Abstract
The coordinated movement of the eyes and hands under visual guidance is an essential part of goal-directed behavior. Several cortical areas known to be involved in this process exchange projections with the dorsal aspect of the thalamic pulvinar nucleus, suggesting that this structure may play a central role in visuomotor behavior. Here, we used reversible inactivation to investigate the role of the dorsal pulvinar in the selection and execution of visually guided manual and saccadic eye movements in macaque monkeys. We found that unilateral pulvinar inactivation resulted in a spatial neglect syndrome accompanied by visuomotor deficits including optic ataxia during visually guided limb movements. Monkeys were severely disrupted in their visually guided behavior regarding space contralateral to the side of the injection in several domains, including the following: (1) target selection in both manual and oculomotor tasks, (2) limb usage in a manual retrieval task, and (3) spontaneous visual exploration. In addition, saccades into the ipsilesional field had abnormally short latencies and tended to overshoot their mark. None of the deficits could be explained by a visual field defect or primary motor deficit. These findings highlight the importance of the dorsal aspect of the pulvinar nucleus as a critical hub for spatial attention and selection of visually guided actions.
Introduction
The pulvinar nucleus of the thalamus has expanded greatly in primate evolution, with its expansion linked to cortical areas associated with integration of visual information, attention, and movement planning (Preuss, 2007). Converging evidence suggests that the pulvinar plays a critical role in visual attention. First, visual responses of single neurons throughout the macaque pulvinar are strongly enhanced or attenuated by the behavioral relevance and the animal's perceptual awareness of visual stimuli (Petersen et al., 1985; Bender and Youakim, 2001; Wilke et al., 2009). Second, blood flow and glucose uptake in the human pulvinar are increased by selective attention (LaBerge and Buchsbaum, 1990; Kastner and Pinsk, 2004; Smith et al., 2009). Third, pulvinar lesions in humans (Zihl and von Cramon, 1979; Rafal and Posner, 1987; Karnath et al., 2002; Ward and Arend, 2007; Arend et al., 2008b; Snow et al., 2009) and monkeys (Bender and Butter, 1987; Petersen et al., 1987; Desimone et al., 1990) lead to specific disruptions in attention tasks.
Nevertheless, it is not known whether these attentional control deficits lead to impairments in action planning and whether the pulvinar plays a critical role in coordination of eye and limb movements. Based on anatomical connectivity alone, the dorsal pulvinar is well situated to coordinate visuomotor behavior, as it exchanges projections with relevant cortical areas, including posterior parietal cortex [i.e., lateral intraparietal (LIP) area, area 5, area 7a] and prefrontal cortex [frontal eye fields (FEFs), dorsolateral prefrontal cortex (DLPFC)], and receives direct input from the intermediate layers of the superior colliculus (SC) (Grieve et al., 2000; Gutierrez et al., 2000; Kaas and Lyon, 2007). However, aside from previous electrophysiological studies showing that pulvinar neurons exhibit firing rate changes during and after eye (Robinson et al., 1990, 1991; Benevento and Port, 1995) and limb (Acuña et al., 1990) movements, it is not yet known whether the pulvinar is critical for the selection and visual guidance of movements. The results of two previous studies involving bilateral ablation were mixed on whether the pulvinar might have a specific role in oculomotor function (Ungerleider and Christensen, 1977; Bender and Baizer, 1990).
In the present study, we investigate several measures of eye and upper limb movement behavior after reversible unilateral inactivation of the dorsal pulvinar, including target selection, movement execution, and spontaneous visual exploration.
Materials and Methods
Experiments
Three adult monkeys (Macaca mulatta), including two females (A and B) and one male (C), weighing between 4.5 and 8 kg, were tested in these experiments. All experimental procedures were performed in accordance with the guidelines of the National Institutes of Health and were approved by the Animal Care and Use Committee of the National Institute of Mental Health.
Surgery
Monkeys were implanted under general isoflurane anesthesia with a scleral search coil and a custom-designed fiberglass head holder. In monkey A, we implanted a 23 gauge fused silicate guide cannula (Plastics One) providing chronic access to the pulvinar. During implantation of the cannula, a small hole was drilled into the skull, exposing a region of 3–5 mm of dura mater. A small incision was made in the dura, and the end of the cannula was positioned just short of its target in the dorsal pulvinar. The top portion of the cannula was set in a corrugated ceramic cylinder, which was affixed to the skull with ceramic screws and dental acrylic and served as a guide for (acute) insertion of the 30 gauge internal cannula during the experiments. In monkeys B and C, inactivation was achieved through cannulae advanced transdurally through a chronically implanted, magnetic resonance (MR)-compatible cylindrical chamber (19 mm inner diameter), which was attached to the skull via ceramic screws and dental acrylic.
Both chamber and cannulae positions were determined using MR-guided frameless stereotaxy (Rogue Research). Before each surgery, we acquired high-resolution anatomical magnetic resonance images (MRIs) (see below, Structural MRI image acquisition). Anatomical MRI scans were rotated into conventional stereotaxic coordinates and then compared with the combined MRI and histology atlas of the macaque brain (Saleem and Logothetis, 2007). During the MRI scans, we fit an array of MR-visible fiducial markers to the monkey's headpost, which were then localized off-line in the MR images. During implantation surgery, the array was brought into the same position so that individual markers could be spatially coregistered with the previously identified positions on the anatomical scan. This registration facilitated the correct positioning and angle of the cannula or chamber on the skull using on-line three-dimensional visualization during surgery (Brainsight; Rogue Research) (Frey et al., 2004). The surgical techniques were conducted aseptically, and vital signs were recorded throughout the procedure. After surgery, all animals received a minimum recovery period of 2 weeks. In addition, animals received analgesic treatment (ketoprofen, 2.2 mg/kg) for the first few days after surgery, as well as antibiotic treatment (cefazolin, 15 mg/kg) for a period of 10 d. Behavioral testing resumed after this recovery period.
Electrophysiological recordings
Single-unit activity in the pulvinar was recorded in monkeys B and C (for details on receptive field mapping and electrophysiological setup, see Wilke et al., 2009). Consistent with a previous study (Benevento and Port, 1995), many neurons in the dorsal portion were visually responsive [78% in monkey B (11 of 14 units) and 37.5% (24 of 64) in monkey C] and typically had large receptive fields with rather undefined borders, often encompassing the central visual field and peripheral positions in the contralateral hemifield (diameter, 2.2–25°).
Pulvinar inactivation
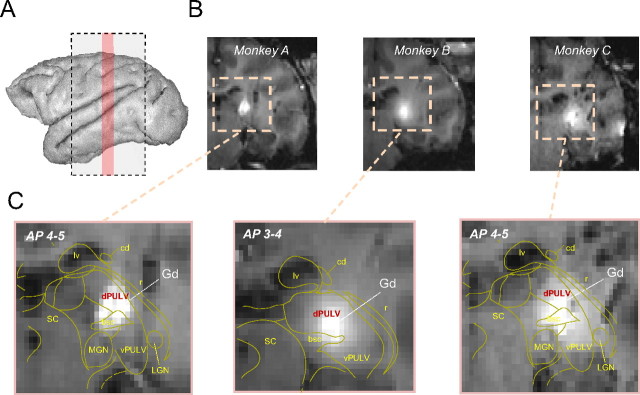
We conceptually divided the pulvinar into ventral and dorsal aspects, separated at the level of the brachium of the SC based on an MRI atlas (Saleem and Logothetis, 2007). This scheme is similar to the parcellation proposed in previous anatomical studies (Olszewski, 1952; Gutierrez et al., 1995, 2000) and was also applied in a previous electrophysiological study (Wilke et al., 2009). The injection center in all three animals corresponded to the dorsal portion of the classically defined lateral pulvinar, with relatively less volume injected in the medial pulvinar (see Fig. 1).
Figure 1.
Inactivation sites in the three monkeys as visualized with coinjections of gadolinium. A, Coronal anatomical MR images were collected (gray rectangle) after pulvinar inactivation. In all cases, the monkeys were fully awake and accustomed to being in the scanner. For each monkey, the center of the injection was between +3 to +5 mm in Horseley–Clark coordinates (pink rectangle). B, Coronal images depicting slices (1.75 mm thickness) around the tip of the cannula after injection, with the gadolinium appearing white. The full extents of the injections are shown in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). C, Magnified view of rectangles in B shown for the three monkeys. The yellow lines correspond to thalamic landmarks derived from the anatomical monkey atlas (Saleem and Logothetis, 2007). These coronal slices were acquired between 0.5 and 1 h after infusion. Injections shown represent the maximum injection volume used in the current study (4 μl). bc, Brachium of superior colliculus; cd, caudate; dPULV, dorsal pulvinar (target structure); Gd, gadolinium; LGN, lateral geniculate nucleus; lv, lateral ventricle; r, reticular nucleus; SC, superior colliculus; vPULV, ventral pulvinar.
Microinfusions of vehicle (buffered saline) and/or GABAA agonist in solution were made through a fused silica or steel cannula (28 gauge; Plastics One). The MR contrast agent gadolinium (5 mm Magnevist; Berlex Imaging) was included in the solution to aid MR visualization. For the inactivation sessions, one of two different GABAA agonists was used: (1) muscimol or (2) 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP) (6.67 μg/μl; Tocris). In each case, the drug was freshly dissolved in vehicle (along with the gadolinium), and the solution, pH 7.0–7.5, was sterile filtered (Corning) before injection. Separate control sessions were conducted in monkeys B and C, in which only the vehicle and gadolinium were injected. Total injection volumes ranged from 2.0 to 4.0 μl and were delivered at a rate of 0.5–1.0 μl/min using a gas-tight Hamilton syringe driven by a digital infusion pump (Harvard Apparatus). The infusions were performed while the animals were awake and sitting in their primate chair, with their heads restrained via implanted head posts.
Overall, we performed 12 inactivation sessions in monkey A (7 muscimol: 3 right hemisphere, 4 left hemisphere; 5 THIP: 3 right hemisphere, 2 left hemisphere), 7 in monkey B (7 THIP, left hemisphere), and 11 in monkey C (3 muscimol, 8 THIP, left hemisphere). Control data collection was interleaved with drug injection sessions. Behavioral effects after THIP injections into the pulvinar typically lasted several hours, whereas behavioral effects after muscimol injections could sometimes be observed after >12 h, reflecting the substantially greater affinity and binding rate of muscimol than THIP for the GABAA receptors [muscimol > GABA > THIP (Waszczak et al., 1980; Jones and Balster, 1998)]. The minimum interval between two injections was 2 d.
Structural MRI image acquisition
Anatomical images were acquired in a 4.7T, 60 cm vertical monkey scanner (Biospec 47/60; Bruker) equipped with a Siemens AC44 gradient coil. Monkeys were well adapted to the scanner environment and sat awake in a custom-made MR-compatible chair (see Materials and Methods) (Maier et al., 2008). Scans were performed via custom-made transmit-and-receive surface coils surrounding the back portion of the animal's head. Coronal anatomical images were acquired using a modified driven equilibrium Fourier transform (MDEFT) sequence with an in-plane resolution of 0.5 mm and a slice thickness of either 1 or 1.75 mm, which were post hoc rotated into the stereotaxic plane to compare with the atlas (Saleem and Logothetis, 2007).
Monkey training for the scanner environment
Monkeys were acclimated to the scanner environment by (1) conditioning them to sit in their chairs in the magnet room, (2) raising and lowering them into the scanner bore, (3) familiarizing them with the sound of the scanner sequences, first when they were outside the scanner and then inside. In all cases, these experiences were reinforced with juice, and the monkeys were observed with a camera for any signs of distress. All three monkeys became quickly accustomed to the magnet.
Behavioral tasks
Behavioral data were acquired in the period between 30 and 120 min after the start of the injection. Control and inactivation sessions were conducted in an alternating manner, applying the following tasks.
Manual retrieval (decision) task.
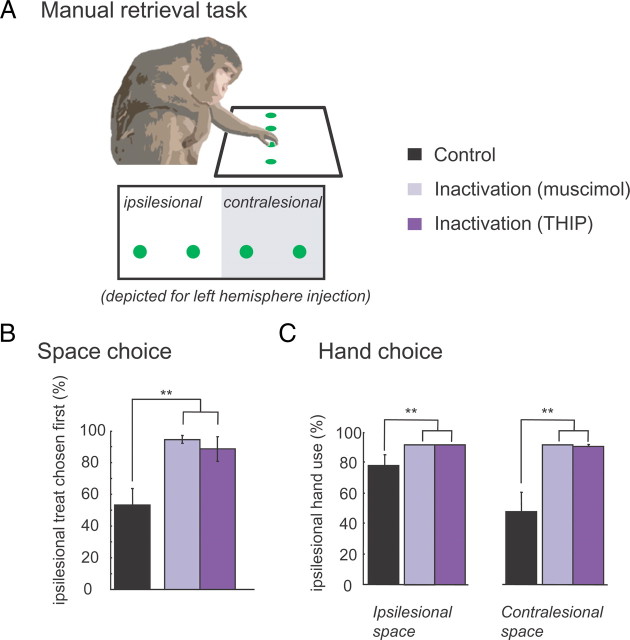
Monkeys sat with their head unconstrained in a primate chair. The chair was open in front, allowing the animals to reach for small pieces of fruit placed on a table in front of them. On each trial (9–31 trials per session), four equally sized food items were placed in each of four positions on the table (see Fig. 2A). Monkeys were free to choose any fruit piece in any order using either hand within a 30 s period.
Figure 2.
Effects of pulvinar inactivation on reaching decisions. A, Task: treats were placed at all four tabletop positions. Monkeys sat in front of the table and retrieved the food-treats. B, Percentage trials in which an ipsilesional treat was selected first in control and inactivation sessions [Ncontrol = 12; Nmuscimol = 6 (3 sessions for each monkey A and C); NTHIP = 6 (3 sessions for each monkey A and C)]. Note that, after the injection, the first reach was strongly biased toward the ipsilesional hemifield. C, Percentage ipsilesional hand usage as a function of food treat position. Whereas in control trials monkeys used both hands, inactivation resulted in nearly exclusive use of the ipsilesional hand in both animals. Error bars indicate SE across sessions. **p < 0.01.
Manual retrieval (instructed hand) task.
The paradigm is the same as that of the manual retrieval (decision) task, but with a barrier placed in front of one hand, requiring the animal to use either the contralesional or ipsilesional hand to grasp the fruit pieces (6–10 trials per hand and session). The barrier was opaque, but the monkeys were able to look over it onto the table surface.
Free viewing task.
Monkeys sat awake in a lit room with their head restrained as their spontaneous oculomotor behavior was monitored by means of a scleral search coil.
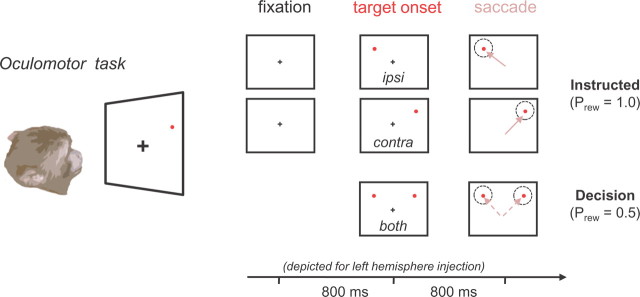
Saccade (instructed location) task.
Monkeys were required to fixate a point in the center of the screen for 800 ms, after which a single peripheral target (0.3° radius) was presented at one of four possible positions [horizontal, −15 or 15° (−8 or 8° in monkey A); vertical: −5 or 5°] in randomly interleaved order. Monkeys received reward when the whole eye movement sequence, consisting of fixation and saccade toward the new target, was successfully completed. Only when the monkeys' performance exceeded 95% correct during training sessions would we start the injection experiments. In most experiments, target onset and fixation spot offset coincided in time. However, we also conducted a delayed version of this task in which the time between cue onset and fixation spot offset was randomized from 300 to 4000 ms. During the delayed saccade task, monkeys were required to maintain fixation on the central point for 1000 ms before the peripheral saccade target was turned on, and then hold fixation until the central fixation point was switched off (Ncontrol = 4; NTHIP = 4).
Saccade (decision) task.
In a subset of trials that were randomly interleaved with the instructed trials (ratio, 1:3), two targets were presented simultaneously at horizontal positions, symmetrically positioned −15 and 15° in the visual fields, either 5° above or below the horizontal meridian. The trial sequence is the same as described above [see Saccade (instructed location) task]. Monkeys were required to saccade to either one or the other, with a reward probability of 50% assigned to each target. Approximately even selection of both targets was furthermore encouraged by rewarding monkeys with three times more juice for a saccade made to the less frequented target (assessed by a counter). The same reward schedule was applied during control and inactivation sessions.
Monkey training for the saccade task
Monkeys were trained using positive juice reinforcement to have their head restrained in the chair as eye movements were continuously measured using an implanted scleral search coil (Robinson, 1963). During testing, eye position was sampled and recorded at 200 Hz. To train the monkeys to fixate in the middle of the screen, they initially received juice reward whenever the measured eye position was within a radius of 5° of a centrally presented fixation spot. As monkeys became more proficient, the fixation window was systematically decreased to 1° and fixation duration was systematically increased to several seconds. During testing, reward was only delivered when the whole eye movement sequence consisting of fixation and saccade toward the new target was successfully completed.
Data analysis
Visually guided grasping
Videotaped data were analyzed off-line. Spatial grasping choices were calculated by dividing the table into four zones, including far (two peripheral) and near (two central) zones in the ipsilesional and contralesional hemifields (see Fig. 2A). The behavioral deficits associated with the far zones were more pronounced than those associated with the near zones; however, we pooled the near and far data to focus on the lateralization of the deficit. The proportions of both hand usage and hemifield choice were computed from trials with four treat items, with results from the two monkeys, A and C, considered together. Reaching times were computed starting from when the table was placed within reach of the monkey to the time of contact with the treat, independently of whether the treat was retrieved correctly. Unless otherwise stated, statistical comparisons between performance in control versus inactivation sessions were tested by means of a two-way ANOVA (hemifield by inactivation), followed by statistical comparisons separately for each hemifield. Reaching movements were qualitatively evaluated by careful examination of the digital movies and classified into three categories: “normal,” in which the hand moved accurately to the target position; “corrected,” in which the hand missed the target initially but reached the correct position after some attempts with a targeted movement; and “uncorrected,” in which the monkey missed the target and either failed to reach it within the 30 s period or hit it by chance (e.g., by using a hand-sweeping strategy) (for a similar classification scheme, see Perenin and Vighetto, 1988). Reaching time was calculated from the time the treat was placed within reach of the monkey to the time the hand touched the treat (independent of whether or not the grasp was correct). Grasping behavior was analyzed off-line, beginning with the monkey moving his hand to the correct treat position. A corrected grasp error was defined as the accurate grasping of a treat after an initially inappropriate hand shaping while approaching the target or on initial contact. An uncorrected grasp error was defined as either a complete failure to grasp and retrieve the treat or dropping the object during the retrieval process.
Eye movement analysis
Eye position was sampled at 200 Hz, and data were stored off-line. Saccades were identified using custom-written software in MATLAB on the basis of velocity criteria. The beginning of a saccade was defined as the time point when velocity (the resultant of the horizontal and velocity vectors) exceeded a threshold of 100°/s. The end of the saccade was defined as the time when velocity fell below 50°/s (for saccade amplitudes >10°) and, consequently, reflects the eye position at this time. Peak velocity was determined as the maximum velocity reached during the saccade. Two-way ANOVAs (hemifield by inactivation) were computed unless otherwise specified, followed by statistical comparisons separately for each hemifield.
Spontaneous eye movements
We calculated the proportion of time spent in either hemifield by analyzing 4 min of horizontal eye traces in each session and subdividing the data into horizontal zones: left (>5° left of center), center (within 5° of center), and right (>5° right of center). Mean viewing position was calculated by averaging the horizontal eye traces first within a given session and then over sessions.
Instructed and decision saccade-task
Only those trials were analyzed in which monkeys kept fixating at least until the onset of the saccade cue. Oculomotor sessions in which monkeys developed a nystagmus and became unable to fixate were eliminated (two muscimol and one THIP injection sessions in monkeys B and C). A correct trial was defined as one in which the monkey acquired the target position within 400 ms after fixation spot offset and fixated this position for at least another 100 ms, and the saccade was within a 5° radius of the target for target eccentricities >15° and within a 3° radius for target eccentricities <15°. This relatively large window was chosen to allow for potential saccade endpoint inaccuracies resulting from the inactivation. Incorrect trials were defined as not acquiring the loosely defined target position in time or breaking fixation of the target position prematurely. Saccade latencies were computed from correct trials.
Results
Three monkeys underwent behavioral testing after injection of a GABAA agonist into the dorsal pulvinar [muscimol (10 sessions) or THIP (20 sessions)]. Data from the three monkeys were qualitatively similar and therefore were pooled unless otherwise stated. Injection locations were identified by means of presurgical anatomical MRI and repeatedly verified by imaging the spread of gadolinium associated with the injection (see Materials and Methods). Imaging data indicate that inactivation was primarily in the dorsal pulvinar (Fig. 1; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Throughout the paper, the terms “ipsilesional” and “contralesional” are used with respect to the inactivated hemisphere (e.g., after an injection into the left hemisphere, the left visual hemifield is ipsilesional).
General behavioral deficits
After unilateral pulvinar inactivation, the monkeys exhibited overt spatial neglect in the contralesional field, particularly after injection with muscimol. Waving pieces of fruit, clapping, and making threatening gestures in the contralesional hemifield were generally ineffective at capturing the interest of the animal, whereas the same actions elicited the expected responses in the ipsilesional hemifield. In addition, squeezing of the contralesional hand or foot frequently did not elicit a behavioral response. In some sessions, particularly after muscimol injection, monkeys exhibited a horizontal oculomotor drift that developed into a nystagmus with the quick phase toward the ipsilesional space. Consistent with previous observations (Waszczak et al., 1980; Jones and Balster, 1998), THIP at the same dose produced more moderate symptoms than muscimol and allowed for systematic testing of oculomotor behavior.
We also observed changes in intentional motor behavior, including reduction of spontaneous movements of the contralesional limbs while the animals were sitting in the testing chair. The contralesional hand was often held in a flexed position in front of the chest. After inactivation, several aspects of visually guided behavior were disrupted. When their ipsilesional hand was restricted, the animals would typically reach inaccurately for a treat with the contralesional hand and grasp it awkwardly. Overall, their appearance was similar to reports of optic ataxia in patients after damage to the parietal lobe (Perenin and Vighetto, 1988). On several occasions, monkeys let the treat drop to the floor (supplemental Fig. 2A, available at www.jneurosci.org as supplemental material), a behavior never observed with either hand in the control sessions or with the ipsilesional hand after injections. Also, as previously reported in patients with parietal lesions (Ropper, 1982), we occasionally observed “self-grasping,” whereby the ipsilesional hand grasped the contralesional forearm and released it 1–2 s later after visual inspection (supplemental Fig. 2B, available at www.jneurosci.org as supplemental material). Other aspects of motor behavior, such as walking on four limbs in the home cage, appeared normal.
Effects on visually guided reaching and grasping
To assess whether the dorsal pulvinar contributes to the selection and coordination of reaching and grasping movements, we designed a simple task in which monkeys sat in front of a table with four treats distributed in an evenly spaced row extending from the ipsilesional to the contralesional hemifield (Fig. 2A). In these experiments, the animals sat in an open chair without head restriction or eye fixation requirements, and were thus able to engage in approximately natural visually guided grasping behavior. The animals were given 30 s to retrieve all items on the table (in control sessions, only a few seconds were typically required). We analyzed the position of items taken, time required, and order of retrieval, and rated the accuracy of both reaching and grasping movements (see Materials and Methods).
The most prominent deficit associated with inactivation was a strong tendency to retrieve ipsilesional targets first, possibly because of a lack of awareness of treats in the contralesional field. Figure 2B shows the percentage of trials in which the animals selected their first treat from the ipsilesional hemifield, before and after inactivation. Whereas in control sessions, selection was divided evenly between the sides, inactivation strongly skewed the initial selections to the ipsilesional side (two-way ANOVA; hemifield by inactivation, p < 0.001). Spatial deficits with muscimol were more severe than those with the same amount of THIP. For example, monkeys completely failed to retrieve contralesional treats in 30.9% of the trials after muscimol injection, compared with 5.7% after THIP injection and 1.1% in control sessions (two-way ANOVA; hemifield by muscimol inactivation, p < 0.05) (data not shown). The second obvious inactivation effect was the exclusive use of the ipsilesional hand to reach for objects when monkeys were free to use either. Whereas monkeys showed only a modest hand preference in control sessions, they strongly favored the ipsilesional hand after inactivation (Fig. 2C). This hand bias was found for ipsilesional and contralesional treat positions (two-way ANOVA; hand by inactivation, p < 0.001).
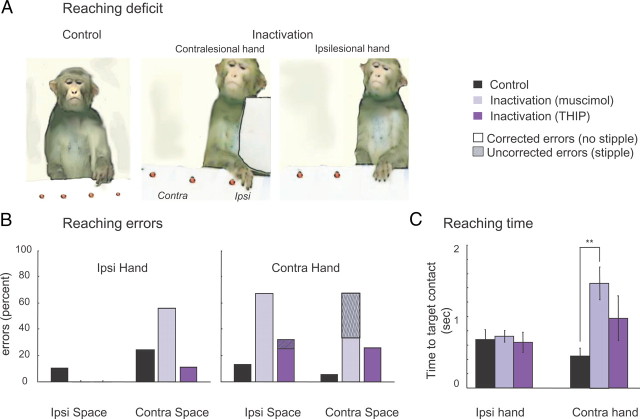
We next tested the reaching performance for each hand separately (i.e., the “instructed hand” condition) (Fig. 3A). As shown in Figure 3B, reaching errors with the ipsilesional hand were rare. When errors did occur, mainly after muscimol inactivation, they were in the contralesional space and of the corrected type (see Materials and Methods). In contrast, reaching movements of the contralesional limb were impaired toward both sides of the table. Although monkeys were nearly always able to correct their reaching errors in control and THIP sessions (Fig. 3B, “uncorrected errors” in THIP sessions), uncorrected errors did occur in 33% of reaches toward contralesional targets after muscimol injection.
Figure 3.
Effects of pulvinar inactivation on reaching errors and time. A, Typical reaching behavior after pulvinar inactivation. Monkey was free to choose the treat at any position and hand usage was enforced by placing a barrier in front of one hand. Note the reaching position inaccuracy when the contralesional (i.e., right) hand was used. B, Distribution of reaching errors as a function of food treat position. Shown is the proportion of corrected and uncorrected errors. Data for the two monkeys are pooled [Ncontrol = 12; Nmuscimol = 6 (3 sessions for each monkey A and C); NTHIP = 6 (3 sessions for each monkey A and C)]. Note the increase of reaching errors in both sides of space when the contralesional hand was used for retrieval. C, Reaching times on trials in which monkeys were instructed to use either the ipsilesional or contralesional hand by placing a barrier in front of the opposite hand. Depicted are the reaching times as analyzed from the digital movies (one frame is 0.03 s). Note the increase in reaching time after inactivation, which was especially pronounced after muscimol injection. Error bars indicate SE across sessions. **p < 0.01.
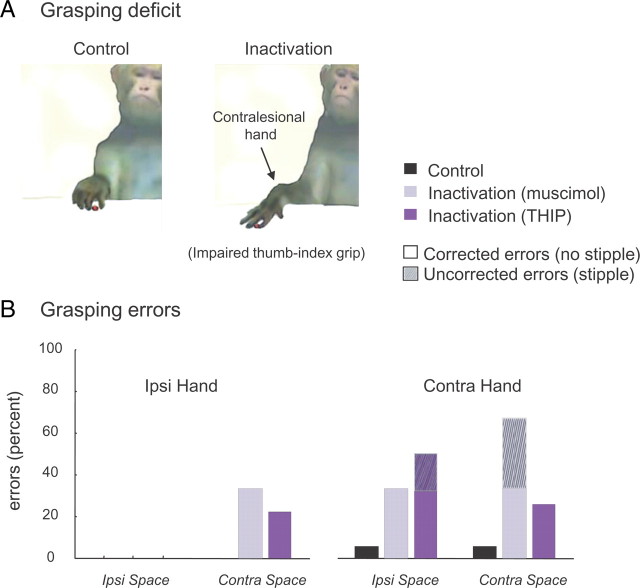
In general, contralesional limb movements were hesitant and uncoordinated, often consisting of broad, sweeping movements, and this was also reflected in increased reaching times (Fig. 3C). Inactivation also affected grasping, disrupting the capacity of the animals to preshape the contralesional hand when approaching the object and to adopt adequate finger postures to grasp it once the correct position was reached (Fig. 4A). The most common mistake was that fingers were overextended during the transport phase and the precision grip was impaired. Like reaching errors, grasping errors (see Materials and Methods) were most prominent during contralesional hand usage (Fig. 4B).
Figure 4.
Effects of pulvinar inactivation on grasping behavior. A, Typical grasping behavior after pulvinar inactivation. Note the extended fingers and failure to shape the contralesional hand appropriately after inactivation. B, Distribution of grasping errors as a function of food treat position. Shown is the proportion of corrected and uncorrected errors. Data for the two monkeys are pooled [Ncontrol = 12; Nmuscimol = 6 (3 sessions for each monkey A and C); NTHIP = 6 (3 sessions for each monkey A and C); same sessions as in Figs. 2 and 3]. Note the significant increase in grasping errors on both sides of space when the contralesional hand was used for retrieval.
Effects on spontaneous visual exploration
As described above, pulvinar inactivation biased limb movement decisions toward using ipsilesional limbs and toward ipsilesional space. To determine whether a similar bias would be observed during spontaneous oculomotor exploration, we measured eye movements while monkeys sat in a lit room in a head-fixed position. Since eye movements after muscimol injection were often confounded by nystagmus, we focused on the effects of THIP injections.
Figure 5A shows typical raw eye movement traces before and after inactivation for two monkeys. Between 1.5 and 2 h after the injection, gaze was almost exclusively directed to the ipsilesional (in these cases, left) hemifield. As shown in the average over sessions in Figure 5B, pulvinar inactivation resulted in a significant increase in time spent in the ipsilesional hemifield (two-way ANOVA, p < 0.001), and consequently, the mean gaze position was shifted 7.7° toward the ipsilesional hemifield (Fig. 5C).
Figure 5.
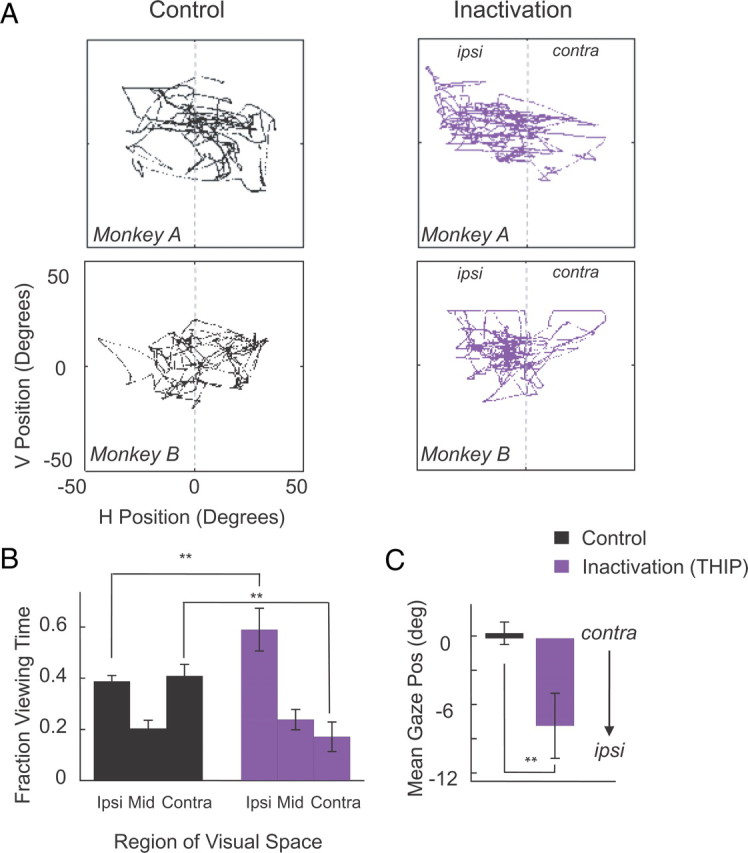
Spontaneous eye movements. Eye movements were recorded while monkeys were sitting with head fixed in a lit room. A, Spontaneous eye movement traces during representative sessions in monkeys A and B before injection (black) and after pulvinar inactivation (purple) with 3 μl of THIP. Each panel shows eye traces collected during a period of 60 s. Note the systematic shift of eye movements toward the ipsilesional hemifield in both monkeys A and B after pulvinar inactivation. B, Proportion of viewing time spent in different zones of the visual field for control and inactivation sessions; data pooled for monkeys A–C (Ncontrol = 6; NTHIP = 6; 2 sessions for each monkey). Zones are defined as follows: ipsilesional (>5° from vertical meridian on ipsilesional side), middle (within 5° of the vertical meridian), contralesional (>5° from vertical meridian on contralesional side). Each bar in the panel on the right represents average viewing time over 4 min during each session collected 60–180 min after injection start. C, Mean eye position in control and inactivation sessions. Note the average gaze position shift toward the ipsilesional hemifield. Error bars indicate SE across sessions. **p < 0.01.
Effects on saccades to single targets
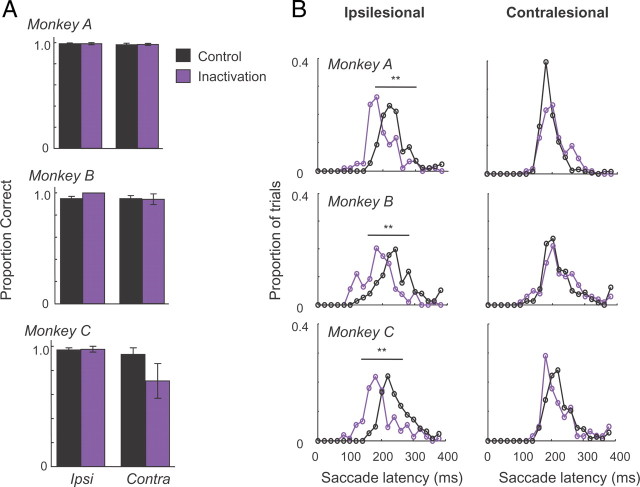
We next evaluated the effects of pulvinar inactivation on instructed, visually guided eye movements toward single targets (Fig. 6, “Instructed”). After inactivation, there was no significant impairment in the monkeys' ability to move their eyes to the correct target (Fig. 7A). At the same time, saccade latencies were strongly affected in all three monkeys (Fig. 7B). This was expressed chiefly in a significant shortening of response times for ipsilesional targets, with that for contralesional targets only minimally affected (ANOVA, main effect of inactivation for ipsilesional targets: p < 0.0001; but not for contralesional targets: p > 0.1). Specifically, pulvinar inactivation decreased the mean saccade latency for ipsilesional targets by 35 ms in monkey A (control, 232 ms, vs inactivation, 197 ms), 56 ms in monkey B (control, 247 ms, vs inactivation, 191 ms) and 49 ms in monkey C (247 vs 198 ms). In addition, peak velocities for ipsilesional saccades were significantly increased (p < 0.0001). Finally, although inactivation did not disrupt the ability to respond correctly in the task, there was a slight ipsilesional shift (i.e., an overshoot) in saccade endpoint positions for ipsilesional targets (mean difference between control and inactivation: 1.1° in monkey A, 0.2° in monkey B, and 0.6° in monkey C; p < 0.0001).
Figure 6.
Task used to evaluate saccades before and after pulvinar injections. Monkeys were trained to move their eyes to a single target, which could appear either ipsilateral or contralateral to the injection site or in both hemifields simultaneously. A trial always started with an 800 ms period of fixation in the middle of the screen, after which the targets could appear in one of four possible peripheral positions on the screen (top right, bottom right, top left, or bottom left), at eccentricities of 5° elevation and 15° azimuth. In the instructed condition, a single target appeared, to which the monkey was required to make a saccade for a reward. In the decision condition, two targets appeared simultaneously, one on the right and one on the left, and the animal was required to make a saccade to either one.
Figure 7.
Saccade performance toward instructed, single targets during control and inactivation sessions. A, Proportion of correct eye movements to targets in the ipsilesional or contralesional hemifield during control and inactivation sessions, plotted separately for the three monkeys [monkey A (Ncontrol = 4; Nmuscimol = 4); monkey B (Ncontrol = 4; NTHIP = 4); monkey C (Ncontrol = 5; NTHIP = 5)]. B, Saccade latency during the direct saccade task for monkeys A–C (see Materials and Methods). The sessions are the same as in A. On all panels, the purple lines represent data obtained after pulvinar inactivation, and the black lines represent data from control sessions. The top and bottom field data are pooled. Note that saccade latencies toward the ipsilesional field became shorter in all three monkeys after inactivation in comparison with the control data, whereas saccade latencies toward contralesional targets remained mostly unaffected. Error bars indicate SE across sessions. **p < 0.01.
Effects on oculomotor decisions
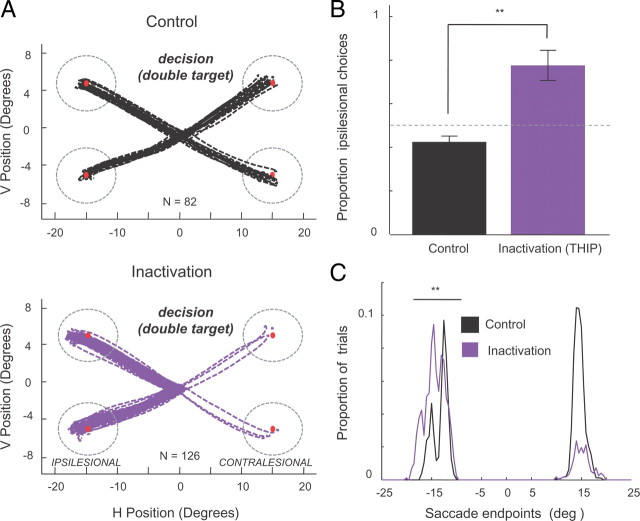
Finally, we tested whether inactivation altered saccadic responses when monkeys were given the choice between two targets shown simultaneously to the left and right visual field (Fig. 6, “Decision”) (see Materials and Methods). This decision condition was randomly interleaved with the single target (“Instructed”) condition described above. Figure 8A shows the eye traces from representative sessions and illustrates a bias after inactivation toward choosing ipsilesional targets. This result demonstrates that, although neither ipsilesional nor contralesional saccade performance is disrupted after inactivation, the monkeys' selection between two targets is severely affected. Figure 8B shows the population data from all nine sessions from the two monkeys tested with this task and shows that, after pulvinar inactivation, there is almost exclusive selection of the target in the ipsilesional hemifield (p < 0.001). As in the single target condition, saccade endpoints were shifted toward the ipsilesional field after pulvinar inactivation (mean difference between control and inactivation, 0.7° in monkey B and 1.1° in monkey C; p < 0.0001) (Fig. 8C).
Figure 8.
Effects of pulvinar inactivation on saccade decisions. A, Typical example of eye movements performed in control and inactivation sessions (monkey B). Raw eye traces (50 ms before saccade start to 50 ms after end) are overlaid from each trial for all four target positions, which were randomly interleaved during testing. The top panel depicts eye movement traces in the control session (black), whereas the bottom panel shows eye movement traces during decision trials after inactivation with THIP (purple). Note the decrease of decisions toward the contralesional targets after inactivation, even though the instructed saccades toward contralesional targets appeared normal (Fig. 7A). B, Proportion of eye movement decisions toward the ipsilesional field. Note the strong increase of saccades toward ipsilesional targets during inactivation sessions. The bar heights represent the mean percentage of ipsilesional target decisions over sessions. Error bars indicate SE across sessions [monkey B and C combined (monkey B (Ncontrol = 4; NTHIP = 4); monkey C (Ncontrol = 5; NTHIP = 5); same sessions as in Fig. 7]. **p < 0.01. C, Histograms of saccade endpoints in control and inactivation sessions in double target trials. Note the systematic shift of saccade endpoints toward the ipsilesional hemifield after inactivation. Error bars indicate SE across sessions. **p < 0.01.
Similar inactivation effects on ipsilesional saccade latencies (control, 290 ms, vs inactivation, 270 ms; p < 0.01) and ipsilesional bias during oculomotor decisions (control, 0.31, vs inactivation, 0.7; p < 0.05) were found in the context of a delayed saccade task (see Materials and Methods). At the same time, the proportion of fixation aborts toward ipsilesional targets in the delayed task was not significantly increased (p > 0.1), indicating that the spatial decision bias cannot be fully explained by a deficit to inhibit saccades toward ipsilesional targets.
Discussion
The main finding of the present study is that unilateral inactivation of the dorsal pulvinar leads to a constellation of deficits involving the selection of eye and hand movement targets and coordination of visually guided manual reaching and grasping. Since monkeys were able to perform saccades to single targets in either hemifield and were able to reach with the contralesional arm on request, the deficits cannot be attributed to either a primary visual field (i.e., scotoma) or motor effect (i.e., hemiparesis). In the following, we discuss the potential role of the dorsal pulvinar in the organization of oculomotor and manual behavior.
The pulvinar and visual extinction
We found that inactivation diminished, and in some cases abolished, exploration of the contralesional field, particularly when left and right space was simultaneously stimulated. This result is consistent with previous studies in both monkeys and human patients showing visual attention impairments after pulvinar lesions (Zihl and von Cramon, 1979; Petersen et al., 1987; Desimone et al., 1990; Karnath et al., 2002; Arend et al., 2008a). Although our tasks did not directly discriminate between attentional and motor decisional components, the effector-independent ipsilesional decision bias shows that pulvinar inactivation severely disrupts overt spatial behavior. This result fits well with previous work showing that inactivation of the dorsomedial pulvinar affects covert spatial selection, interfering with monkeys' capacity to shift attention into the contralesional field (Petersen et al., 1987). Our findings suggest that the disruption of covert orienting is part of a more general syndrome involving action selection.
In general, the extinction symptoms in the present study resembled those previously reported after extended parietal or temporoparietal cortical lesions, and may thus directly reflect the temporary dysfunction of this cortical circuitry (Lynch and McLaren, 1989; Karnath, 2001; Kerkhoff, 2001; Wardak et al., 2002a; Husain and Nachev, 2007), which is conceivable given its strong anatomical connectivity with these areas. Parietal lesions in monkeys rarely lead to full-blown visual neglect but instead result in contralesional extinction under conditions of bilateral stimulation (Lynch and McLaren, 1989; Wardak et al., 2002b; Schiller and Tehovnik, 2003). We found that large doses of muscimol to the dorsal pulvinar led to pronounced spatial neglect, whereas lower doses of muscimol or THIP more consistently led to contralesional extinction without overt signs of neglect.
There is considerable evidence that anatomical disconnection of frontoparietal circuits is an important contributor to spatial neglect symptoms (Bartolomeo et al., 1994; Gaffan and Hornak, 1997). One possibility is that the neglect and extinction symptoms we observed after pulvinar inactivation reflect such a disconnection. It has been previously suggested that corticocortical loops through the pulvinar may serve to coordinate communication between distant brain areas (Crick and Koch, 1998; Sherman, 2005), and this long-range coordination may be particularly important for the complex visuomanual behavior of primates (Preuss, 2007). Thus, one interpretation of the diverse behavioral deficits we observed is that inactivation of the dorsal pulvinar led to a temporary disruption of information flow between cortical areas involved in spatial attention and action programming, such as posterior parietal, dorsolateral prefrontal, and superior temporal cortex (Gutierrez et al., 2000; Cappe et al., 2007).
The dorsal pulvinar and saccade generation
After inactivation, the execution of saccades to contralesional targets remained intact. The absence of contralesional oculomotor impairments per se after pulvinar lesions is consistent with a previous study investigating oculomotor behavior after chronic bilateral pulvinar lesions in monkeys (Bender and Baizer, 1990). Albeit not reported in previous studies, the shortened ipsilesional latencies found in the present study are reminiscent of a previous observation in human patients with pulvinar lesions showing reduced saccade latency inhibition during the presence of a fixation spot (Rafal et al., 2004). Given the strong interconnectivity of the dorsal pulvinar with oculomotor networks in the parietal and frontal cortex, as well as the caudal superior temporal sulcus, effects on eye movements may well be understood as reflecting dysfunction of these cortical areas.
Support for this proposal comes from previous lesion studies of brain structures, such as the LIP area, that have strong connections with the dorsal pulvinar. For example, Li and Andersen (1999) reported that, after LIP inactivation, saccades toward the ipsilesional hemifield became hypermetric. At the same time, and also similar to our findings, structural or reversible lesions of area LIP had either modest (Lynch and McLaren, 1989; Li et al., 1999; Liu et al., 2010) or no effect on saccade latencies to contralesional targets (Wardak et al., 2002b). However, neither of those studies investigating oculomotor behavior after LIP lesions reported a decrease in saccade latencies toward ipsilesional targets, suggesting that this effect of pulvinar inactivation does not reflect LIP function. Several other cortical and subcortical structures must also be considered based on anatomical connectivity. For example, the FEFs, known to be important for voluntary oculomotor behavior, are interconnected with the dorsal pulvinar. However, the pattern of behavioral effects in the present study did not resemble those of pharmacological FEF inactivation (Dias et al., 1995; Sommer and Tehovnik, 1997). Although FEF inactivation led to increases in saccadic latency, increases in saccadic error, and failure to perform saccades toward contralesional space, we found none of those oculomotor deficits. Another structure interconnected with the dorsal pulvinar is the DLPFC. Although there are to our knowledge no experiments that directly correspond to ours, there is some evidence from human transcranial magnetic stimulation and human patients studies, that a “virtual” or structural lesion of the DLPFC leads to shorter than normal saccade latencies toward the ipsilesional side (Pierrot-Deseilligny et al., 2005; Coubard and Kapoula, 2006), consistent with that particular aspect of our findings. Finally, the SC projects to the pulvinar and receives input from cortical areas interconnected with the pulvinar. However, reported oculomotor deficits after SC lesions are far more pronounced than those in the present study (Hikosaka and Wurtz, 1985; Schiller et al., 1987). Based on the absence of saccade deficits to contralesional targets presented alone, the pronounced decision bias, and the decreased latencies toward ipsilesional targets, we conclude that dorsal pulvinar lesion effects on oculomotor behavior are most consistent with a hypofunction in posterior parietal (i.e., LIP) and possibly dorsolateral prefrontal circuits.
The pulvinar and limb movements
Based on its connectivity, the pulvinar has been thought to play a role in such functions as multimodal integration, transformation between reference frames, and volitional limb movements (Grieve et al., 2000). In support of the latter hypothesis, some cells in the lateral pulvinar of monkeys were found to be activated by intentional movement of the upper limbs (Acuña et al., 1983, 1990).
We found that dorsal pulvinar inactivation severely disrupted reaching for treats and grasping them with the contralesional hand. After inactivation, monkeys tended to overestimate or underestimate target positions, and the anticipatory shaping of the thumb–index grip was impaired during contralesional hand use. Similar deficits have been described in monkeys with large lesions of the posterior parietal cortex, including areas 5 and 7 (Faugier-Grimaud et al., 1978; Lamotte and Acuña, 1978) and in humans with lesions in the superior parietal lobe, a disorder termed “optic ataxia” (Perenin and Vighetto, 1988; Battaglia-Mayer and Caminiti, 2002). Based on its anatomical interconnectivity, dorsal pulvinar inactivation could lead to hypofunctioning of cells in the posterior parietal cortex that signal spatial relationships between body parts and code for egocentric space (Andersen et al., 1993; Andersen and Cui, 2009).
In addition, directional errors in human patients with optic ataxia have been attributed to a mismatch between target location and eye and hand movement parameters because of a breakdown of frontoparietal communication (Battaglia-Mayer and Caminiti, 2002). Since the inferior parietal lobule and dorsolateral prefrontal cortex project to extensively overlapping locations within the dorsal pulvinar (Gutierrez et al., 2000), it is conceivable that, after its inactivation, reaching and grasping deficits are attributable to the functional breakdown of those frontoparietal cortical networks.
Anatomical interconnections of the dorsal pulvinar also suggest that its inactivation would impair cognitive functions that rely on parietomedial temporal cortex interactions, such as knowing where objects are located in respect to each other in allocentric space (Burgess, 2008). However, this question was not investigated here and has to await additional studies.
Are the reaching deficits explained by visual attention effects? Since reaching times as well as positional errors were increased for both ipsilesional and contralesional targets during contralesional hand usage, reaching deficits cannot be entirely attributed to the visual neglect symptoms. Pulvinar inactivation also resulted in grasping impairments such as inappropriate wrist orientation and inappropriate hand shaping before and during treat retrieval. In addition, monkeys tended to ignore stimulation of the contralesional hand such as touching or manipulating by the experimenter. Therefore, somatosensory and proprioceptive deficits may have contributed to the observed grasping deficits. Together, our data are consistent with the view that the visually guided reaching and grasping deficits after pulvinar inactivation result from a hypofunction in frontoparietal circuits, which in turn leads to a disintegration of sensorimotor transformation processes.
Footnotes
This work was supported by the National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and National Eye Institute Intramural Research Programs. We thank C. Zhu and Dr. F. Ye for help with MRI anatomical scans. We thank Dr. I. Kagan for comments on this manuscript.
References
- Acuña C, Gonzalez F, Dominguez R. Sensorimotor unit activity related to intention in the pulvinar of behaving Cebus apella monkeys. Exp Brain Res. 1983;52:411–422. doi: 10.1007/BF00238034. [DOI] [PubMed] [Google Scholar]
- Acuña C, Cudeiro J, Gonzalez F, Alonso JM, Perez R. Lateral-posterior and pulvinar reaching cells—comparison with parietal area 5a: a study in behaving Macaca nemestrina monkeys. Exp Brain Res. 1990;82:158–166. doi: 10.1007/BF00230847. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Li CS, Stricanne B. Coordinate transformations in the representation of spatial information. Curr Opin Neurobiol. 1993;3:171–176. doi: 10.1016/0959-4388(93)90206-e. [DOI] [PubMed] [Google Scholar]
- Arend I, Machado L, Ward R, McGrath M, Ro T, Rafal RD. The role of the human pulvinar in visual attention and action: evidence from temporal-order judgment, saccade decision, and antisaccade tasks. Prog Brain Res. 2008a;171:475–483. doi: 10.1016/S0079-6123(08)00669-9. [DOI] [PubMed] [Google Scholar]
- Arend I, Rafal R, Ward R. Spatial and temporal deficits are regionally dissociable in patients with pulvinar lesions. Brain. 2008b;131:2140–2152. doi: 10.1093/brain/awn135. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, D'Erme P, Gainotti G. The relationship between visuospatial and representational neglect. Neurology. 1994;44:1710–1714. doi: 10.1212/wnl.44.9.1710. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Caminiti R. Optic ataxia as a result of the breakdown of the global tuning fields of parietal neurones. Brain. 2002;125:225–237. doi: 10.1093/brain/awf034. [DOI] [PubMed] [Google Scholar]
- Bender DB, Baizer JS. Saccadic eye movements following kainic acid lesions of the pulvinar in monkeys. Exp Brain Res. 1990;79:467–478. doi: 10.1007/BF00229317. [DOI] [PubMed] [Google Scholar]
- Bender DB, Butter CM. Comparison of the effects of superior colliculus and pulvinar lesions on visual search and tachistoscopic pattern discrimination in monkeys. Exp Brain Res. 1987;69:140–154. doi: 10.1007/BF00247037. [DOI] [PubMed] [Google Scholar]
- Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol. 2001;85:219–234. doi: 10.1152/jn.2001.85.1.219. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Port JD. Single neurons with both form/color differential responses and saccade-related responses in the nonretinotopic pulvinar of the behaving macaque monkey. Vis Neurosci. 1995;12:523–544. doi: 10.1017/s0952523800008439. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial cognition and the brain. Ann N Y Acad Sci. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Cappe C, Morel A, Rouiller EM. Thalamocortical and the dual pattern of corticothalamic projections of the posterior parietal cortex in macaque monkeys. Neuroscience. 2007;146:1371–1387. doi: 10.1016/j.neuroscience.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Coubard OA, Kapoula Z. Dorsolateral prefrontal cortex prevents short-latency saccade and vergence: a TMS study. Cereb Cortex. 2006;16:425–436. doi: 10.1093/cercor/bhi122. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Constraints on cortical and thalamic projections: the no-strong-loops hypothesis. Nature. 1998;391:245–250. doi: 10.1038/34584. [DOI] [PubMed] [Google Scholar]
- Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harb Symp Quant Biol. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- Dias EC, Kiesau M, Segraves MA. Acute activation and inactivation of macaque frontal eye field with GABA-related drugs. J Neurophysiol. 1995;74:2744–2748. doi: 10.1152/jn.1995.74.6.2744. [DOI] [PubMed] [Google Scholar]
- Faugier-Grimaud S, Frenois C, Stein DG. Effects of posterior parietal lesions on visually guided behavior in monkeys. Neuropsychologia. 1978;16:151–168. doi: 10.1016/0028-3932(78)90103-3. [DOI] [PubMed] [Google Scholar]
- Frey S, Comeau R, Hynes B, Mackey S, Petrides M. Frameless stereotaxy in the nonhuman primate. Neuroimage. 2004;23:1226–1234. doi: 10.1016/j.neuroimage.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120:1647–1657. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- Grieve KL, Acuña C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends Neurosci. 2000;23:35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Yaun A, Cusick CG. Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J Comp Neurol. 1995;363:545–562. doi: 10.1002/cne.903630404. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Cola MG, Seltzer B, Cusick C. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J Comp Neurol. 2000;419:61–86. doi: 10.1002/(sici)1096-9861(20000327)419:1<61::aid-cne4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Balster RL. Muscimol-like discriminative stimulus effects of GABA agonists in rats. Pharmacol Biochem Behav. 1998;59:319–326. doi: 10.1016/s0091-3057(97)00413-9. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO. New insights into the functions of the superior temporal cortex. Nat Rev Neurosci. 2001;2:568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA. Visual attention as a multilevel selection process. Cogn Affect Behav Neurosci. 2004;4:483–500. doi: 10.3758/cabn.4.4.483. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Spatial hemineglect in humans. Prog Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte RH, Acuña C. Defects in accuracy of reaching after removal of posterior parietal cortex in monkeys. Brain Res. 1978;139:309–326. doi: 10.1016/0006-8993(78)90931-9. [DOI] [PubMed] [Google Scholar]
- Li CS, Mazzoni P, Andersen RA. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol. 1999;81:1827–1838. doi: 10.1152/jn.1999.81.4.1827. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yttri EA, Snyder LH. Intention and attention: different functional roles for LIPd and LIPv. Nat Neurosci. 2010;13:495–500. doi: 10.1038/nn.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC, McLaren JW. Deficits of visual attention and saccadic eye movements after lesions of parietooccipital cortex in monkeys. J Neurophysiol. 1989;61:74–90. doi: 10.1152/jn.1989.61.1.74. [DOI] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J. Basel: Karger; 1952. The thalamus of the Macaca mulatta, an atlas for use with the stereotaxic instrument. [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111:643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann N Y Acad Sci. 2005;1039:239–251. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagosto M, editors. Primate origins: evolution and adaptations. New York: Springer; 2007. pp. 625–675. [Google Scholar]
- Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci U S A. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal R, McGrath M, Machado L, Hindle J. Effects of lesions of the human posterior thalamus on ocular fixation during voluntary and visually triggered saccades. J Neurol Neurosurg Psychiatry. 2004;75:1602–1606. doi: 10.1136/jnnp.2003.017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson DL, McClurkin JW, Kertzman C. Orbital position and eye movement influences on visual responses in the pulvinar nuclei of the behaving macaque. Exp Brain Res. 1990;82:235–246. doi: 10.1007/BF00231243. [DOI] [PubMed] [Google Scholar]
- Robinson DL, McClurkin JW, Kertzman C, Petersen SE. Visual responses of pulvinar and collicular neurons during eye movements of awake, trained macaques. J Neurophysiol. 1991;66:485–496. doi: 10.1152/jn.1991.66.2.485. [DOI] [PubMed] [Google Scholar]
- Ropper AH. Self-grasping: a focal neurological sign. Ann Neurol. 1982;12:575–577. doi: 10.1002/ana.410120612. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. Atlas of the rhesus monkey brain. London: Elsevier; 2007. [Google Scholar]
- Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci. 2003;18:3127–3133. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- Smith AT, Cotton PL, Bruno A, Moutsiana C. Dissociating vision and visual attention in the human pulvinar. J Neurophysiol. 2009;101:917–925. doi: 10.1152/jn.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci U S A. 2009;106:4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res. 1997;116:229–249. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Christensen CA. Pulvinar lesions in monkeys produce abnormal eye movements during visual discrimination training. Brain Res. 1977;136:189–196. doi: 10.1016/0006-8993(77)90146-9. [DOI] [PubMed] [Google Scholar]
- Ward R, Arend I. An object-based frame of reference within the human pulvinar. Brain. 2007;130:2462–2469. doi: 10.1093/brain/awm176. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. Neglect in monkeys: effect of permanent and reversible lesions. Oxford: Oxford UP; 2002a. [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002b;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak BL, Hruska RE, Walters JR. GABAergic actions of THIP in vivo and vitro: a comparison with muscimol and GABA. Eur J Pharmacol. 1980;65:21–29. doi: 10.1016/0014-2999(80)90204-6. [DOI] [PubMed] [Google Scholar]
- Wilke M, Mueller KM, Leopold DA. Neural activity in the visual thalamus reflects perceptual suppression. Proc Natl Acad Sci U S A. 2009;106:9465–9470. doi: 10.1073/pnas.0900714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J, von Cramon D. The contribution of the “second” visual system to directed visual attention in man. Brain. 1979;102:835–856. doi: 10.1093/brain/102.4.835. [DOI] [PubMed] [Google Scholar]