Abstract
Background
Health plans that increase prescription cost-sharing for their patients may increase overall plan costs. We analyzed the impact on health plan spending of a switch in public drug insurance from full coverage to a prescription copayment (copay), and then to income-based deductibles plus coinsurance (IBD).
Methods
We studied British Columbia residents 65 years of age or older who were dispensed inhaled steroids, β2 agonists or anticholinergics on or after January 1996. Multivariable linear regression was used to estimate health plan costs for the population using inhalers by the Ministry of Health (MOH) during the copay and IBD policies. We estimated costs for excess physician visits and emergency hospitalizations based on data from a previously published cohort study and cost data from the MOH. We estimated the net change in MOH spending as the sum of changes in spending for inhalers, physician visits, hospitalizations, and policy administration costs.
Results
Net health plan spending increased by C$1.98 million per year during the copay policy [95% confidence interval (CI): 0.10–4.34], and C$5.76 million per year during the first 10 months of the IBD policy (95% CI: 1.75–10.58). Out-of-pocket spending by older patients increased 30% during the copay policy (95% CI: 24–36) and 59% during the IBD policy (95% CI: 56–63).
Conclusions
British Columbia’s experience indicates that cost containment focused on cost-shifting to patients may increase net expenditures for the treatment of some diseases. Health plans should consult experts to anticipate the potential cross-program impacts of policy changes.
Keywords: pharmacoeconomics, inhaled medications, asthma, COPD, health care utilization, drug benefit plans, health services research
Patients who join the new Medicare prescription drug plan gain much needed assistance but still have to pay a substantial portion of their medication costs through deductible and coinsurance payments. Those cost-sharing mechanisms give patients a financial incentive to use less expensive therapies because they pay more for higher priced drugs. Drug plans that use cost sharing typically require patients to pay some portion of a prescription regardless of whether the drug is relatively expensive or inexpensive. One exception is a reference pricing (RP) policy in which the drug plan pays fully for the lowest cost brand among therapeutically equivalent drugs, and requires patients who use more expensive brands to pay the difference.1 In this sense, RP is similar to a tiered copayment (copay) plan where the lowest tier, consisting of the least expensive drug, does not require a copay.
Previous studies have examined the effects of copays, tiered copays, and RP on drug spending by plans and patients,2–4 drug switching,2,5–9 and drug stopping.5–7,10–12 Some studies have estimated the effect of cost-sharing policies on health-related outcomes other than prescription drug use.11,13,14 RP of angiotensin-converting enzyme inhibitors was found to reduce net health plan spending in British Columbia (BC).3 In 2005, a review of 30 studies concluded that cost sharing reduces the consumption of prescription drugs but may have unintended effects on the process and outcomes of therapy.15 Although patient and societal consequences of cost-sharing policies are also important, from a health plan’s perspective it is important to understand how changes in prescription drug cost sharing between patients and the plan causes changes in spending on other insured services.
It is plausible that cost containment measures that focus on price or reimbursement level, but unlike RP do not fully reimburse therapeutically equivalent alternatives, may result in a higher risk of reduced drug utilization that could lead to increased health care utilization and adverse health outcomes. The publicly funded drug plan in the Province of BC, Canada, has provided an opportunity to study this effect in a natural experiment involving most residents of BC over 65 years of age. In January 2002, after 28 years of paying all eligible prescription ingredient costs for residents over 65 years of age, BC Pharmacare, the provincial drug plan operated by the Ministry of Health (MOH), introduced a copay policy for older residents of $25 per prescription. Sixteen months later the copay policy was replaced with an income-based deductible plus coinsurance policy (IBD).
Older patients with asthma or chronic obstructive pulmonary disease were at particular risk of reducing their use of inhaled medications in response to cost sharing because their drugs are relatively expensive. Before this study we estimated a 6% reduction in use of inhaled β2 agonists and a 13% reduction in use of inhaled steroids during the 16 months of the copay policy and the first 14 months of the IBD policy.10 To determine if the policies adversely affected health outcomes we conducted a cohort study of older chronic users of inhalers who likely had chronic obstructive pulmonary disease, asthma, or emphysema (CAE). Both policies were associated with significant increases in physician visits (3% during the copay policy, 7% during the IBD policy), and the IBD policy was associated with a significant increase of 29% in emergency hospital admissions for CAE in the first 10 months. We found no evidence of a significant change in CAE mortality or all-cause mortality.16
The objective of this study was to estimate the net change in health plan spending by the BC MOH from the copay and IBD policies compared with the previous policy of full coverage in patients over 65 years of age who chronically used inhaled medications. In a secondary analysis, we estimated the impact of the policy changes on patient out-of-pocket spending for inhalers.
METHODS
Drug Policy Changes
Residents of BC ≥65 years of age had full coverage for prescription ingredient costs until December 31, 2001, for drugs listed on the provincial formulary. We defined 3 exposure levels denoted by the preexisting full coverage policy and 2 subsequent policy changes. The first policy change was a fixed copay of $25 per prescription for seniors ($10 for seniors with incomes less than C$22,000/yr) that started on January 1, 2002, and lasted until April 30, 2003. The second policy was the Fair PharmaCare IBD policy that was implemented on May 1, 2003. The IBD policy had 3 components: a family deductible of 0% to 2% based on family income, a coinsurance payment of 25% for prescriptions after passing the deductible, and an out-of-pocket ceiling equal to 1.25%, 2%, or 3% of income.17 Families were responsible for all drug costs under their deductible but may have had supplemental private insurance.
Data
Our source population included all Medical Services Plan eligible BC residents over 65 years of age who were not federally insured for drug benefits (approximately 487,000 people in 1996 and 563,000 million in 2003). All prescriptions for inhaled steroids, inhaled β2 agonist, and inhaled anticholinergics dispensed at community pharmacies were obtained from the province’s PharmaNet database. Errors in the PharmaNet database should be low because the system performs data quality checks when prescriptions are processed. We used MOH administrative databases for physician services and hospitalizations to estimate physician and hospital expenditures. Completeness and misclassification in those databases is probably comparable to similar administrative databases that have been evaluated for accuracy.18–22
The impact on MOH spending for policy-related changes in physician visits and emergency CAE hospitalizations were based on results of our earlier study on health-related outcomes associated with the copay and IBD policies, shown in Table 1.16 In the analysis, we observed an overall increase of 29% in CAE emergency admissions during the first 10 months of the IBD policy in patients over 65 years of age [95% confidence interval (CI): 9%–52%], and a non-statistically significant increase of 13% during the copay policy (95% CI: −3% to 32%). Furthermore, we observed significant overall increases in patient visit days of 3% during the copay policy (95% CI: 1%– 4%) and 7% during the IBD policy (95% CI: 5%– 8%). The cohort was described in detail in the original study. Briefly, it included long-term inhaler users 65 years of age or older at the study baseline date (January 1, 2001). Long-term inhaler use was defined as ≥3 inhaler prescriptions within 6 months prior to baseline.
TABLE 1.
Emergency Hospital Admissions and Physician Visits of 75,628 Chronic Users of Inhaled Medications Before and After the Copay and IBD Policy Changes
| Outcome | Baseline Period (Months 1–12) Events/1000Person-Years* | Copay Period (Months 13–28) |
IBD Period (Months 29–40) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Events/1000 Person-Years* | Ajusted RR* | Excess Events/1000 Person-Years† | (95% CI) | Events/1000 Person-Years* | Ajusted RR* | Excess Events/1000 Person-Years† | (95% CI) | ||
| Admission via emergency room for COPD, asthma, or emphysema | |||||||||
| Policy group (≥65 yr) | 67 | 72 | 1.13 | 9.0 | (−1.8–21.5) | 79 | 1.29 | 19.2 | (6.1–34.7) |
| Control group (55–64 yr) | 36 | 35 | 36 | ||||||
| Physician visits | |||||||||
| Policy group (≥65 yr) | 22,013 | 22,298 | 1.03 | 605 | (324–889) | 19,068 | 1.07 | 1476 | (1103–1852) |
| Control group (55–64 yr) | 17,739 | 17,626 | 14,520 | ||||||
Events and adjusted rate ratios (RR) were previously reported in health outcomes study.16
Excess events calculated as [(events/1000 person-years at baseline) × adjusted rate ratio] − events/1000 person-years at baseline.
Change in Health Plan Spending for Inhaled Medications
All expenditures in our study were converted to 2003 Canadian dollars using the Canadian Consumer Price Index.23 MOH spending on inhaler prescriptions was summed for each calendar month. Linear regression analysis was used to estimate monthly spending by the MOH during a full coverage period between January 1996 and November 2001, and to predict spending during the December 2001 to April 2003 copay period and the May 2003 to February 2004 IBD period. The models included a linear time trend, 11 indicator variables for month, and an autoregressive lag term. Overall change in spending for inhalers during each policy period was estimated by summing differences between observed spending and predicted spending from the linear regressions. The impact of the IBD policy may have been overestimated because of the assumption that copay period effects did not carry over to the IBD period.
Change in Spending for Emergency Hospitalizations
The actual cost of hospital stays are not calculated in Canada. Instead, hospitals receive global funding from their provincial MOH, and discharge records include a resource intensity weight (RIW) that can be used to estimate cost. RIWs are calculated by the Canadian Institute for Health Information. The RIW is an estimate of the relative amount of resources used by a patient during a hospital stay. Detailed information on RIW methodology can be obtained from www.cihi.ca. The BC MOH estimates a dollar value for an RIW by considering a number of factors, including the total number of RIWs that a hospital accumulated and the amount of funding it received. As of June 30, 2005, the MOH in BC estimated the value of 1 RIW in the fiscal year 2003/2004 to be C$5220.24
We extracted emergency hospitalization records between April 1, 2003 and February 29, 2004 that had a primary diagnosis in the CAE definition (ICD-10 codes J40–J45). To estimate the average expenditure for an emergency hospitalization for CAE we multiplied the average RIW by C$5220. To estimate total spending for policy-induced excess hospitalizations to the MOH, we multiplied the estimate of average expenditure for 1 emergency CAE admission by the number of excess admissions estimated from our previous cohort study (Table 1), and adjusted for inflation according to the year in which excess admissions occurred. In a sensitivity analysis, we varied the average cost of an emergency CAE hospital stay from C$4000 to C$8000, and varied the estimated number of excess admissions using the 95% lower and upper confidence limits from the cohort study.16 For the copay policy, the lower 95% confidence limit indicated a 3% decrease in emergency CAE admissions, while the upper limit indicated a 32% increase. For the IBD policy, the lower 95% confidence limit indicated a 9% increase and the upper limit indicated a 52% increase.
Change in Spending for Physician Visits
We extracted all physician visit records between January 1, 2003 and December 31, 2003. Each record included the amount paid to the physician by the MOH. All MOH payments to physicians for each patient and day were summed to obtain the total amount paid per patient visit day. To estimate MOH spending for excess physician use we multiplied the average cost per patient visit day by the number of excess patient visit days estimated in the cohort study and adjusted for inflation. In a sensitivity analysis, we varied the average cost of a patient visit day from C$40 to C$80, and varied the estimated number of excess patient visit days using the 95% lower and upper confidence limits from the cohort study.
Out-of-Pocket Inhaler Spending by Patients
Out-of-pocket spending for inhalers was summed for each calendar month. For the purpose of contrast we also analyzed out-of-pocket spending for inhalers by patients younger than 65 years of age. Linear regression analysis was used on the adjusted data to estimate total monthly out-of-pocket spending during the full coverage period, and to predict spending during the copay and IBD periods. The models included a linear time trend, 11 indicator variables for month, and an autoregressive lag term.
Spending on Policy Development, Implementation, and Administration
No formal estimates of policy implementation and development costs were available from the MOH.25 Instead, we estimated development and implementation spending in consultation with a senior business analyst who was involved in many aspects of the development and implementation of the copay and IBD policies.26 Preparing the policy required the skills of data analysts, managers, technical experts, and clerks. For each of these workers we assigned an aggregate annual full-time salary (FTE) of C$100,000 for consultants, managers and technical experts, C$65,000 for analysts who were employees, and C$50,000 for administrative professionals. For fixed and variable costs we estimated the number of FTEs of each kind that were needed and multiplied those estimates by the respective FTE salary. Fixed costs included analysis and preparation, construction of a registration database (IBD only), system interface enhancements, and initial registration of patients. Variable costs for ongoing registration and administration were also included for the IBD policy. Because both policy changes applied to all drug classes and not just inhalers, the amount of overall development and implementation spending in our analysis attributable to the inhaler portion of the MOH budget was chosen to be the percentage of BC Pharmacare expenditures devoted to inhalers in 2001 among beneficiaries 65 years of age and older who were subject to the policy change. These percentages were 5.57% for the copay policy and 4.18% for the IBD policy.
Net Change in Health Plan Spending
The net change in spending by the MOH from each policy change was calculated as the sum of estimated spending for inhaled medications, excess emergency CAE admissions, excess physician use, and policy development and implementation costs. Net changes in spending were estimated for the entire 16-month duration of the copay policy (plus December of 2001 because of policy-related medication stockpiling) and the first 10 months of the IBD policy. We annualized results by multiplying estimates of changes in expenditures by 12/17 for the copay policy and 12/10 for the IBD policy.
RESULTS
Spending for Policy Development, Implementation, and Administration
Development and implementation costs for the policies are shown in Table 2. The copay policy was replaced with the IBD policy and therefore did not have ongoing administrative costs, and it did not require capital enhancements. Approximately C$170,000 was spent on developing the copay policy and communicating it to BC Pharmacare beneficiaries. The total additional cost of administering the copay policy was estimated to be C$370,000, of which we attributed 5.57% (C$20,597) to the inhaler portion of MOH drug spending. For the IBD policy, building a registration system, modifying other systems and initial registration of families cost approximately C$9.2 million. Ongoing administrative costs were approximately C$1.4 million per year, and policy analysis and preparation were approximately C$345,000. The total estimated additional cost of administering the IBD policy for the first 10 months was estimated to be C$10.9 million, of which we attributed 4.18% (C$457,000) to the inhaler portion of MOH drug spending.
TABLE 2.
Estimated Costs for Development and Implementation of the Copay and IBD Policies (2003 Canadian Dollars)
| Component | Sub-Component | Copay Policy Labor | IBD Policy |
|
|---|---|---|---|---|
| Labor | Capital | |||
| Fixed costs | (June 2001–November 2001) | (June 2001–April 2003) | ||
| Analysis and preparation | Analysis | 32,500 | 132,500 | |
| Management | 25,000 | 200,000 | ||
| Administrative support | 12,500 | 12,500 | ||
| Sub-totals | 70,000 | 345,000 | ||
| Building registration system | Hardware/software | 5,000,000 | ||
| System interface enhancements | Technical | 200,000 | 900,000 | |
| Management | 50,000 | |||
| Administrative support | 50,000 | |||
| Sub-totals | 200,000 | 1,000,000 | ||
| Initial registration volumes | Retrofit existing databases to bulk load families | 100,000 | ||
| Assisting clients registration | 500,000 | |||
| One-time communications push | 100,000 | 100,000 | 2,000,000 | |
| Administrative review/exceptions processing | 500,000 | |||
| Sub-totals | 100,000 | 1,200,000 | 2,000,000 | |
| Variable costs | (May 2003–February 2004) | |||
| Ongoing costs | New registrations | 500,000 | ||
| Maintenance of family membership list | 300,000 | |||
| Facilities management | 250,000 | |||
| Administrative review/exceptions processing | 250,000 | |||
| Annual renewal process | 100,000 | |||
| Sub-totals | 1,400,000 | |||
| Total | 370,000 | 3,945,000 | 7,000,000 | |
| Portion of drug plan expenditure spent on inhalers for affected patients | ×5.57% | ×4.18% | ×4.18% | |
| Inhaler therapy share of policy development costs | 20,597 | 164,839 | 292,490 | |
Ministry of Health Spending for Inhaled Medications
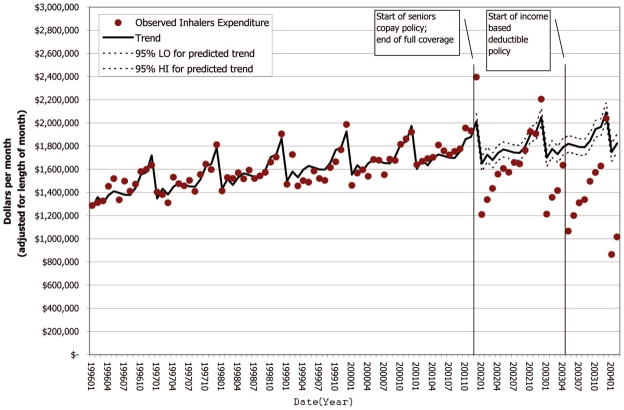
Monthly spending on inhalers by the MOH is plotted in Figure 1 and shows a cyclical pattern of stockpiling medication in December of each year, followed by a poststockpiling bounce in January. Both policy changes were associated with reduced MOH spending for inhalers (Table 3). MOH spending on inhalers decreased C$1.93 million per year from expected spending under full coverage during the copay period (95% CI: 0.26–3.59). MOH spending for inhaled medications during the first 10 months of the IBD period decreased by C$6.13 million per year (95% CI: 4.44–7.82).
FIGURE 1.
Trend in MOH expenditure on inhalers (steroids + β2 agonists + anticholinergics) before and after the copay and IBD policies, for patients 65 years of age and older (2003 Canadian dollars).
TABLE 3.
Estimated Change in Annual Spending for the Copay and IBD Policies by the Ministry of Health for Users of Prescription Inhalers Over 65 Years of Age (2003 Canadian Dollars)
| Expenditure Component | Decrease (−) or Increase (+) in Millions |
|||||
|---|---|---|---|---|---|---|
| Copay Policy |
IBD Policy |
|||||
| 95% Low | Base | 95% High | 95% Low | Base | 95% High | |
| Inhaled prescription medications* | −0.264 | −1.927 | −3.590 | −4.436 | −6.129 | −7.822 |
| Excess emergency CAE hospitalizations†‡ | 30.464 | 2.379 | 5.690 | 2.039 | 6.456 | 11.671 |
| Excess physician visitsठ| 0.809 | 1.512 | 2.223 | 3.650 | 4.884 | 6.129 |
| Policy development and implementation costs (variable) | 0.178 | 0.198 | 0.218 | |||
| Policy development and implementation costs (fixed) | 0.014 | 0.015 | 0.017 | 0.316 | 0.351 | 0.386 |
| Total | 0.095 | 1.979 | 4.340 | 1.747 | 5.760 | 10.581 |
| Total (excluding fixed costs) | 0.081 | 1.963 | 4.323 | 1.431 | 5.409 | 10.195 |
| Total (excluding physician visits) | −0.714 | 0.467 | 2.117 | −1.903 | 0.876 | 4.452 |
Estimated amount spent by the MOH on ingredient costs and dispensing fees for inhaler medications for patients 65 years of age and older, adjusted for inflation to 2003 Canadian dollars.
The estimated number of excess emergency CAE admissions was 9.0 per 1000 person-years [copay; 95% confidence interval (CI), −1.8 to 21.5] and 19.2 admissions per 1000 person-years (IBD; 95% CI, 6.1–34.7). There were 55,500 person-years of follow-up time contributed by older chronic inhaler patients during the copay policy, and 45,000 person-years contributed during the first 10 mo of the IBD policy.
The mean cost for CAE admissions in fiscal year 2003/2004 was $6232.
The estimated number of excess physician visits was 605 per 1000 person-years (copay; 95% CI, 324–889) and 1476 visits per 1000 person-years (IBD; 95% CI, 1103–1852).
The mean observed paid cost per visit day in 2003 was $60.10.
Ministry of Health Spending for Emergency Hospitalizations and Physician Visits
Additional spending on emergency CAE hospitalizations during the copay period was C$2.38 million per year (95% CI: −0.46 to 5.69), and during the IBD period was C$6.46 million per year (95% CI: 2.04–11.67), making the increased of hospitalizations during the first 10 months of IBD approximately equal to the savings on inhaler expenditures (Table 3). MOH spending for physician services increased C$1.51 million per year during the copay policy (95% CI: 0.81–2.22) and C$4.88 million per year during the IBD policy (95% CI: 3.65–6.13).
Net Change in Ministry of Health Spending
After summing reductions in MOH spending for inhalers with additional costs for hospital and physician services plus administrative costs, the copay policy increased net MOH spending by C$1.98 million per year (95% CI: 0.10–4.34) compared with the full coverage baseline period (Table 3), and by C$5.76 million per year during the IBD policy compared with full coverage (95% CI: 1.75–10.58). The sensitivity analysis showed that changes in spending during both policies were highly influenced by spending for additional hospitalizations and to a lesser degree by additional spending for physician services (Table 4). However, the analysis of net change in expenditure in Table 3 also showed that physician costs contributed substantially to net change in expenditure.
TABLE 4.
Sensitivity Analysis of Change in Annual Spending by the Ministry of Health for the Copay and IBD Policies, for Patients Over 65 Years of Age Who Used Inhaled Medications (2003 Canadian Dollars)
| No. Excess Emergency CAE Admissions and Physician Visits* Variation in Excess Admissions and Visits | Unit Cost Category† Variation in Unit Cost | Mean Cost per CAE ER Admission‡ | Mean Cost Per Physician Visit Day§ | Net Decrease (−) or Increase (+) in Ministry of Health Expenditure (millions) Variation in Decreased Expenditure on Inhalers||¶ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Copay Policy |
IBD Policy |
||||||||
| 95% Low | Mean | 95% High | 95% Low | Mean | 95% High | ||||
| 95% low | Low | $4000 | $40 | −0.017 | −1.681 | −3.344 | −0.547 | −2.240 | −3.933 |
| Mean | $6232 | $60 | 0.081 | −1.582 | −3.245 | 1.451 | −0.242 | −1.935 | |
| High | $8000 | $80 | 0.229 | −1.434 | −3.098 | 3.145 | 1.452 | −0.241 | |
| Mean | Low | $4000 | $40 | 2.240 | 0.576 | −1.087 | 3.093 | 1.401 | −0.292 |
| Mean | $6232 | $60 | 3.626 | 1.963 | 0.300 | 7.102 | 5.409 | 3.716 | |
| High | $8000 | $80 | 4.743 | 3.080 | 1.417 | 10.425 | 8.733 | 7.040 | |
| 95% high | Low | $4000 | $40 | 4.798 | 3.135 | 1.793 | 7.253 | 5.560 | 3.867 |
| Mean | $6232 | $60 | 7.649 | 5.986 | 4.323 | 13.561 | 11.868 | 10.175 | |
| High | $8000 | $80 | 9.859 | 8.196 | 6.533 | 18.745 | 17.052 | 15.359 | |
Ninety-five percent confidence limits for excess emergency CAE admissions were −1.8 to 21.5 admissions per 1000 person-years (copay), and 6.1–34.7 admissions per 1000 person-years (IBD). There were 55,500 person-years of follow-up time contributed by older chronic inhaler patients during the copay policy, and 45,000 person-years contributed during the first 10 months of the IBD policy.
Low and high unit cost categories were chosen arbitrarily and were approximately equal to adding or subtracting one-third of the mean unit cost from the mean.
The mean cost for CAE admissions in fiscal year 2003/2004 was $6232.
The mean observed paid cost per visit day in 2003 was $60.10.
Ninety-five percent confidence limits for policy-related changes in annual inhaler spending were −$0.264 million to −$3.590 million (copay) and −$4.436 million to −$7.822 million (IBD).
Fixed policy development and implementation costs were excluded and variable costs were held constant at 0 (copay) and $0.20 million (IBD).
Out-of-Pocket Spending
Patients under 65 years of age were not part of the copay policy but were subject to the IBD policy. There was no significant change in total out-of-pocket spending during the IBD policy for the population under 65 years of age. This coincides with a nearly neutral change in net spending on inhalers by the MOH for that age group. In the patient population greater than 65 years of age, total out-of-pocket spending for inhalers increased 30% during the copay policy (C$1.70 million per year; 95% CI: 1.39, 2.01) and 59% during the first 10 months of the IBD policy (C$5.81 million per year; 95% CI: 5.50, 6.12). Median monthly out-of-pocket spending for older patients was C$6 during the full coverage period (equivalent to the dispensing fee), C$14 during the copay period, and C$27 during the IBD period.
DISCUSSION
Policy Impact on Spending by the Ministry of Health
MOH expenditure for inhalers decreased 3 times as much under the IBD policy than under the copay policy compared with full coverage, but the greater decrease did not translate into a similar magnitude decrease in net MOH spending on the patients who used those drugs. The 2 policy changes, as they pertained to users of inhaled medications, had different effects on MOH spending for hospitalizations and physician services. These results agree with a review of 30 studies that concluded cost sharing reduces the consumption of prescription drugs may have unintended effects on health outcomes.15 This analysis shows that net changes in health plan spending from those policies can cost rather than save money in some patient groups. When cross-program impacts were taken into account there was an increase in net spending during the copay policy and an even larger increase during the IBD policy. The increase in emergency CAE hospitalizations during the copay policy, which was estimated in our previous cohort study, was included as an expense in our analysis of net spending even though the 95% CI for the effect on hospitalizations was not statistically significant in our cohort study.10 If there was no actual increase in hospitalizations during the copay policy then the change in net spending relative to full coverage would have instead equaled a nonsignificant decrease of C$0.40 million per year (95% CI: 0.60–1.35).
Spending on inhalers and hospitalizations should also be considered separately from physician costs because inhaler patients take other medications that were also subject to cost-sharing. If the policies caused patients to change their use of other drugs, and those changes led to more physician visits, then is not appropriate to compare total change in physician spending to only to changes in inhaler spending as we have done. When physician costs are excluded from the analysis of net change in spending (Table 3), estimated net spending by the MOH still increased for its older inhaler patients (copay, C$0.47 million per year; IBD, C$0.88 million per year), but when the lower 95% confidence limits were used the net change was in the direction of a savings for the MOH (copay, C$0.71 million per year; IBD, C$1.9 million per year).
The observational study that provided the estimates of policy-related increases in emergency CAE admissions and physician visits analyzed the policies in 2 ways.16 One method compared older chronic users of inhalers to a historical control group, and a second method used a concurrent control group. Our analysis only used estimates from the concurrent control group analysis because the time-varying nature of changes to physician and hospital budgets, and how RIWs and their costs are calculated over time, could have made an economic analysis of the historical control group prone to substantial bias. The historical design showed a 41% increase in emergency CAE admissions during IBD compared with a 29% increase observed using the concurrent control group. However, physician visits increased approximately half as much in the historical control group design during both policies. Based on those results we can anticipate that the historically controlled event data would have shown the copay policy to be less costly to the MOH, and the IBD policy to be slightly more costly.
Policy Impact on Out-of-Pocket Spending
Our secondary analysis of out-of-pocket spending revealed that the median patient over 65 years of age paid twice as much per month for inhalers because of the copay policy, and 4 times as much because of the IBD policy, both compared with full coverage. Out-of-pocket spending did not change in inhaler patients under 65 years of age, but the population estimate might have masked substantial shifts between families of different income strata. The increase in out-of-pocket spending by older patients is substantial but should be considered in relation to the modest amount they were paying for dispensing fees before the policy changes.
Relationship of Changes in Out-of-Pocket Spending to Inhaler Use
The decrease in MOH spending for inhalers was not significantly greater than the increase in patient out-of-pocket spending for inhalers during both policies. In our prior study of changes in inhaler use before and after the copay and IBD policy changes, we observed statistically significant decreases in the use of inhaled medications of 6% for β2 agonists and 13% each for steroids and anticholinergics.10 The apparent discrepancy between reduced drug use and a lack of a significant change in total drug spending (MOH + patient out-of-pocket) can be attributed to a concurrent secular shift away from less expensive single preparation inhalers toward newly introduced and much more expensive combination inhalers.
Are Copayments Preferable to Coinsurance?
From a health plan’s perspective, a coinsurance policy is preferable to a copay policy because patients automatically pay their share of any price increases. However, a coinsurance policy may burden patients who need expensive drugs when less expensive therapeutic alternatives are unavailable. A copay, because it is a fixed amount, must be increased by the drug plan whenever it wishes to offset a price increase. However, our analysis indicates that what works in theory may not hold in reality because the MOH health plan faired better under its copay policy than its IBD policy, at least with regards to its inhaler patients. Even so, there were several limitations in our analysis that prevent us from making strong conclusions about the relative superiority of 1 policy over another. One limitation is that our analysis was confined to patients of inhalers. An expanded analysis including all insured drugs and health care services would be useful but large undertaking. In the meantime, it is important to recognize that population-level conclusions would change if the MOH captured savings on other drug classes without adversely affecting hospital and physician services.
Other factors may have biased our spending estimates. Among these were attributing all increases in physician and hospital services to reduced inhaler use rather than to changes in the use of all drugs (a bias in our analysis toward increased net spending by the MOH), assuming there was no collateral effects on health care system use in patients under 65 (bias toward lower spending if the policy was harmful, higher spending if helpful), assuming the policy changes increased physician and hospital spending in only chronic inhaler users rather than all users (bias toward lower spending), and assuming that impacts of the copay policy did not carry-over into the IBD policy period (bias toward the copay costing less and IBD costing more). Also, additional out-of-pocket patient expenses due to their deteriorating health were not measured.
Another possible limitation is the assumption that the MOH increased its physician and hospital spending in response to the impacts of the policy changes. Because the impacts on physician and hospital use were unknown to the MOH, and because spending on hospitals and physicians is done using global-style budgeting, it is unknown whether the collateral effects were accommodated by increased funding for physicians and hospitals, or by reductions in service for other patients. Our MOH cost estimates could be biased upward if the collateral policy effects were absorbed by unintentionally reducing services for other patients. Finally, our estimates of policy development costs relied on an interview with a senior business analyst who worked on the implementation of the policies.26 This was necessary because the Ministry of Health did not track those costs separate from their larger administrative budgets. We decided it was more valid to include reasonable estimates for development and implementation costs rather than to ignore them.
CONCLUSIONS
BC’s experience with copays, coinsurance, and deductibles indicates that these forms of cost sharing do not necessarily lower health plan spending relative to full coverage for the treatment of some diseases. This does not mean that cost-sharing policies are faulty in principle since there are drugs that are over used by patients who would not suffer by discontinuing or reducing their use. The weakness of these cost-sharing policies lies in their tendency to use a one-size-fits-all approach. We recommend that policy-makers consider the potential collateral costs of policies using cross-program analyses that include policy development and implementation costs. Such a process would review existing evidence to understand what types of patients may change their drug use, and consulting clinical experts as to the risks posed by those changes. This is necessary because, as we have shown, savings in 1 part of the health care system may produce extra costs in another. At the same time, it needs to be recognized that some outcomes, including perhaps those observed in this study, cannot be anticipated.
Acknowledgments
Supported by the National Institute on Aging (R01-AG021950) and the Agency for Healthcare Research and Quality (2-RO1-HS10881), Department of Health and Human Services, Rockville, MD (to S.S.) and a doctoral research award from the Canadian Institutes of Health Research (award 68359) (to C.R.D.).
References
- 1.Schneeweiss S, Maclure M, Walker AM, et al. On the evaluation of drug benefits policy changes with longitudinal claims data: the policy-maker’s versus the clinician’s perspective. Health Policy. 2001;55:97–109. doi: 10.1016/s0168-8510(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 2.Huskamp HA, Epstein AM, Blumenthal D. The impact of a national drug formulary on prices, market share, and spending: lessons for medicare? Health Aff (Millwood) 2003;22:149–158. doi: 10.1377/hlthaff.22.3.149. [DOI] [PubMed] [Google Scholar]
- 3.Schneeweiss S, Dormuth C, Grootendorst P, et al. Net health plan savings from reference pricing for angiotensin-converting enzyme inhibitors in elderly British Columbia residents. Med Care. 2004;42:653–660. doi: 10.1097/01.mlr.0000129497.10930.a2. [DOI] [PubMed] [Google Scholar]
- 4.Grootendorst PV, Dolovich LR, O’Brien BJ, et al. Impact of reference-based pricing of nitrates on the use and costs of anti-anginal drugs. Can Med Ass J. 2001;165:1011–1019. [PMC free article] [PubMed] [Google Scholar]
- 5.Huskamp HA, Deverka PA, Epstein AM, et al. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med. 2003;349:2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 6.Schneeweiss S, Walker AM, Glynn RJ, et al. Outcomes of reference pricing for angiotensin-converting enzyme inhibitors. New Engl J Med. 2002;346:822–829. doi: 10.1056/NEJMsa003087. [DOI] [PubMed] [Google Scholar]
- 7.Schneeweiss S, Soumerai SB, Maclure M, et al. Clinical and economic consequences of reference pricing for dihydropyridine calcium channel blockers. Clin Pharm Ther. 2003;74:388–400. doi: 10.1016/S0009-9236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 8.Briesacher B, Kamal-Bahl S, Hochberg M, et al. Three-tieredcopayment drug coverage and use of nonsteroidal anti-inflammatory drugs. Arch Intern Med. 2004;164:1679–1684. doi: 10.1001/archinte.164.15.1679. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JK, Grootendorst PV, O’Brien BJ, et al. Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia. Can Med Ass J. 2002;166:1655–1662. [PMC free article] [PubMed] [Google Scholar]
- 10.Dormuth CR, Maclure M, Glynn RJ, et al. Impact of two sequential drug cost-sharing policies on the use of inhaled medications in older patients with COPD/asthma. Clin Ther. 2006;28:964–978. doi: 10.1016/j.clinthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Tamblyn R, Laprise R, Hanley JA, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA. 2001;285:421–429. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- 12.Kozyrskyj AL, Mustard CA, Cheang MS, et al. Income-based drug benefit policy: impact on receipt of inhaled corticosteroid prescriptions by Manitoba children with asthma. Can Med Ass J. 2001;165:897–902. [PMC free article] [PubMed] [Google Scholar]
- 13.Soumerai SB, Ross-Degnan D, Avorn J, et al. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med. 1991;325:1072–1077. doi: 10.1056/NEJM199110103251505. [DOI] [PubMed] [Google Scholar]
- 14.Motheral B, Fairman KA. Effect of a three-tier prescription copay on pharmaceutical and other medical utilization. Med Care. 2001;39:1293–1304. doi: 10.1097/00005650-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gibson T, Ozminkowski R, Goetzel R. The effects of prescription drug cost sharing: a review of the evidence. Am J Manag Care. 2005;11:730–740. [PubMed] [Google Scholar]
- 16.Dormuth CR, Maclure M, Glynn RJ, et al. Emergency hospital admissions after income-based deductibles and prescription copayments in older users of inhaled medications. Clin Ther. 2008;30P1:1038–1050. doi: 10.1016/j.clinthera.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Details of the IBD policy. Available at: www.healthservices.gov.bc.ca/pharme/plani/planiinfo.html#M.
- 18.Williams JI, Young W. Inventory of Studies on the Accuracy of Canadian Health Administrative Databases. Toronto, Ontario: Institute for Clinical Evaluative Sciences; 1996. [Google Scholar]
- 19.Folwes JB, Lawthers AG, Weiner JP, et al. Agreement between physicians’ office records and medicare part B claims data. Health Care Financ Rev. 1995;16:189–199. [PMC free article] [PubMed] [Google Scholar]
- 20.Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32:81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, Monane M, Gurwitz JH, et al. Agreement between drug treatment data and a discharge diagnosis of diabetes mellitus in the elderly. Am J Epidemiol. 1999;149:541–549. doi: 10.1093/oxfordjournals.aje.a009850. [DOI] [PubMed] [Google Scholar]
- 22.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: progress has been made but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Data Source: Statistics Canada, Consumer Price Indexes for Canada, Monthly, 1914–2003 (P100000 series).
- 24.British Columbia Ministry of Health. Ministry Contact: Stephen Lee, Information Support Consultant, Knowledge, Management and Technology Division, (File CWC04 V4b.xls) Victoria, British Columbia: British Columbia Ministry of Health; 2005. [Google Scholar]
- 25.British Columbia Ministry of Health. Director, Pharmacare Decision Support. Victoria, British Columbia: British Columbia Ministry of Health; Conversation With George Day. [Google Scholar]
- 26.Burnett Sean. Business Analyst. Victoria, British Columbia: PFIA Corporation; [Google Scholar]