML1 gene expression is significantly elevated when lysosome biogenesis is induced (Sardiello et al. 2009). As membrane fission is Ca2+-dependent, it is likely that TRPML1 and, more specifically, its Ca2+ conduction, is required for the membrane fission from LEL compartments or late endosome-lysosome hybrids. Notably, intraluminal Ca2+ release has been demonstrated to play an essential role in the in vitro re-formation of lysosomes from endosome–lysosome hybrids (Pryor et al. 2000, Luzio et al. 2007a). Consistent with the requirement of TRPML1 in lipid migration from late endosomes and lysosomes to the TGN, Ca2+ has been shown to be required for the formation and stabilization of specific transport vesicles (Ahluwalia et al. 2001, Luzio et al. 2007a, Luzio et al. 2007b).
TRPML1 is also involved in the membrane fusion of endo-lysosomes. Both the transport of fluid-phase markers to lysosomes and the lysosomal degradation of internalized growth factor receptors are delayed in TRPML1−/− cells (Treusch et al. 2004, Thompson et al. 2007), suggesting a defect in trafficking of endocytosed materials into the LEL compartment. These findings could indicate that the Ca2+ permeability of TRPML1 is required for the Ca2+-dependent membrane fusion between early endocytic compartments, or for the formation of transport vesicles from early to late endosomes. In addition to its interaction with the late endosome, a lysosome can also incompletely (“kiss-and-run”) or completely fuse with the plasma membrane (Luzio et al. 2007b) resulting in the exocytosis of lysosomal contents (lysosomal exocytosis). This process has been implicated in cellular waste elimination, membrane repair, and neurotransmitter release (Reddy et al. 2001, Zhang et al. 2007). Lysosomal exocytosis is triggered by a rise of [Ca2+]cyt and the subsequent binding of Ca2+ to the C2 domains of synaptotagmin VII localized in the lysosomal membrane.
The lumen of the lysosome is a major sources of the Ca2+ involved in lysosomal exocytosis (Luzio et al. 2007a, Luzio et al. 2007b). The mechanisms underlying lysosomal Ca2+ release, however, are still unclear. Moreover, the molecular identities of the putative lysosomal Ca2+ release channels remain elusive. Lysosomal exocytosis is reduced in ML4 cells (LaPlante et al. 2006) but increased in HEK cells expressing gain-of-function TRPML1 mutations (Dong et al. 2009). Increased lysosomal exocytosis results in increased cell surface expression of TRPML1, which may explain the measurable whole-cell current of TRPML1Va (Dong et al. 2009). To elaborate this hypothesis, it is necessary to investigate whether or not the blockade of lysosomal exocytosis reduces ITRPML1-Va amplitude. Consistent with a role of TRPML1 in exocytosis, shRNA knockdown of TRPML1 leads to the reduced transport of major histocompatibility complex II (MHCII) to the plasma membrane in macrophages (Thompson et al. 2007). In summary, TRPML1 participates in multiple transport steps of late endocytic pathways by its involvement in the mechanisms of both membrane fusion and fission.
TRPML2 and TRPML3 appear to play similar roles to TRPML1 in postendocytic membrane trafficking. Zeevi et al recently reported that siRNA knockdown of endogenous TRPML2 in HEK or Hela cells results in inclusion bodies in the LEL compartment (Zeevi et al. 2009). Inactivation of TRPML2 by a dominant-negative approach reduced recycling of internalized plasma membrane proteins back to the plasma membrane (Karacsonyi et al. 2007). Conversely, overexpression of TRPML2 caused a constitutive activation of Arf6, leading to increased exocytosis (Karacsonyi et al. 2007). These studies suggest that TRPML2 is required for membrane fusion in the early endocytic pathways, and membrane fusion and fission in the late endocytic pathways. Consistent with a positive role of TRPML3 in the late endocytic pathways, siRNA knockdown of TRPML3 leads to inclusion bodies in the LEL compartment of HEK and Hela cells (Zeevi et al. 2009). Two recent studies report that overexpression of TRPML3 results in enlarged endo-lysosomes, decreased endocytosis, and reduced lysosomal degradation (Kim et al. 2009, Martina et al. 2009). These results initially appear inconsistent with Zeevi et al’s TRPML3 findings and the proposed functions for TRPML1 and TRPML2. These apparent disparities can be reconciled if we consider the dual functions of TRPMLs in membrane fusion and fission. As mentioned above, enlarged endo-lysosomes might result from either excessive fusion or reduced fission. TRPML3 is expressed in both early endosomes and the LEL compartment (Kim et al. 2009, Martina et al. 2009). Dysregulated organellar Ca2+ release resulting from TRPML3 overexpression may possibly increase membrane fusion events in the early and/or late endocytic pathways. The vacuolar phenotype resulting from overexpression may not be limited to TRPML3. Indeed, we have observed enlarged LEL compartments in HEK cells expressing high levels of TRPML1 (Cheng and Xu, unpublished observation; also see Ref. (Vergarajauregui et al. 2009)). An overexpression of C-terminal, but not N-terminal, EGFP fusion constructs of TRPML1 or TRPML2 in B-lymphocytes was reported to cause enlarged LEL compartments (Song et al. 2006). These C-terminal fusion constructs appear to exhibit high levels of expression in B-lymphocytes (personal communication with Scharenberg A.). Consistent with a role of TRPML3 in membrane fusion, TRPML3 overexpression also leads to an increased number of autolysosomes, which results from the fusion of autophagosomes and lysosomes(Kim et al. 2009). TRPMLs may thus play important roles in membrane traffic by regulating both Ca2+-dependent membrane fusion and fission. As different Ca2+ sensors and effectors are implicated in fusion and fission, selective inhibitors of Ca2+ sensors may prove informative regarding the nature (effecting fusion versus fission) of the defects leading to enlarged endolysosomes.
A key open question is how TRPML channels are regulated by various cytosolic and luminal factors, and/or proteins and lipids in the endo-lysosomal membranes, especially those which are known to be involved in endo-lysosomal trafficking. In addition to Ca2+, PIPs and G proteins have been found to regulate intracellular trafficking (Roth 2004, Stenmark 2009). It is conceivable that these signaling cascades may be involved in relaying extracellular signals to those intracellular pathways that mediate membrane trafficking. Signaling by lipid and G proteins is known to modulate plasma membrane TRP channels (Clapham 2003, Montell 2005, Nilius et al. 2007); it is likely that PIPs and Rabs regulate membrane traffic by modulating vesicular TRPMLs. Direct evidence to support this hypothesis is still lacking.
TRPMLs in signal transduction
TRPML1 has been proposed to be an important participant in lysosome-mediated signal transduction. As mentioned previously, Ca2+ release from lysosomes or lysosome-related acidic stores, similar to Ca2+ release from the ER, plays an indispensable role in the transduction of many extracellular signals (Galione et al. 2009). For example, endothelin-1 (ET-1) and integrin ligands mobilize lysosome-related Ca2+ stores in smooth muscle cells (Galione et al. 2009). Accumulated evidence suggests that nicotinic acid adenine dinucleotide phosphate (NAADP) may act as a common second messenger downstream of these receptors, and that the putative NAADP receptor is likely to be found in lysosomes (Zhang & Li 2007, Calcraft et al. 2009, Galione et al. 2009). As Ca2+ channels in the LEL compartment, TRPMLs are natural candidates to mediate the NAADP response. By reconstituting lysosomal membranes into a lipid bilayer, Zhang et al recently reported that NAADP upregulates a cationic current that is dependent on TRPML1 expression (Zhang & Li 2007). The pharmacological properties of INAADP in the lipid bilayer are similar to those of the putative NAADP receptor. However, INAADP is a Cs+-permeable current and exhibits a linear I–V curve, two properties that are inconsistent with ITRPML1. It is possible that other lysosomal membrane proteins may form heteromultimers with TRPML1, leading to a novel current. For example, TPC2 protein has been recently shown to mediate NAADP-induced Ca2+ release from lysosomes (Calcraft et al. 2009, Galione et al. 2009). Future work may reveal the relative contributions of TRPML1, TRPM2 (see following), and TPC2 to the endogenous NAADP response in different cell types.
TRPMLs in vesicular ion homeostasis
In the endo-lysosome system, H+, Ca2+, and membrane fusion have been found to be interconnected (Luzio et al. 2007a, Luzio et al. 2007b). In addition to Ca2+, TRPML channels are permeable to other cations in the LEL lumen (Fig. 3) and thus may have functions distinct from Ca2+ signaling. Although these findings are highly controversial, ML4 or TRPML1 knockdown cells (TRPML1−/−) cells appear to have an overly acidified pH in LEL compartments (Soyombo et al. 2006, Miedel et al. 2008). Given that the TRPML1-Va channel exhibits no permeability to protons, it is not immediately clear what may cause lysosomal hyperacidification in the absence of TRPML1. Although the TRPML3 channel is not proton-permeable and is indeed inhibited by low pH, overexpression of TRPML3 results in the alkalization of endo-lysosomes (Martina et al. 2009). Therefore, it is likely that endo-lysosomal acidification is secondary to Ca2+ release (see Ref. (Cheng 2010) for discussion).
TRPML1 and TRPML2, but not TRPML3, are also permeable to Fe2+, Mn2+, and other heavy trace metals (Dong et al. 2008). ML4 cells exhibit a cytosolic Fe2+ deficiency and a concurrent lysosomal Fe2+ overload, suggesting that the iron efflux pathway is blocked in ML4 cells and that TRPML1 is essential for lysosomal Fe2+ release (Dong et al. 2008). Under oxidative conditions, lysosomal Fe2+ overload may dramatically increase the production of reactive hydroxyl radicals (OH•; Fenton reaction), which in turn facilitate the formation of lipofuscin(also called aging pigment) (Kurz et al. 2008).
In summary, TRPMLs participate in multiple endo-lysosome-mediated functions including signal transduction, ionic homeostasis, and more than one aspect of membrane trafficking. A major challenge of TRPML research is to understand how one single TRPML protein can play such diverse roles. Multiple ionic conductances in a single membrane channel may certainly contribute to multifaceted functions. In addition to their divalent permeability, TRPMLs are also permeable to Na+ and K+ (Xu et al. 2007). TRPMLs may therefore regulate organelle dynamics by regulating endo-lysosomal membrane potentials. Rabs and PIPs exist in “microdomains” in the membranes of endo-lysosomes, participating in multiple functions by recruiting distinct effector proteins (Poccia & Larijani 2009, Stenmark 2009). TRPMLs may differentially associate with Rabs, PIPs, and Ca2+ sensors (for example, CaM, Syt, and ALG-2), giving them the ability to generate multiple cellular outputs.
TRPM2 in the LEL compartment
Initially characterized as a plasma membrane channel gated by free cytosolic ADP-ribose (ADPR) (Perraud et al. 2001), recent evidence suggests that TRPM2 is also localized in the LEL compartment (Lange et al. 2009). Rather than simply being cargo in the degradative pathway, TRPM2 can function as a lysosomal Ca2+ release channel in response to cytosolic ADPR (Lange et al. 2009). Plasma membrane TRPM2 is reportedly activated by NAADP (Beck et al. 2006). It is conceivable that TRPM2 also has a role in lysosomal NAADP signaling (see above). In addition, plasma membrane TRPM2 is activated by increases in [Ca2+]i (Du et al. 2009). Thus, TRPM2 might mediate Ca2+-induced Ca2+ release (CICR) in the LEL compartment, analogous to IP3 R/ RyR in the ER/SR (Berridge et al. 2003). Although CICR has not been demonstrated in mammalian LELs, such mechanism has been proposed to exist in yeast vacuoles (Palmer et al. 2001).
TRP channels in recycling endosomes
Like the transferrin receptor, plasma membrane TRP channels constantly undergo endocytosis and enter the recycling pathway (Maxfield & McGraw 2004). The steady-state location of TRPs is determined by the balance of endocytosis and membrane insertion, which is under tight regulation by a variety of extracellular signals and cellular cues (Maxfield & McGraw 2004). TRPV5 and TRPV6 are Ca2+-selective channels involved in Ca2+ transport in the kidney (van de Graaf et al. 2006). In epithelial cells, TRPV5 and 6 are found to be localized to recycling endosomes and physically interact with Rab11, a small G protein that is predominantly localized in recycling endosomes (van de Graaf et al. 2006). Locking Rab11 in a inactive GDP-bound state results in the reduced surface expression of TRPV5 and 6 (van de Graaf et al. 2006).
Several TRPs have been shown to undergo regulated exocytosis. In hippocampal neurons, TRPC5 is localized in the intracellular vesicles of neurites (Bezzerides et al. 2004). Using total internal reflection fluorescence (TIRF) microscopy to image events within 100 nm of the plasma membrane, Berrerides et al. demonstrated that, in response to growth factor stimulation, TRPC5-residing vesicles undergo rapid insertion into the plasma membrane (Bezzerides et al. 2004). Similarly TRPC3, residing in VAMP2-positive compartments, was reported to undergo GPCR receptor-stimulated translocation to the plasma membrane (Singh et al. 2004). In response to mechanical shear stress, TRPM7-residing vesicles undergo translocation to the plasma membrane of vascular smooth muscle cells (Oancea et al. 2006). TRPV1 is found in intracellular vesicles and physically interacts with the vesicular proteins Snapin and Synaptotagmin IX (Morenilla-Palao et al. 2004). Upon stimulation, TRPV1-containing vesicles undergo PKC-dependent exocytosis, allowing a novel form of channel regulation (Morenilla-Palao et al. 2004). Although the molecular identities of these vesicles are poorly defined, they can be tentatively classified as belonging to an intracellular pool that is derived from recycling endosomes.
Are these vesicular TRPs functional? It was shown that activation of TRPC3 by diacylglycerol (DAG) may further increase the cell surface expression of the channel (Singh et al. 2004). It is not clear whether DAG was activating plasma membrane or vesicular TRPC3, or perhaps both. Conducting this experiment in the presence of a membrane-impermeable TPRC3 inhibitor (not reported yet) would prove informative. If vesicular TRPs were purely cargo that did not actively participate in vesicular trafficking, it would be important that they keep inactive during transport to avoid the misregulation of membrane traffic. There are several reasons to suggest that this might be the case. The lipid composition of intracellular membranes is quite different from that of the plasma membrane. Many TRPs require PI(4,5)P2 for their function (Ramsey et al. 2006). Although abundant in the plasma membrane, this lipid is usually excluded from endocytic vesicles (Roth 2004, Poccia & Larijani 2009). In addition, most TRPs are inhibited by low pH (Clapham 2003), such as that found in many vesicles. Therefore, it is likely that even the presence of agonist may not sufficiently activate TRPs in the intracellular reserve pool. This hypothesis could be tested by measuring the [Ca2+]cyt response upon stimulating the cells using membrane-permeable agonists (if available) in the absence of extracellular Ca2+. As intracellular vesicles may be heavily dependent on extracellular Ca2+ to fill/refill their Ca2+ stores, caution is necessary regarding to the time course of such experiments.
III: TRP Channels in the Biosynthetic/Secretory Pathway
Like all other plasma membrane proteins, TRPs have to go through various stages of biosynthetic pathways before they reach their destination. For example, in order to carry out normal functions, plasma membrane TRPs are glycosylated in the ER and Golgi apparatus (Cohen 2006). Recent works, however, indicate that several TRPs are constitutively localized in the ER and Golgi and, more importantly, can be activated in these compartments by their agonists (Koulen et al. 2002, Prasad et al. 2008, Thebault et al. 2005, Turner et al. 2003). As multiple transport steps in the ER and the Golgi are regulated by intraluminal Ca2+ release (Ahluwalia et al. 2001, Hay 2007), these studies point to active roles of TRPs in ER or Golgi-mediated signal transduction and/or membrane trafficking.
Ca2+ release from organelles in the secretory pathways (ER and Golgi) is important for signal transduction (Berridge et al. 2003). For example, the “classic” Ca2+ release channels, i.e., IP3Rs/RYRs in the ER/SR, couple numerous extracellular signals to intracellular events such as hormone scretion (Berridge et al. 2003). Several TRP Ca2+ channels (see below) in the ER and Golgi may also be activated to completely or partially deplete the Ca2+ stores, which may be coupled with unidentified cellular events. A most likely possibility is that TRP-mediated ER Ca2+ release activates IP3R/RYR-mediated Ca2+-induced-Ca2+ release (CICR) (Berridge et al. 2003). In addition, there exist poorly characterized “ER leak” pathways that are known to be important in regulating ER Ca2+ homeostasis, although their molecular identities are not known (Camello et al. 2002, Berridge et al. 2003).
Ca2+ release from the ER and the Golgi is also important for membrane trafficking. In vivo studies revealed distinct sensitivities to BAPTA-AM versus EGTA-AM for many steps of membrane trafficking in the secretory pathways including, for example, anterograde intra-Golgi transport and retrograde endosome-to-TGN transport (Ahluwalia et al. 2001, Burgoyne & Clague 2003, Chen et al. 2002, Hay 2007). Inhibitory effects by BAPTA but not EGTA were also observed in cell-free intra-Golgi transport assays (Porat & Elazar 2000). CaM inhibitors have also been shown to block the intra-Golgi transport (Ahluwalia et al. 2001, Chen et al. 2002, Porat & Elazar 2000). Therefore, it is likely that uncharacterized Ca2+ release channels in the ER or Golgi may be activated by trafficking cues to regulate membrane fusion/fission events in the secretory pathways.
TRPP1 channels in the endoplasmic reticulum
TRPP1 (also called polycystin-2, PC2, and TRPP2) is one of two genes mutated in polycystic kidney disease (PKD) (Zhou 2009). TRPP1 is localized to the ciliary membranes of kidney epithelial cells where it mediates fluid flow-induced Ca2+ influx (Nilius et al. 2007). However, both heterologously-expressed and endogenous TRPP1 proteins are also found to be localized to the ER (Koulen et al. 2002, Geng et al. 2008). By reconstitution into the lipid bilayer, TRPP1 was shown to be activated by Ca2+ on the cytoplasmic side (Koulen et al. 2002, Geng et al. 2008). TRPP1 may therefore function as a calcium-induced calcium release channel like the IP3R and the RyR, participating in signal amplification. Consistent with this, receptor-mediated IP3-dependent ER Ca2+ release is increased by the overexpression of wild-type, but not the disease-causing mutant TRPP1 (Koulen et al. 2002, Geng et al. 2008). In TRPP1-deficient cells, the basal [Ca2+]cyt is significantly lower than in control cells (Geng et al. 2008). In contrast, overexpression of TRPP1 leads to an increase of [Ca2+]cyt and a concurrent decrease of ER Ca2+ content (Geng et al. 2008). These results suggest that TRPP1 may function as an ER leak channel. TRPP1 is also found to interact with Syntaxin-5, an ER and Golgi-associated t-SNARE; this interaction inhibits the ITRPP1(Geng et al. 2008). While Ca2+ is presumed to regulate SNARE complex formation, this study suggests that SNARE proteins can in turn regulate Ca2+ release channels.
TRPM8 in the endoplasmic reticulum
TRPM8 is a cold- and menthol-activated channel in the plasma membrane of somatosensory neurons (Ramsey et al. 2006). In human prostate epithelial cells, however, TRPM8 is highly localized to the ER (Thebault et al. 2005). In the absence of extracellular Ca2+, activation of TRPM8 by menthol or cold induces [Ca2+]i increases that is sensitive to thapsigargin (TG), suggesting that the ER store is the source of the Ca2+ release (Thebault et al. 2005). TRPM8-agonist-inducced ER Ca2+ store depletion results in the activation of the store-operated Ca2+ release-activated Ca2+ (CRAC) channel(Thebault et al. 2005). Therefore, in some cell types, TRPM8 may function as an ER Ca2+ release channel similar to IP3R and RyR. It is not clear whether TRPM8 can function as a Ca2+ release channel in the ER of other cell types, such as sensory neurons. Although the cellular mechanism activating TRPM8 in the ER is not known, it is conceivable that endogenous menthol-like molecules exist, functioning as novel Ca2+-mobilizing second messengers.
TRPV1 in the endoplasmic reticulum and the trans-Golgi network
TRPV1 is a true sensory channel that is expressed in somatosensory neurons and is activated by heat, protons, and capsaicin (Clapham 2003). Subcellular localization studies suggest that both heterologously-expressed and endogenous TRPV1 are also localized to the ER and Golgi compartments (Turner et al. 2003). Activation of TRPV1 by capsaicin induces Ca2+ release from an IP3-sensitive but TG-insensitive store. While ER Ca2+ stores are maintained by the TG-sensitive SERCA (Sarco/Endoplasmic Reticulum Ca2+) pump, Ca2+ gradients in the Golgi are established by both SERCA and the TG-insensitive SPCA (Secretory Pathway Ca2+) pump. Like the ER, the Golgi also contains functional IP3Rs (Michelangeli et al. 2005). Due to the TG-insensitivity of TRPV1-induced Ca2+ release, the most likely location for TRPV1 is in SPCA-positive Golgi compartments. Mobilization of TRPV1-containing Ca2+ stores, however, is not sufficient to activate CRAC channels (Turner et al. 2003). Nevertheless, the evidence sufficiently supports a role of TRPV1 in intracellular Ca2+ release.
The endogenous agonist of TRPV1 in intracellular membranes has remained elusive. As the Golgi is acidic relative to the ER (Fig. 1), transport of TRPV1-positive vesicles from the ER to the Golgi may readily activate or sensitize TRPV1. Anandamide, a weak agonist of TRPV1, is known to induce intracellular Ca2+ release via a PLC-independent mechanism (Felder et al. 1993). TRPV1 in the Golgi compartments may serve as a natural candidate for this release.
TRPA1 in secretory vesicles and secretory granules
TRPA1 is expressed in somatosensory neurons and responds to a variety of noxious sensory compounds (Ramsey et al. 2006). In mast cells, TRPA1is localized in secretory vesicles (SVs) and secretory granules (SGs) and physically interacts with vesicular proteins in SGs (Prasad et al. 2008). When heterologously expressed in HEK cells, TRPA1 can mediate intracellular Ca2+ release, presumably from SVs, in response to the TRPA1 agonist icilin (Prasad et al. 2008). As intracellular Ca2+ activates TRPA1 (Zurborg et al. 2007), TRPA1 may function as another CICR-like channel in SV or SG. As SVs or SGs undergo Ca2+-regulated exocytosis, this mechanism may allow a feed-forward induction of cell-surface expression of TRPA1, contributing to a sudden increase in the ITRPA1.
Other TRP channels in the endoplasmic reticulum, trans-Golgi network, secretory vesicles and granules
All plasma membrane TRP channels need to go through the biosynthetic pathways, i.e. ER, TGN, and secretory vesicles/granules before being inserted into the plasma membrane. Are these nascent TRP channels functional in these compartments? As many synthetic and natural products have been found to activate specific TRPs in the plasma membrane (Ramsey et al. 2006), for these TRPs, these relatively selective agonists may be useful for future studies to reveal whether they are functional in intracellular compartments.
All four TRPs mentioned above (TRPP1, TRPM8, TRPV1, and TRPA1) are active intracellularly. Is intracellular activation a general feature for TRPs in the biosynthetic pathways? While PI(4,5)P2 is a limiting factor to prevent the activation of TRPs in the recycling pathways, the ER and the Golgi do contain significant amounts of PI(4,5)P2 in their membranes (Roth 2004). There are factors, however, that may prevent the full functionality of TRPs in the ER and Golgi membranes. For example, the lipid bilayers of the ER and the Golgi favor proteins with shorter transmembrane domains than those of the plasma membrane (Watson & Pessin 2001). In addition, several TRPs require post-translational modification such as glycosylation for function (Cohen 2006). Nevertheless, since all four TRPs studied so far are active intracellularly, it is conceivable that TRPs in the biosynthetic pathways might be active. Although activation of many other vesicular TRPs (Fig. 1) has not been reported, some of them, for example, TRPC3 and TRPC7, have been shown to actively participate in trafficking/exocytosis (Lavender et al. 2008).
IV: TRP Channels in the Cell-type Specific Compartments
Several TRPs are found in cell-type specific vesicular compartments, for example, lysosome-related organelles (LROs) and synaptic vesicles (SyVs). These may participate in cell-type specific functions in these compartments independent of their general roles.
TRPM7 in synaptic vesicles
TRPM7 is a protein with two functional domains: a cation channel module and a kinase domain (Ramsey et al. 2006). Under physiological conditions, ITRPM7 is outwardly rectifying with an inward non-selective (Ca2+, Mg2+, Na+) conductance and an outward monovalent conductance (Clapham 2003). Although ITRPM7-like current can be recorded in almost every cell, in sympathetic neurons and PC12 cells TRPM7 is found to be mainly localized in acetylcholine (ACH)-containing synaptic vesicles (SyVs) (Krapivinsky et al. 2006, Brauchi et al. 2008). Consistent with its vesicular localization, Kapivinsky et al provided additional biochemical evidence that TRPM7 forms a molecular complex with the fusion machinery components snapsin, a SNARE protein, and synaptotagmin I (Krapivinsky et al. 2006). Electrophysiological analyses revealed that both amplitude and frequency of EPSP are reduced in neurons transfected with TRPM7-specific siRNA or dominant-negative TRPM7 constructs. Further analysis suggest that the reduced EPSP amplitude is due to a smaller quantal size of neurotransmitter release, which reflects a reduced amount of ACH release in a single fusion event (fusion of a SyV with the plasma membrane). As TRPM7 is outwardly rectifying, one possibility is that TRPM7 may provide counter ions for the release of positively-charged neurotransmitters (Krapivinsky et al. 2006). However, vesicular ITRPM7 is presumed to exhibit a linear I–V as low pH from the luminal side may potentiate the inward (cations flowing out the vesicular lumen) conductance (Jiang et al. 2005). An alternative possibility is that the ITRPM7 controls the ionic homeostasis and membrane potential of the SyV, which may indirectly affect the neurotransmitter release. The role of TRPM7 in controlling transmitter release probability and/or frequency has been confirmed using a direct assay for the fusion of acidic vesicles with the plasma membrane (Brauchi et al. 2008). In this assay, TIRF microscopy was employed to monitor the fluorescent reporter pHluorin, which is quenched in the acidic lumen of the vesicle but increases in fluorescence upon exposure to extracellular pH, thus indicating fusion of the vesicle with the plasma membrane. Reduced EPSP amplitude may cause a portion of the fusion events to fall out of the assay detection limit, which might indirectly cause the reduced frequency phenotype. It is also possible that TRPM7 may play a direct role in controlling the probability of transmitter release. For example, the Ca2+ permeability of TRPM7 and its association with synaptotagmin may allow TRPM7 to regulate membrane fusion. A Ca2+-impermeable TRPM7 pore mutant with intact outward conductance would allow a test of this possibility. Finally, the kinase domain of TRPM7 appears to play a role in vesicle mobility (Brauchi et al. 2008). Thus TRPM7 may be a key regulator of multiple steps of exocytosis involved in neurotransmitter release.
The key question remains unanswered is how TRPM7 is activated in the SyV. Synaptic, like endocytic, vesicles are deprived of the PI(4,5)P2 that is abundant in the plasma membrane (Krapivinsky et al. 2006). PI(4,5)P2 binding activates TRPM7 in the plasma membrane (Runnels et al. 2002). One interesting possibility is that when a SyV is tethered to the plasma membrane via the SNARE complex, the cytoplasmic portion of TRPM7 is exposed to PI(4,5)P2 from the inner leaf of the plasma membrane, and is activated. PIP2 is indispensable for exocytosis and neurotransmission (Suudhof 2008). Upon Ca2+ binding, the cytoplasmic part of the synaptotagmins may interact with PIP2 in the plasma membrane, and this interaction is important for the membrane penetration of synaptotagmin and subsequent vesicle fusion (Bai et al. 2004). As TRPM7 has been shown to complex with synaptotagmin I (Krapivinsky et al. 2006), it is possible that TRPM7-mediated Ca2+ release further enhances the interaction of synaptotagmin and the plasma membrane which is necessary for bilayer fusion.
TRPs in Lysosome-Related Organelles
TRPMLs are also localized in cell-type specific LROs, which resemble LEL compartments but have distinct morphologies, content, and functions (Blott & Griffiths 2002). For example, TRPML3 is localized in melanosomes of melanocytes (Xu et al. 2007). In response to light stimulation, melanosomes undergo rapid translocation to the plasma membrane and release melanin (Blott & Griffiths 2002). The pigmentation defects seen in the Va mice could indicate a role of TRPML3 in melanin release. However, Va is a severe gain-of-function phenotype and may cause melanocyte cell death due to Ca2+ overload in the cytosol. To dissect the mechanism underlying the pigmentation defect, it is necessary to conduct loss-of-function studies of TRPML3 using, for example, TRPML3 knockout mice. Rab38 and Rab27 are two small G proteins that colocalize with TRPML3 in the melanosomes (Stenmark 2009). Mice with mutations of Rab38 and Rab27 exhibit pigmentation phenotypes (Blott & Griffiths 2002). This is suggestive of an interaction between Rab proteins and TRPML3 being involved in the regulation of melanosome trafficking. Recently, Oancea et al reported that TRPM1, although not localized in melanosomes, can regulate the melanin content and pigmentation in melanocytes (Oancea et al. 2009). It is intriguing how vesicular TRPs indirectly regulate functions of LROs.
Gastric parietal cells contains a specialized vesicular compartment that is involved in the regulated transport of vesicles containing an H+/K+-ATPase for acid secretion into the gastric lumen (Puertollano & Kiselyov 2009, Slaugenhaupt 2002). ML4 patients exhibit reduced gastric acid secretion, suggesting that TRPML1 might be required for the translocation (Slaugenhaupt 2002). In addition, TRPML1, and probably TRPML2 as well, is localized in the MHCII compartments of antigen presenting cells such as macrophages and B-lymphocytes (Song et al. 2006, Thompson et al. 2007). Following endocytosis or phagocytosis of antigen receptors, MHCII compartments undergo translocation to the plasma membrane (Thompson et al. 2007). Overexpression of TRPML1 or TRPML2 results in enlarged compartments that are positive for MHCII (Song et al. 2006). Consistent with this trafficking defect, knockdown of TRPML1 results in a delayed translocation of MHCII compartments to the plasma membrane in cultured macrophages.
Summary, Perspectives, and Future Directions
Many TRP channels have been found to be localized to intracellular vesicles and to interact with a variety of vesicular proteins. Functional studies suggest that many intracellularly-localized TRPs are not simply passive cargo, but instead play active roles in membrane fusion and fission, signal transduction, and vesicular homeostasis. In the future we hope to see the advancement of our knowledge of intracellular TRPs in the following areas:
Real-time live imaging methods will be used to capture the local Ca2+ transients mediated by release from vesicles. These seemingly spontaneous events might be able to be correlated with the membrane fusion events, which can be monitored with fluorescence imaging approaches. Ca2+ release could possibly be altered by genetic or pharmacological manipulation of the vesicular proteins involved in membrane fusion and fission.
Organellar identities of TRP-resident compartments will be defined for most TRPs. It will be revealed whether TRPs are also in group II organelles.
More information will be provided for the electric properties of intracellular compartments and vesicles. Ion imaging methods will be applied to accurately measure luminal ion concentrations at both basal and stimulated states.
Molecular identification will be performed of Ca2+ release channels and respective activation mechanisms. Agonists and antagonists of intracellular TRPs may provide useful for altering membrane traffic.
Acknowledgements
We apologize to colleagues whose works are not cited due to space limitations; in many cases review articles were referenced at the expense of original contributions. The work in the authors’ laboratory is supported by startup funds to H.X. from the Department of MCDB and Biological Science Scholar Program, the University of Michigan, a NIH RO1 grant (NS062792 to H.X), pilot grants (to H.X.) from the UM Initiative on Rare Disease Research, Michigan Alzheimer’s Disease Research Center (NIH grant P50-AG08671 to Gilman), and National Multiple Sclerosis Society. We appreciate the encouragement and helpful comments from other members of the Xu laboratory.
References
- Ahluwalia JP, Topp JD, Weirather K, Zimmerman M, Stamnes M. A role for calcium in stabilizing transport vesicle coats. The Journal of biological chemistry. 2001;276:34148–34155. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nature structural & molecular biology. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. Faseb J. 2006;20:962–964. doi: 10.1096/fj.05-5538fje. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nature cell biology. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Blott EJ, Griffiths GM. Secretory lysosomes. Nature reviews. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8304–8308. doi: 10.1073/pnas.0800881105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Clague MJ. Calcium and calmodulin in membrane fusion. Biochimica et biophysica acta. 2003;1641:137–143. doi: 10.1016/s0167-4889(03)00089-2. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camello C, Lomax R, Petersen OH, Tepikin AV. Calcium leak from intracellular stores--the enigma of calcium signalling. Cell calcium. 2002;32:355–361. doi: 10.1016/s0143416002001926. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Ahluwalia JP, Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. The Journal of biological chemistry. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- Cheng X, Shen D, Samie M, Xu H. Mucolipins:Intracellular TRPML1–3 channels. FEBS letters. 2010 doi: 10.1016/j.febslet.2009.12.056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cohen DM. Regulation of TRP channels by N-linked glycosylation. Seminars in cell & developmental biology. 2006;17:630–637. doi: 10.1016/j.semcdb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends in biochemical sciences. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Samie MA. The varitint-waddler mouse phenotypes and the TRPML3 ion channel mutation: cause and consequence. Pflugers Arch. 2008;457:463–473. doi: 10.1007/s00424-008-0523-4. [DOI] [PubMed] [Google Scholar]
- Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. The Journal of cell biology. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Shen D, et al. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. The Journal of biological chemistry. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Xie J, Yue L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7239–7244. doi: 10.1073/pnas.0811725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca(2+) release channels. Pflugers Arch. 2009;458:869–876. doi: 10.1007/s00424-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Boehmerle W, Maeda Y, et al. Syntaxin 5 regulates the endoplasmic reticulum channel-release properties of polycystin-2. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15920–15925. doi: 10.1073/pnas.0805062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO reports. 2007;8:236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C, Kistner U, Annaert W, Jahn R. Fusion of endosomes involved in synaptic vesicle recycling. Molecular biology of the cell. 1999;10:3035–3044. doi: 10.1091/mbc.10.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. The Journal of general physiology. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nature cell biology. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Karacsonyi C, Miguel AS, Puertollano R. Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic (Copenhagen, Denmark) 2007;8:1404–1414. doi: 10.1111/j.1600-0854.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Muallem S. Gain-of-function mutation in TRPML3 causes the mouse Varitint-Waddler phenotype. The Journal of biological chemistry. 2007;282:36138–36142. doi: 10.1074/jbc.C700190200. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Soyombo AA, Muallem S. A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. Embo J. 2008;27:1197–1205. doi: 10.1038/emboj.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic (Copenhagen, Denmark) 2009;10:1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nature cell biology. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008;129:389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Science signaling. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlante JM, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt SA, Vassilev PM. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS letters. 2002;532:183–187. doi: 10.1016/s0014-5793(02)03670-0. [DOI] [PubMed] [Google Scholar]
- LaPlante JM, Sun M, Falardeau J, Dai D, Brown EM, Slaugenhaupt SA, Vassilev PM. Lysosomal exocytosis is impaired in mucolipidosis type IV. Molecular genetics and metabolism. 2006;89:339–348. doi: 10.1016/j.ymgme.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Lavender V, Chong S, Ralphs K, Wolstenholme AJ, Reaves BJ. Increasing the expression of calcium-permeable TRPC3 and TRPC7 channels enhances constitutive secretion. The Biochemical journal. 2008;413:437–446. doi: 10.1042/BJ20071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochemical Society transactions. 2007a;35:1088–1091. doi: 10.1042/BST0351088. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nature reviews. 2007b;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113(Pt 9):1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nature reviews. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- Martina JA, Lelouvier B, Puertollano R. The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway. Traffic (Copenhagen, Denmark) 2009;10:1143–1156. doi: 10.1111/j.1600-0854.2009.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nature reviews. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Current opinion in cell biology. 2005;17:135–140. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Miedel MT, Rbaibi Y, Guerriero CJ, Colletti G, Weixel KM, Weisz OA, Kiselyov K. Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. The Journal of experimental medicine. 2008;205:1477–1490. doi: 10.1084/jem.20072194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. The Journal of biological chemistry. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:353–358. doi: 10.1073/pnas.0707963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiological reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Science signaling. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circulation research. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. CUPpling calcium to lysosomal biogenesis. Trends in cell biology. 2004;14:471–473. doi: 10.1016/j.tcb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Poccia D, Larijani B. Phosphatidylinositol metabolism and membrane fusion. The Biochemical journal. 2009;418:233–246. doi: 10.1042/BJ20082105. [DOI] [PubMed] [Google Scholar]
- Porat A, Elazar Z. Regulation of intra-Golgi membrane transport by calcium. The Journal of biological chemistry. 2000;275:29233–29237. doi: 10.1074/jbc.M005316200. [DOI] [PubMed] [Google Scholar]
- Prasad P, Yanagihara AA, Small-Howard AL, Turner H, Stokes AJ. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181:5024–5034. doi: 10.4049/jimmunol.181.7.5024. [DOI] [PubMed] [Google Scholar]
- Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. The Journal of cell biology. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic (Copenhagen, Denmark) 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Kiselyov K. TRPMLs: in sickness and in health. American journal of physiology. 2009;296:F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annual review of physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Roth MG. Phosphoinositides in constitutive membrane traffic. Physiological reviews. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nature cell biology. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Saito M, Hanson PI, Schlesinger P. Luminal Chloride-dependent Activation of Endosome Calcium Channels: PATCH CLAMP STUDY OF ENLARGED ENDOSOMES. The Journal of biological chemistry. 2007;282:27327–27333. doi: 10.1074/jbc.M702557200. [DOI] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science (New York, N.Y. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. The Journal of biological chemistry. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- Singh BB, Lockwich TP, Bandyopadhyay BC, et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Molecular cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Slaugenhaupt SA. The molecular basis of mucolipidosis type IV. Curr Mol Med. 2002;2:445–450. doi: 10.2174/1566524023362276. [DOI] [PubMed] [Google Scholar]
- Song Y, Dayalu R, Matthews SA, Scharenberg AM. TRPML cation channels regulate the specialized lysosomal compartment of vertebrate B-lymphocytes. European journal of cell biology. 2006;85:1253–1264. doi: 10.1016/j.ejcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. The Journal of biological chemistry. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature reviews. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Suudhof TC. Neurotransmitter release. Handbook of experimental pharmacology. 2008:1–21. doi: 10.1007/978-3-540-74805-2_1. [DOI] [PubMed] [Google Scholar]
- Thebault S, Lemonnier L, Bidaux G, et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. The Journal of biological chemistry. 2005;280:39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- Thompson EG, Schaheen L, Dang H, Fares H. Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC Cell Biol. 2007;8:54. doi: 10.1186/1471-2121-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, Fares H. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. The Biochemical journal. 2003;371:341–350. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Graaf SF, Chang Q, Mensenkamp AR, Hoenderop JG, Bindels RJ. Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane. Molecular and cellular biology. 2006;26:303–312. doi: 10.1128/MCB.26.1.303-312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog Neurobiol. 2003;71:269–303. doi: 10.1016/j.pneurobio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, Montell C. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal B, Browning MF, Curcio-Morelli C, Varro A, Michaud N, Nanthakumar N, Walkley SU, Pickel J, Slaugenhaupt SA. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. American journal of human genetics. 2007;81:1070–1083. doi: 10.1086/521954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. The Journal of biological chemistry. 2009;284:36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Transmembrane domain length determines intracellular membrane compartment localization of syntaxins 3, 4, and 5. Am J Physiol Cell Physiol. 2001;281:C215–C223. doi: 10.1152/ajpcell.2001.281.1.C215. [DOI] [PubMed] [Google Scholar]
- Xu H, Delling M, Li L, Dong X, Clapham DE. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint waddler mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18321–18326. doi: 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi DA, Frumkin A, Offen-Glasner V, Kogot-Levin A, Bach G. A potentially dynamic lysosomal role for the endogenous TRPML proteins. The Journal of pathology. 2009 doi: 10.1002/path.2587. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li PL. Reconstitution and Characterization of a Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP)-sensitive Ca2+ Release Channel from Liver Lysosomes of Rats. The Journal of biological chemistry. 2007;282:25259–25269. doi: 10.1074/jbc.M701614200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nature cell biology. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annual review of physiology. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- Zhou XL, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nature neuroscience. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]