Abstract
The BH3-interacting domain death agonist Bid has been shown to be critical for Fas-induced hepatocellular apoptosis. Furthermore, some studies have suggested that phosphorylation of Bid may determine the apoptotic function of Bid and may act as a switch to non-apoptotic functions. The aim of this study was to evaluate the role of Bid and phosphorylated Bid for FasL-induced apoptosis in murine livers. The monoclonal antibody Jo2 and a hexameric form of sFasL (MegaFasL) were used to induce apoptosis in wildtype, Bid-deficient (Bid−/−), Bid transgenic mice expressing a non-phosphorable form of Bid and Fas receptor-deficient lpr mice. Apoptosis sensitivity was determined in healthy mice and in mice following bile duct ligation, partial hepatectomy or suramin pretreatment. As previously reported, loss of Bid protects mice against Jo2-induced liver failure. Remarkably however, Bid−/− mice are highly sensitive to MegaFasL-induced apoptosis. MegaFasL-treated Bid−/− mice showed a typical type I cell signaling behavior with activation of caspase-3 without Bax translocation to the mitochondria and no cytochrome C/Smac release into the cytosol. In contrast to previous in vitro findings, phosphorylation of Bid does not affect the sensitivity of hepatocytes to Fas receptor-mediated apoptosis in vivo. Our data suggest that Bid mainly amplifies a weak death receptor signal in quiescent and non-quiescent hepatocytes rendering the liver more sensitive to FasL-induced apoptosis. Thus, depending on the efficacy of Fas receptor activation, hepatocytes and non-parenchymal cells can either behave as type I or type II cells.
Introduction
The Fas receptor is constitutively expressed by most tissues including the liver and apoptosis induced by activation of Fas has been implicated in various liver diseases (1, 2). The only known physiological ligand of Fas is the TNF related cytokine FasL. FasL is synthesized as a type II–membrane protein, which undergoes metalloproteinase-mediated proteolytic cleavage resulting in the release of soluble trimeric ligand (3). Upon ligand binding, activated death receptors engage the adapter protein Fas Associated Death Domain (Fadd) (4). Fadd in turn recruits caspase-8 via a homophilic death effector domain (Ded) interaction forming the death-inducing signaling complex (Disc). Disc formation activates caspase-8 through a proximity induced dimerization mechanism requiring no autoproteolysis (5, 6). In some cells, caspase-8 directly activates the effector caspases-3 and -7 and this suffices to kill the cell. In other cells, the amount of active caspase-8 generated at the Disc is not sufficient to induce cell death and a mitochondria-dependent apoptosis pathway is required (7). The model of type I and II Fas signaling has therefore been introduced to differentiate cells that do not require a mitochondrial amplification of the Fas signal (type I) from those that rely on mitochondria for cell death (type II). Subsequently, the BH3 only molecule Bid was identified to mediate the apoptotic signal from the cell surface to mitochondria (8). Upon activation of caspase-8, Bid is cleaved to its active form, which translocates to the mitochondria mediating the activation of Bak/ Bax and subsequently the release of cytochrome C (9). Once in the cytosol, cytochrome C induces the formation of the apoptosomal complex, which activates caspase-9 and subsequently the effector caspases-3 and -7. When Bid−/− mice are injected with the monoclonal anti-mouse Fas-activating antibody Jo2 (Fas mAb), they are protected from hepatocellular apoptosis, whereas WT mice die from fulminant liver failure (9–11). Based on these data hepatocytes are considered type II cells. Interestingly, some in vitro studies argued against the two-pathway concept because the differences were not found when cells were stimulated with FasL rather than with an agonistic antibody and questioned that antibodies accurately reflect Fas-signaling induced by the physiological ligand and are therefore of clinical relevance (12).
Recent studies suggest that Bcl-2 like proteins do not only regulate apoptosis, but exert also other non-apoptotic functions such as cell cycle progression (13, 14). At this point however it is not clear which factors switch Bid-mediated signaling from primarily apoptotic to non-apoptotic. The pro-apoptotic activity of Bcl-2 like family members can be regulated in multiple ways including proteolytic cleavage, dimerization and phosphorylation/ dephosphorylation (15). Phosphorylation of Bid on ser61 renders the molecule resistant to cleavage by caspase-8 in vitro (16, 17) and may also prevent the translocation of Bid to the mitochondria (18). Moreover, we have shown that sustained phosphorylation of Bid correlates with resistance to Fas-induced apoptosis in the liver (19). Together these data suggest that phosphorylation of Bid may determine the apoptotic function of Bid and may act as a switch to non-apoptotic signaling pathways. Interestingly, two recent studies have shown that Bid is phosphorylated by ataxia telangiectasia-mutated kinase (ATM) on ser61 and 78 in vitro leading to an accumulation of cells in S-phase and implicating Bid in the DNA damage response (20, 21).
The aim of this study was to evaluate the role of Bid for hepatocellular apoptosis using different stimuli of the Fas receptor in vivo. Furthermore, we were interested to determine the role of Bid phosphorylation for the sensitivity of hepatocytes to Fas-induced apoptosis using a genetic mouse model in which endogenous Bid has been replaced with a gene that cannot be phosphorylated.
Materials and Methods
Mice
Eight weeks old Bid−/− mice and WT littermates in the C57Bl/ 6 background were used (10). Lpr mice and age-matched wild type mice (C57Bl/ 6 background) were obtained from Jackson Laboratories (Bar Harbor, MA). The generation of BidS61A/ S78A mice is described in the supplementary methods.
Mice were housed in rooms with constant temperature, humidity and 12h light/ dark cycles. Food and water were available ad libitum. The animal experiments were all in accordance with the guidelines of district government of Lower Saxony, Germany, the government of Israel, the University of Kansas Medical Center and the National Research Council for the Care and Use of Laboratory Animals in Research.
In vivo apoptosis experiments
Mice were injected with anti-Fas antibody (Jo2) (purified anti mouse CD95 (Fas); Becton Dickinson GmbH, Heidelberg, Germany) or Mega-Fas Ligand (h-ACRP30, h-FasL; Apoxis SA, Lausanne, Switzerland) to induce apoptosis (22). Some animals were treated with the pan-caspase inhibitor EP1013 (10 mg Z-VD-fmk/ kg; a generous gift from Dr. S.X. Cai, Epicept Corp., San Diego, CA) dissolved in 0.05 M Tris buffer or the solvent 30 min before injecting the MegaFasL intraperitoneally (i.p). Groups of animals were sacrificed 0, 1, 2 and 3 hours after MegaFasL administration. Suramin (Fluka/ Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) pretreatment, partial hepatectomy and bile duct ligations were performed as previously described (19, 23). The animals were injected i.p. with Jo2 or MegaFasL 24 hours after partial hepatectomy or 14 days after bile duct ligation and sacrificed at indicated time points.
Histology and immunhistochemistry
Liver tissue was fixed in 10% phosphate-buffered formalin, pH 7.4, dehydrated in 100 % ethanol, and embedded in paraffin wax at 58°C. Five-micrometer sections were rehydrated and stained with hematoxylin/ eosin (H&E). The terminal deoxynucleotidyl transferase-mediated dUDP nick-end labeling (TUNEL) assay (Roche, Mannheim, Germany) was performed according to the manufacturer's recommendations. Nuclei in the tissue section were targeted with the nucleic acid probe DAPI.
Caspase-8, -9 and -3 activity assay
Caspase-8 and -3 activities were assayed using fluorescence probes (DEVD-MCA; IETD-AFC) in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk (10 µM) as described (19). Caspase-9 activities were assessed using the Caspase-Glo 9 Assay (Promega, Madison, WI, USA).
Western Blot Analysis
Frozen liver tissue were homogenized in cell lysis buffer, containing Complete Protease Inhibitor mixture (Roche, Mannheim, Germany). Western Blotting was performed as described (24). Ponceau red staining was performed with each blot to demonstrate equal protein loading of the samples. Antibody specifications are described in the supplementary methods.
Statistical analysis
Data are expressed as mean ± SEM determined by 2-way analysis of variance followed by Student’s t-test to determine significance. P<0.05 was considered significant.
Results
Loss of Bid protects mice against Jo2-induced apoptosis
Previously, it has been shown that Bid−/− mice are protected from Jo2-induced apoptosis (10). In agreement with this observation, almost no apoptotic hepatocytes were detected in livers of Bid−/− mice injected with 0.5 µg/ g mouse Jo2 (Fig. 1A). In contrast, histology and TUNEL assay revealed massive hemorrhagic liver failure in wild type control mice (Fig. 1A). Accordingly, a significant stronger caspase-8, -9 and -3 activation was evident in WT mice in comparison to Bid−/− mice (Fig. 1B). To determine whether Bid−/− mice are also protected against higher doses of Jo2, up to 100 µg Jo2 per mouse were injected into knock out mice. Remarkably, even with high doses of Jo2 only a few scattered apoptotic hepatocytes were seen in livers of Bid−/− mice (Fig. 1A, B) suggesting that loss of Bid severely impairs Fas mAb-induced apoptosis in the liver irrespective of the dose of the antibody used.
Figure 1.
Bid−/− mice are protected from Jo2 induced apoptosis. WT and Bid−/− mice were treated with (0.5 µg/ g) Jo2 or saline (C, control) and sacrificed 6h after injection or shortly before death. Subsequently, Bid−/− mice were challenged with up to 100 µg Jo2 (HD). [A]: H&E staining showed disruption of liver tissue only in Jo2 injected WT mice. Similarly, multiple TUNEL positive hepatocytes were only observed in WT mice. In contrast, only scattered TUNEL positive cells were seen in livers of Bid−/− mice. [B]: Caspase-8, -9 and -3 activities were measured using the substrates DEVD (caspase-3 marker), IETD (putative caspase-8 marker) and LEHD (putative caspase-9 marker) in control and Jo2 challenged mice (*p< 0.05).
Loss of Bid does not protect mice against FasL-induced apoptosis
Next, we aimed to determine whether the Bid mediated mitochondrial amplification loop is also required when Fas is activated with a stronger trigger. Previously, it has been shown that mere trimerization of Fas in some cells is insufficient to induce apoptosis and that only membrane bound or multimerized FasL consistently induces cell death (22). In this study, a hexameric form of sFasL (MegaFasL) was used. MegaFasL was produced by fusing the dimer forming serum protein stalk of human ACRp30 to the trimeric portion of human sFasL (25, 26).
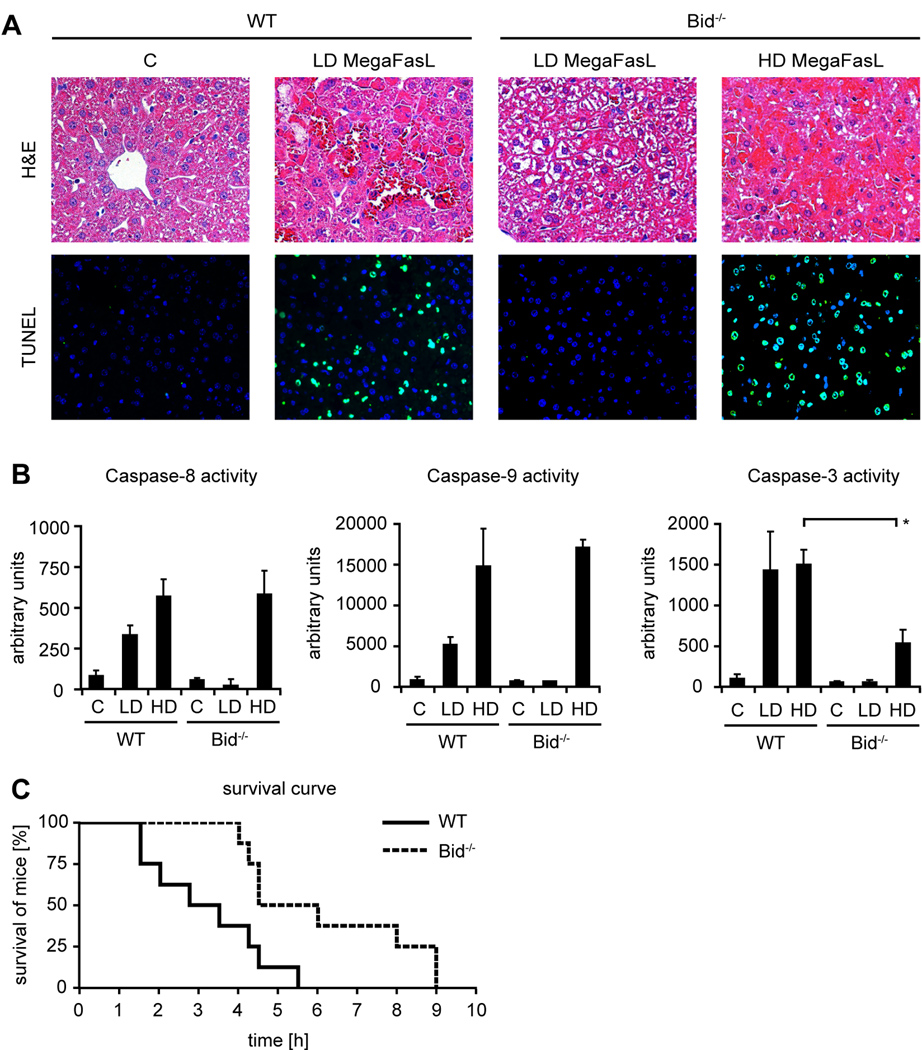
Injection of low doses of MegaFasL (0.05 µg/ g mouse) rapidly induced fulminant liver failure in WT mice, whereas Bid−/− mice survived this dose (Fig. 2A). Remarkably however, a not even two fold higher dose (0.08 µg/ g mouse) of MegaFasL was sufficient to induce severe hemorrhagic liver failure in Bid−/− mice (Fig. 2A). Liver histology and TUNEL staining showed massive hepatic apoptosis in all parts of MegaFasL treated WT (LD) and Bid−/− mice (HD), which was associated with a strong activation of caspase-8 and -3 (Fig. 2B). Additionally, activation of caspase-9 was evident in these mice. However, it is very unlikely that this truly reflects activation of caspase-9, which absolutely requires formation of the apoptosome. There is substantial substrate overlap between individual caspases and recently, it has been shown that the putative caspase-9 LEHD substrate is effectively cleaved by caspase-8 and -3 (27). With the higher MegaFasL dose, not only the WT but also all Bid−/− mice succumbed to this treatment within 12 hours albeit slightly later than the WT controls (Fig. 2C).
Figure 2.
Bid−/− mice do not survive MegaFasL-induced apoptosis. WT and Bid−/− mice were treated with MegaFasL and sacrificed 3h after injection. [A]: H&E staining showed tissue destruction in WT mice treated with LD dose MegaFasL (0.05 µg/ g body weight) and Bid−/− mice treated with HD dose MegaFasL (0.08 µg/ g). Accordingly, multiple TUNEL positive cells were observed. [B]: Caspase-8, -9 and -3 activities were measured using the substrates DEVD (caspase-3 marker), IETD (putative caspase-8 marker) and LEHD (putative caspase-9 marker) (*p< 0.05). [C]: Survival curve of WT and Bid−/− mice (n= 8 per genotype), treated with HD dose MegaFasL
Next, several proteins involved in the death cascade induced by Fas activation were analyzed in livers of control and MegaFasL treated WT and Bid−/− mice. Overall protein levels of Fas-R, Flice-like inhibitory proteins (c-Flip), Bcl-2 like proteins Bcl-xl, Mcl-1 and Bax and the inhibitor of apoptosis cIap-1 were not affected by loss of Bid (Fig. 3A). Analysis of the intracellular distribution of Bax revealed a translocation from the cytosol to the mitochondrial fraction in MegaFasL treated WT mice (Fig. 3B). Activation of Bax and Bak induce structural changes at the mitochondrial membrane resulting in cytochrome C and Smac/ Diablo release (28). Here, both proteins were found in the cytosol of MegaFasL treated WT mice. Similar results were obtained with Jo2 treated controls. Interestingly however, translocation of Bax and release of cytochrome C and Smac/ Diablo were not evident in MegaFasL treated Bid−/− mice (Fig. 3B) suggesting that the mitochondrial pathway was not activated in these mice. In agreement with the caspase activity data, processing of caspase-8, and -3 was clearly evident in both groups. Together, these data indicate that loss of Bid does not protect mice against FasL-induced apoptosis in vivo. MegaFasL treated Bid−/− mice showed a typical type I signaling behavior without activation of the mitochondrial amplification loop.
Figure 3.
[A]: Western blot analysis of total liver tissue lysates was performed for Fas, Flip I, Flip s, Bid, Bcl-xl, Mcl-1, Bax, cIAP1 and Actin. [B]: Additionally, mitochondrial fractions were analyzed for Bax and cytosolic fractions for Cytochrome C, smac/ Diablo, Caspase-8 and cleaved Caspase-3. Time points are shown for LD (0.05 µg/ g) or for HD (*) treatment (0.08 µg/ g). Mice were sacrificed at 0, 1 and 3h.
MegaFasL induced apoptosis is mediated through the Fas receptor and caspases
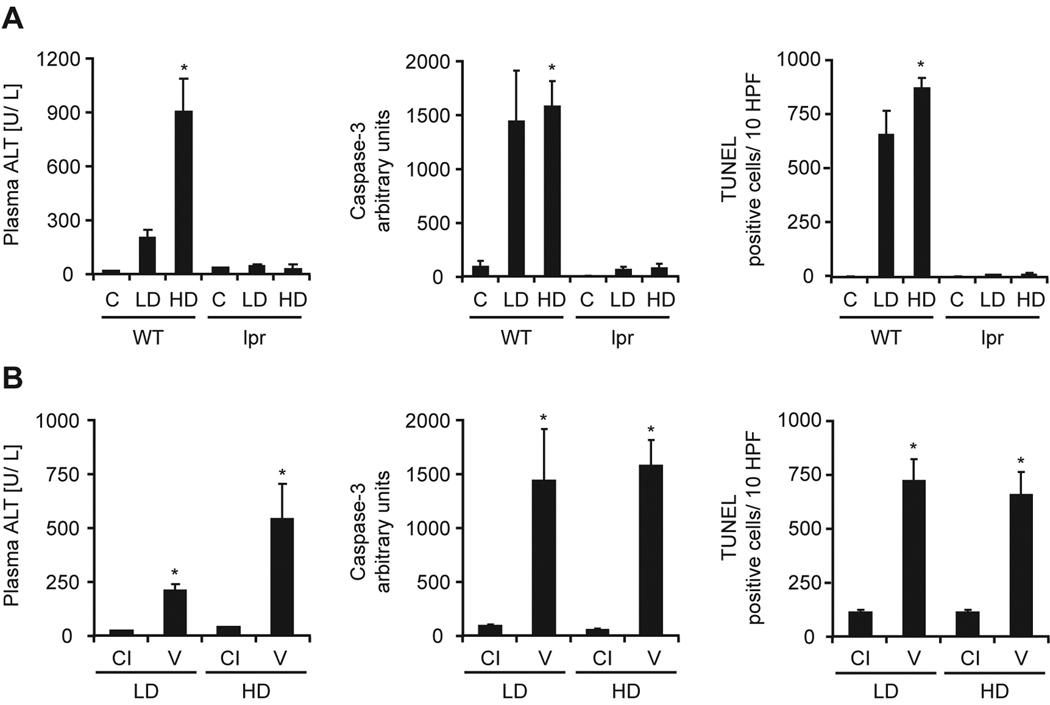
To confirm that MegaFasL signals through Fas-R, Fas receptor-deficient lpr mice and littermate controls were challenged with the low and high dose of MegaFasL. As expected, littermate controls showed massive apoptosis (indicated by activation of caspase-3 and the number of TUNEL-positive hepatocytes), and evidence of secondary necrosis (plasma ALT activities) at 2 hours (Figure 4A). All animals died within 3 hours from severe hemorrhage (not shown). Importantly however, all lpr mice were completely protected from MegaFasL-induced apoptosis (Fig. 4A) and all animals survived. These data indicate that MegaFasL-induced hepatocellular apoptosis is entirely dependent on the Fas receptor. Next, WT mice were pretreated with 10 µg/ g of the pan-caspase inhibitor Z-VD-fmk (Fig. 4B). This pan-caspase inhibitor effectively prevented TNF-induced apoptosis in murine livers (29). Both low and high doses of MegaFasL induced massive caspase activation, DNA fragmentation (TUNEL), and an increase in plasma ALT activities. Z-VD-fmk treatment reduced all parameters by > 95% indicating that MegaFasL-induced apoptosis is completely caspase-dependent in all liver cell types independent of the dose administered.
Figure 4.
The lethal effect of MegaFasL is mediated through the Fas receptor and is dependent on caspase activation. [A]: Fas receptor-deficient lpr mice and littermate controls (WT) were treated with 0.05 µg/ g (LD) or 0.08 µg/ g (HD) MegaFasL. Control mice (C) were injected with saline. [B]: WT were treated with LD or HD MegaFasL 20 min after i.p. injection of 10 µg/ g of a pan-caspase inhibitor (CI). Control mice received vehicle (V). Mice were sacrificed 2h after injection. Secondary necrosis was evident by increased serum ALT levels. Caspase-3 activity is expressed in arbitrary units and the number of TUNEL positive cells was counted in 10 high power fields (HPF). (*p< 0.05 compared to controls or vehicle)
Bid increases the sensitivity to Fas induced apoptosis in apoptosis-resistant mice
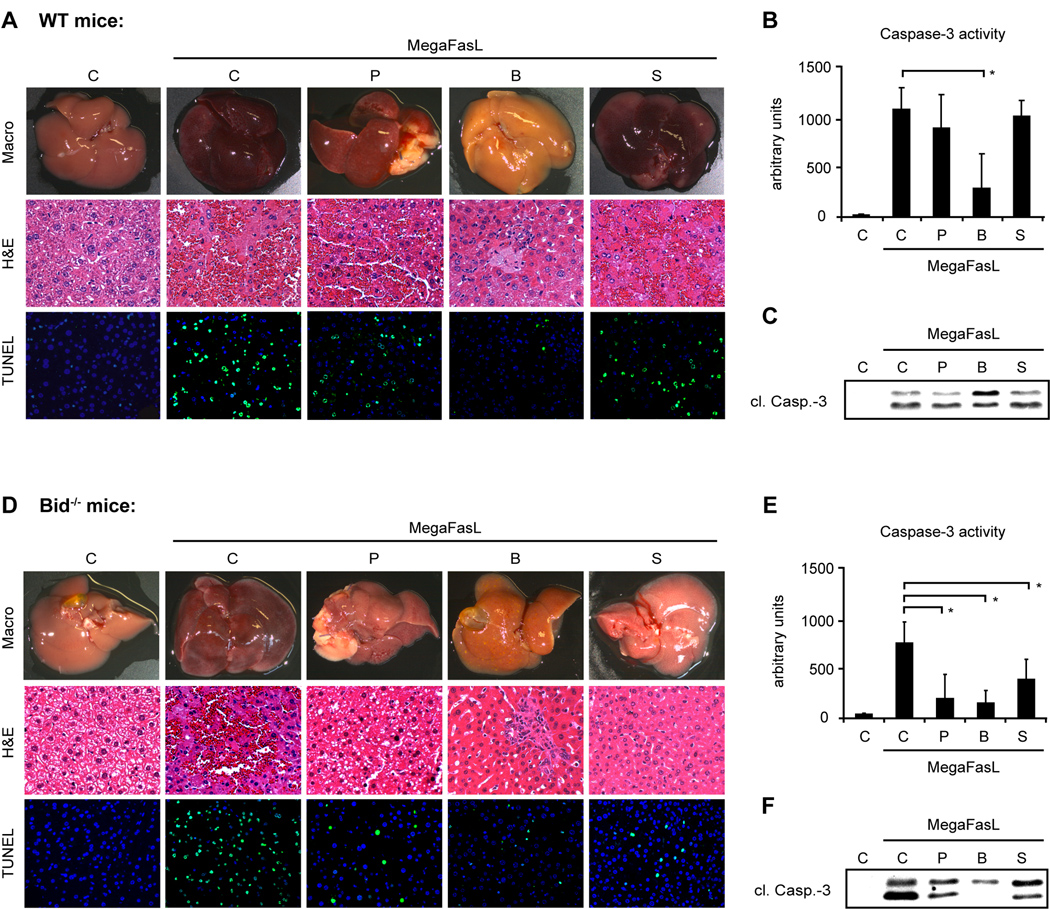
Liver regeneration and also liver injury is in some cases associated with a resistance to Fas mAb-induced apoptosis, which may be viewed as an adaptive response allowing survival during acute or chronic liver distress. We and others have shown that the chronic injury found in the metabolic liver disease hereditary tyrosinemia (HT-1) or after bile duct ligation induce a cell death resistance (19, 24, 30). Resistance to Fas mAb-induced apoptosis can also be found in the regenerating liver following partial hepatectomy and can be induced with the drug suramin (23, 31). The exact mechanisms causing the apoptosis resistance are not yet completely understood. To elucidate the role of Bid and the mitochondrial pathway for the reduced sensitivity of hepatocytes to Fas-induced cell death, preconditioned WT and Bid−/− mice were challenged with MegaFasL. Liver sections of WT mice treated with MegaFasL following partial hepatectomy or suramin injection displayed severe destruction of the parenchyma and multiple TUNEL positive hepatocytes. Accordingly, activation and processing of caspases was clearly evident in these mice suggesting that a stronger apoptosis trigger can overcome the apoptotic resistance in these two models (Fig. 5A–C). In contrast, BDL mice remained protected against MegaFasL induced cell death.
Figure 5.
After 2/ 3 partial hepatectomy (P), bile duct ligation (B) or Suramin (S) pretreatment (n= 8 each), WT and Bid−/− mice were injected with a lethal dose of MegaFasL (WT: 0.05 µg/ g; Bid−/−: 0.08 µg/ g) or saline as control (C) and sacrificed 6h after injection or shortly before death. [A, D]: Images of representative mouse livers. Liver injury and apoptosis are shown by H&E and TUNEL staining. Following MegaFasL challenge, severe liver injury was evident in WT mice pretreated with 2/ 3 partial hepatectomy and Suramin. In contrast, Bid−/− mice displayed significantly less tissue damage. [B, C, E, F]: Bile duct ligation protects both WT and BID−/− against MegaFasL-induced apoptosis. Activation of Caspase-3 was analyzed in control and MegaFasL-challenged mice. (*p< 0.05)
Next, we wondered whether loss of Bid would re-establish the apoptosis resistance in MegaFasL treated mice following partial hepatectomy and suramin treatment. As shown in Fig. 5, livers of all preconditioned Bid−/− mice challenged with MegaFasL exhibited an almost normal appearance and only a few spotted TUNEL positive hepatocytes were detectable (Fig. 5D–F). Together these data show that Bid increases the sensitivity to FasL-induced apoptosis in at least two models of apoptosis resistance supporting the concept that Bid amplifies the apoptotic signal from death receptors and enables execution of cell death when the original signal from the death receptor is not strong enough to directly activate enough caspase-3.
Phosphorylation of Bid does not mediate the resistance against Fas-induced apoptosis
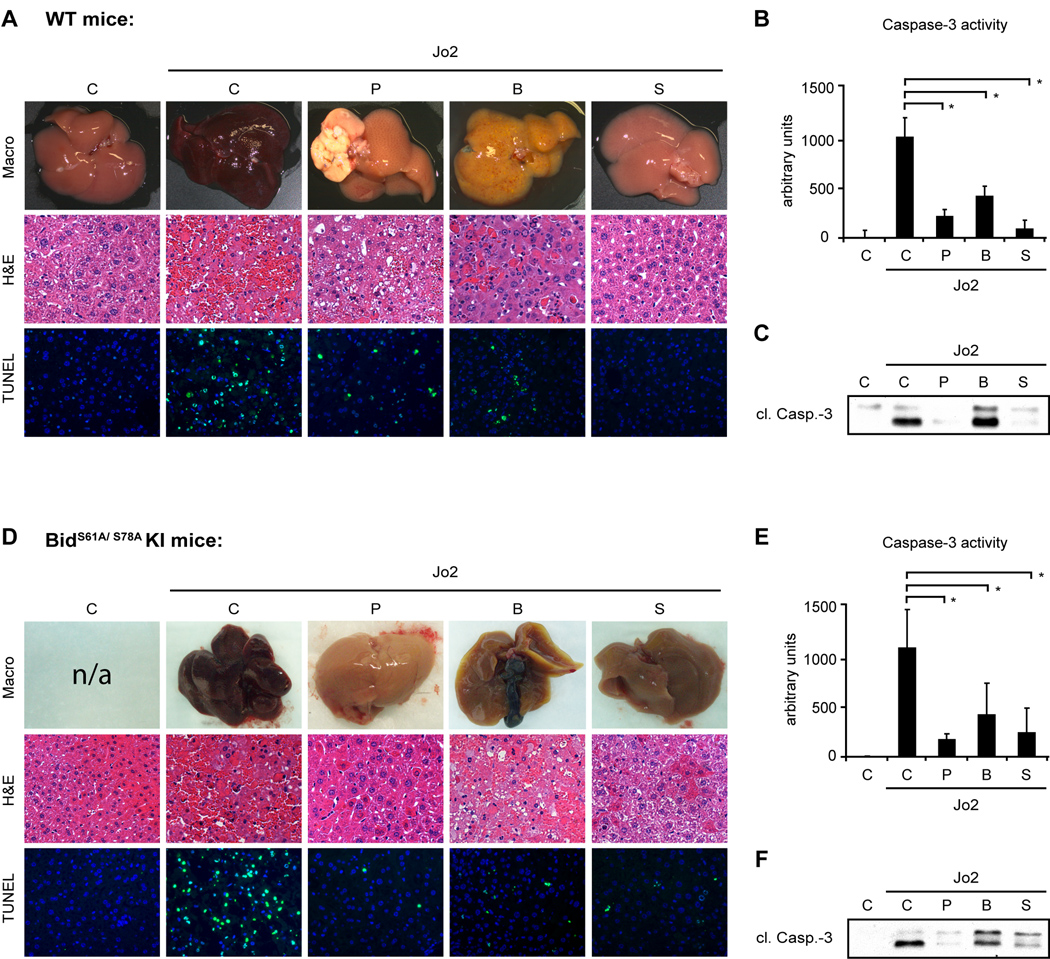
We have previously reported that Bid is not dephosphorylated and cleaved in Fas mAb-resistant mice suggesting that the phosphorylation status of Bid may determine the apoptotic threshold of hepatocytes in vivo (19). Furthermore, several in vitro studies have shown that phosphorylation of Bid prevents cleavage of Bid by caspase-8 (16, 17). Here, BIDS61A/ S78A knock-in mice were used to delineate the role of Bid phosphorylation for the sensitivity of hepatocytes to Fas mAb-induced apoptosis. We hypothesized that BidS61A/ S78A knock-in mice might be more sensitive to Jo2 induced apoptosis. In contrast to the in vitro data however, there was no significant difference between healthy BidS61A/ S78A knock-in mice and littermate controls and all mice died with similar doses of Jo2 (data not shown). Furthermore, BidS61A/ S78A knock-in mice developed the same resistance to Fas mAb-induced apoptosis following bile duct ligation, partial hepatectomy and suramin injection (WT mice: Fig. 6A–C; BidS61A/ S78A KI mice: Fig. 6D–F). Together these data suggest that phosphorylation of Bid does not affect the sensitivity of hepatocytes in vivo.
Figure 6.
BidS61A/ S78A knock-in mice developed the same resistance to Jo2-induced apoptosis as littermate controls. After 2/ 3 partial hepatectomy (P), bile duct ligation (B) or Suramin (S) pretreatment, WT and BidS61A/ S78A KI mice were injected with a lethal dose of Jo2 (0.5 µg/ g) or saline. Mice were sacrificed 6h after injection or shortly before death. [A, D]: Images of representative mouse livers. Liver injury is shown by H&E and TUNEL staining. [B, C, E, F]: Activation and processing of Caspase-3 was analyzed in control and Jo2-challenged mice. (*p< 0.05)
Discussion
In this study, we report that the strength of the Fas-signal determines whether hepatocytes act as type I or II cells in murine livers. Hepatocytes challenged with the anti-Fas antibody Jo2 require Bid to undergo apoptosis as previously reported. Remarkably however, loss of Bid did not completely inhibit apoptosis induced by MegaFasL.
The investigation of Fas-mediated apoptosis in the liver began in 1993 with the isolation of a CD95 specific monoclonal hamster antibody that ligates CD95 and induced acute liver failure in mice due to massive hepatocellular apoptosis. This study demonstrated for the first time that hepatocytes are highly sensitive to Fas-induced apoptosis in contrast to other tissues and organs (32). Subsequent studies revealed that Bcl2-like proteins significantly affect the apoptosis sensitivity of hepatocytes. Particular the pro-apoptotic molecule Bid has been shown to be critically important for Fas-mediated apoptosis induced by Jo2 and by a soluble FLAG-FasL crosslinked with anti-FLAG antibodies (10, 33). Furthermore, mice with targeted deletions of Bax/ Bak are protected from Jo2-induced apoptosis, but also mice expressing Bcl-2 or Bcl-xl as transgene (10, 34–36). Hepatocytes are therefore generally regarded as type II cells. Initial studies have suggested that cells are either type I or type II cells (7). More recent studies however indicate that the Bid dependent and independent pathway can coexist within the same cell. One very recent study for example showed that Fas signaling can switch from Bid-dependent type II signaling in vivo to Bid independent type I pathway in cultured mouse hepatocytes (37). Here, MegaFasL induced severe apoptosis in Bid−/− livers without activation of the mitochondrial amplification loop. Importantly, control experiments using Fas receptor-deficient lpr mice or treatment with an effective pan-caspase inhibitor clearly confirmed that MegaFasL-induced apoptosis is strictly dependent on the Fas receptor and the activation of caspases. These data suggest that hepatocytes and non-parenchymal cells in the liver are not obligatory type II cells as previously assumed but that the strength of the death receptor trigger basically determines whether caspase-8 directly activates effector caspases or the BH3-only molecule Bid in the liver. In this regard, anti-Fas antibodies and soluble FLAG-FasL with crosslinking agents may be viewed as weak or moderate signal, whereas MegaFasL represents a strong signal. Killing by FasL requires extensive receptor aggregation that cannot consistently be induced by mAbs in vitro. The difference between commercially available agents and the physiological ligand to trigger Fas induced apoptosis has been a topic of controversy in the past and it is still not clear which reagent most closely resembles the physiological ligand in vivo. Importantly however, our data show for the first time that a strong Fas-trigger can induce apoptosis in murine livers without activation of the mitochondrial amplification loop. Interestingly, loss of Bid does also not completely protect mice in models of TNF-α-mediated apoptosis and concanavalin A induced liver injury (33, 38) suggesting that the pro-apoptotic role of Bid in hepatocytes may be limited when death receptors are stimulated with a strong trigger in vivo.
The strength of the death receptor signal however does not only depend on the trigger that is used to induce apoptosis, but also depend on the activation of other intracellular signaling pathways that interact with the Fas pathway. Injection of the normally hepatotoxic antibody Jo2 into partially hepatectomized mice does not cause hepatocellular apoptosis but instead accelerates liver regeneration suggesting that Fas mediated signaling can be switched from primarily apoptotic to non-apoptotic (31). Furthermore, chronic liver injury induced by Fah-deficiency or bile duct ligation inhibits apoptotic Fas signaling in hepatocytes (19, 24, 30). Here, we show that the resistance to Fas-induced apoptosis can be overcome with MegaFasL in two models of apoptosis resistance. However, it is interesting to note that deletion of Bid could again significantly inhibit killing of hepatocytes in preconditioned mice. These data support the concept that Bid also amplifies the apoptotic signal from death receptors in non-quiescent hepatocytes. Thus, the balance of the different pro- and anti-apoptotic molecules controlling Fas-induced apoptosis in hepatocytes is quite delicate and a small shift enables execution of cell death when the original signal from the death receptor is not strong enough to directly activate enough caspase-3.
In summary, we provide evidence that Bid is not essentially required for FasL-induced apoptosis in the liver and that phosphorylation of Bid does not affect the sensitivity of hepatocytes to Fas-induced apoptosis. Our data suggest that Bid mainly amplifies a weak death receptor signal in quiescent and non-quiescent hepatocytes rendering the liver more sensitive to Fas-induced apoptosis. However, hepatocytes and non-parenchymal cells can behave as type I cells in murine livers following strong Fas-receptor activation. These findings raise the question whether the pro-apoptotic effect of Bid is really its most relevant function in vivo. Previously, it has been shown that loss of Bid delays diethylnitrosamine-induced liver tumor development in mice by impeding hepatocyte proliferation (14). Therefore, further studies are required to fully elucidate the non-apoptotic functions of Bid in the liver.
Supplementary Material
Acknowledgment
This work was supported by the Deutsche Forschungsgemeinschaft (Vo 959/3-1 to A.V.), the German-Israeli Foundation (Grant to A.V. and A.G.) and the National Institutes of Health grants R01 DK070195 and R01 AA12916 (H.J.).
Footnotes
There is no conflict of interest to disclose
References
- 1.Galle PR, Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18:141–151. doi: 10.1055/s-2007-1007150. [DOI] [PubMed] [Google Scholar]
- 2.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, et al. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 5.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 6.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, et al. Two CD95 (APO-1/Fas) signaling pathways. Embo J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 10.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 11.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 12.Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, et al. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L) Proc Natl Acad Sci U S A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vail ME, Chaisson ML, Thompson J, Fausto N. Bcl-2 expression delays hepatocyte cell cycle progression during liver regeneration. Oncogene. 2002;21:1548–1555. doi: 10.1038/sj.onc.1205212. [DOI] [PubMed] [Google Scholar]
- 14.Bai L, Ni HM, Chen X, DiFrancesca D, Yin XM. Deletion of Bid impedes cell proliferation and hepatic carcinogenesis. Am J Pathol. 2005;166:1523–1532. doi: 10.1016/S0002-9440(10)62368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 16.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 17.Degli Esposti M, Ferry G, Masdehors P, Boutin JA, Hickman JA, Dive C. Post-translational modification of Bid has differential effects on its susceptibility to cleavage by caspase 8 or caspase 3. J Biol Chem. 2003;278:15749–15757. doi: 10.1074/jbc.M209208200. [DOI] [PubMed] [Google Scholar]
- 18.Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. Embo J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel A, Aslan JE, Willenbring H, Klein C, Finegold M, Mount H, Thomas G, et al. Sustained phosphorylation of Bid is a marker for resistance to Fas-induced apoptosis during chronic liver diseases. Gastroenterology. 2006;130:104–119. doi: 10.1053/j.gastro.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, Haimovich G, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichhorst ST, Krueger A, Muerkoster S, Fas SC, Golks A, Gruetzner U, Schubert L, et al. Suramin inhibits death receptor-induced apoptosis in vitro and fulminant apoptotic liver damage in mice. Nat Med. 2004;10:602–609. doi: 10.1038/nm1049. [DOI] [PubMed] [Google Scholar]
- 24.Vogel A, van Den Berg IE, Al-Dhalimy M, Groopman J, Ou CN, Ryabinina O, Iordanov MS, et al. Chronic liver disease in murine hereditary tyrosinemia type 1 induces resistance to cell death. Hepatology. 2004;39:433–443. doi: 10.1002/hep.20077. [DOI] [PubMed] [Google Scholar]
- 25.Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greaney P, Nahimana A, Lagopoulos L, Etter AL, Aubry D, Attinger A, Beltraminelli N, et al. A Fas agonist induces high levels of apoptosis in haematological malignancies. Leuk Res. 2006;30:415–426. doi: 10.1016/j.leukres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 27.McStay GP, Salvesen GS, Green DR. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ. 2008;15:322–331. doi: 10.1038/sj.cdd.4402260. [DOI] [PubMed] [Google Scholar]
- 28.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 29.Jaeschke H, Farhood A, Cai SX, Tseng BY, Bajt ML. Protection against TNF-induced liver parenchymal cell apoptosis during endotoxemia by a novel caspase inhibitor in mice. Toxicol Appl Pharmacol. 2000;169:77–83. doi: 10.1006/taap.2000.9035. [DOI] [PubMed] [Google Scholar]
- 30.Osawa Y, Hannun YA, Proia RL, Brenner DA. Roles of AKT and sphingosine kinase in the antiapoptotic effects of bile duct ligation in mouse liver. Hepatology. 2005;42:1320–1328. doi: 10.1002/hep.20967. [DOI] [PubMed] [Google Scholar]
- 31.Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- 32.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 35.de la Coste A, Fabre M, McDonell N, Porteu A, Gilgenkrantz H, Perret C, Kahn A, et al. Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol. 1999;277:G702–G708. doi: 10.1152/ajpgi.1999.277.3.G702. [DOI] [PubMed] [Google Scholar]
- 36.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter D, Schmich K, Vogel S, Pick R, Kaufmann T, Hochmuth FC, Haber A, et al. Switch from type II to I Fas/CD95 death signaling on in vitro culturing of primary hepatocytes. Hepatology. 2008;48:1942–1953. doi: 10.1002/hep.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, Fan J, et al. Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol. 2007;27:541–553. doi: 10.1128/MCB.01166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.