Abstract
T cells use secreted soluble factors for highly specific intercellular communication and targeted cell killing. This specificity is achieved first through T cell receptor–mediated recognition of complexes of peptide and major histocompatibility complex displayed by appropriate antigen-presenting cells and then by the directed secretion of cytokines and lytic factors into the immunological synapse between the T cell and antigen-presenting cell. Studies have begun to probe the molecular basis for this synaptic secretion and have also shown that T cells release chemokines and certain inflammatory factors through a multidirectional pathway directed away from the synapse. Thus, the mode of secretion seems to be tailored to the intended function of the secreted molecule.
Antigen recognition transforms T cells from quiescent bystanders into highly specialized intercellular communicators. Activated CD4+ T helper cells interact with dendritic cells, natural killer cells, B cells and other T cells, coordinating their respective activities to shape the resultant immune response. CD8+ cytotoxic T lymphocytes (CTLs) can secrete cytokines but also specifically kill infected cells in the periphery. This targeted cell killing constitutes a form of communication by which cells presenting the appropriate complexes of peptide and major histocompatibility complex are ‘instructed’ to die.
A large portion of this intercellular communication is mediated by soluble factors such as cytokines and cytolytic molecules. However, T cells operate in a dense milieu that is packed with bystander cells, which raises the obvious question of how specificity is maintained. Simply stated, how do T cells avoid activating or killing the wrong cell? The answer to this question lies in the cellular context in which these events occur. It has been known for some time that within minutes of activation of the T cell antigen receptor (TCR), a stereotypical junction known as the ‘immunological synapse’ (IS) forms between the T cell and the antigen-presenting cell (APC)1. The classical IS consists of a central cluster containing the TCR surrounded by a peripheral ring enriched in the integrin LFA-1 (αLβ2)2.
The IS functions as a conduit for the transfer of information between the T cell and the APC. Some of this information transfer is mediated by cell surface proteins. For example, CD40L accumulates in the IS of T cell–B cell conjugates, where it facilitates the antigen-specific activation of the B cells3. In addition, it is becoming increasingly apparent that certain soluble factors, including cytolytic molecules and some cytokines, are secreted in a directional way into the IS. This ‘synaptic’ secretion, analogous to what occurs in neuronal synapses, allows T cells to be highly selective in terms of which surrounding cells receive signals. Early evidence of this phenomenon was provided by studies showing that T cells trapped in the pores of a Transwell membrane secrete cytokines ‘preferentially’ on the side of the membrane containing activating antibodies4. Soon after, immunocytochemistry studies showed that certain important cytokines accumulate near the IS in T cell–B cell conjugates5,6. Those discoveries have now been confirmed and extended by more detailed imaging studies of both CTLs and T helper cells. This newer work, which is the subject of this review, has also provided insight into the molecular mechanisms that drive directional secretion at the IS. In addition, it has demonstrated the existence of a second, ‘multidirectional’ pathway that releases molecules away from the synapse. This latter pathway may be important for bystander activation and the establishment of chemokine gradients.
Laying the groundwork: microtubule polarization
Directional secretion depends on the underlying microtubule cytoskeleton. Microtubules radiate with a defined polarity from the microtubule-organizing center (MTOC), with ‘minus’ ends directed inward and ‘plus’ ends directed outward. Within minutes of TCR stimulation, the MTOC polarizes to a position just beneath the IS7,8 (Fig. 1). The MTOC is associated with the Golgi apparatus, where proteins destined for secretion are processed and prepared. Thus, the reorientation of the MTOC effectively aligns the IS with organelles involved in protein secretion and therefore is central to the synaptic release of lytic factors and cytokines.
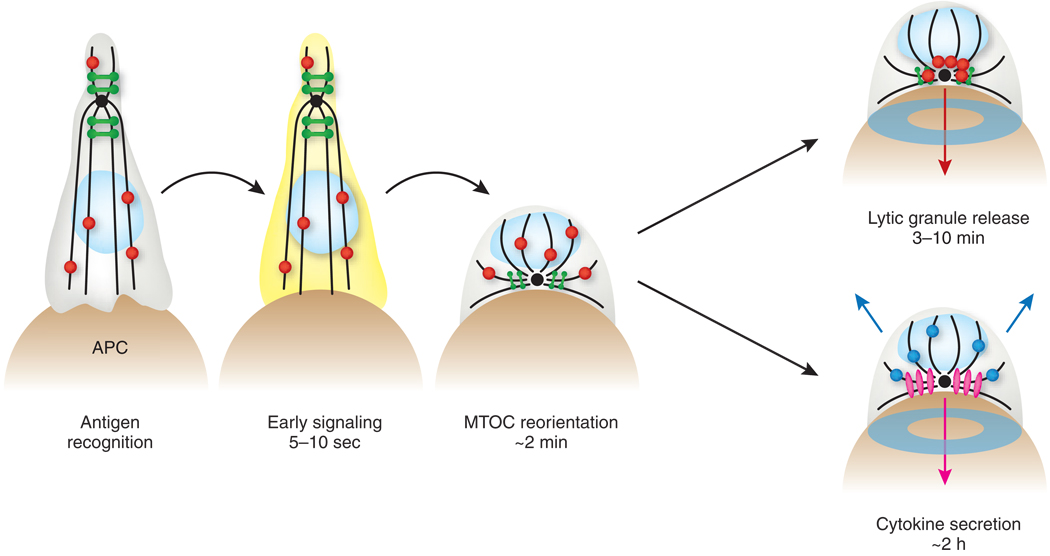
Figure 1.
The time course of T cell secretory responses. Antigen recognition triggers early signaling responses within 10 seconds, followed by MTOC reorientation, synapse formation and the release of lytic granules (for cytolytic cells) in less than 10 min (refs. 2,21,60). Cytokine secretion typically occurs hours after initial antigen recognition23. Beige, APC; gray, T cell; light blue oval, T cell nucleus; black lines, microtubules; black dots, MTOC; green, Golgi apparatus; red, lytic granules; pink and blue, cytokine-containing compartments destined for synaptic and multidirectional secretion, respectively; yellow cytoplasm, early signaling responses; turquoise ring, IS formation. Arrows indicate the directionality of secretion. Although lytic granule release is separated from cytokine secretion here, we do not mean to suggest that T cells exclusively either kill or secrete cytokines. Indeed, CTLs execute both of these effector functions in many conditions.
The molecular mechanisms that drive MTOC polarization are poorly understood. IS formation involves a burst of actin polymerization at the contact site and the subsequent activation of integrin-mediated adhesion1. It has been shown, however, that MTOC reorientation can be decoupled from both actin polymerization and integrin activation9. Instead, in T cells, the process seems dependent on TCR signaling itself. Early signaling effectors of the TCR, such as the kinases Zap70, Lck and Fyn, the adaptors Lat and SLP-76, and the guanine nucleotide-exchange factor Vav, are also required10–12. How these ‘upstream’ events are ‘translated’ into cytoskeletal polarization, however, remains obscure. Presumably, receptor-proximal signals are transduced to a set of cytoskeletal regulators, which in turn recruit effector molecules that carry out the requisite pushing, pulling and modification of intracellular structures.
Studies have identified several candidate regulators and effectors (only some of these proteins are discussed here; others are listed in Table 1). Cytoplasmic dynein, a negatively directed microtubule motor, is recruited to the IS through its interaction with the cytoskeletal adaptor molecule ADAP13. Knockdown of ADAP by means of small interfering RNA (siRNA) inhibits both recruitment of dynein and MTOC polarization in Jurkat cells, consistent with the involvement both proteins in the process. Given that microtubules are oriented with their ‘plus’ ends toward the cell membrane, a ‘negatively’ directed motor anchored at the IS could pull the MTOC toward it. This is a likely hypothesis, in particular because dynein has been linked to MTOC polarization in other cell types. It is suggested that the ADAP-dynein complex is recruited to the synapse through direct interaction between ADAP and SLP-76. However, knockdown of ADAP by siRNA in mouse T cells does not affect MTOC reorientation13, which suggests that other mechanisms exist for dynein recruitment.
Table 1.
Proteins linked to MTOC polarization and the release of lytic granules and cytokines
| Process | Gene product | Reference |
|---|---|---|
| MTOC polarization | ADAP | 13 |
| Dynein | 13 | |
| FMNL1, Dia1 | 14 | |
| IQGAP | 14 | |
| Rac, Cdc42 | 14,20 | |
| Pyk2 (CAKβ) | 57 | |
| Erk | 43,58 | |
| HDAC6 | 59 | |
| Lytic-granule release | Rab27a | 33 |
| AP-3 | 34 | |
| Munc13-4 | 39, 40 | |
| Syntaxin-11 | 41 | |
| Slp1, Slp2 | 42 | |
| Paxillin | 43 | |
| Protein kinase C-δ | 36 | |
| WIP | 37 | |
| Synaptic cytokine secretion | Rab3d, Rab19 | 23 |
| Syntaxin-4, SNAP23 | 46 | |
| Wiskott-Aldrich syndrome protein | 52 | |
| Multidirectional cytokine secretion | Syntaxin-6 | 23 |
The formin family of cytoskeletal regulators has also been linked to MTOC polarization14. Two family members, formin-like 1 (FMNL1) and diaphanous 1 (Dia1), are located in rings surrounding the MTOC in resting T cells and are also recruited to the IS after TCR stimulation. In addition, knockdown of either protein by siRNA inhibits MTOC reorientation. Formins induce the formation of linear actin bundles and are thought to be important for the protrusion of fillapodial structures15. However, they also are involved in the stabilization of microtubules at the cell membrane by interacting with several tip-binding proteins, including EB1 and the adenomatosis polyposis coli protein16. How either of these activities facilitates MTOC reorientation remains to be determined.
Dia1 interacts directly with IQGAP, a cytoskeletal regulator that binds to microtubule tips and is involved in linking them to the overlying actin cortex17,18. IQGAP is required for the localization of Dia1 to the leading edge of fibroblasts and also to phagocytic cups in macrophages, which suggests that it may regulate the spatiotemporal distribution of formin activity18. In T cells, knockdown of IQGAP by siRNA inhibits MTOC polarization, consistent with a function in formin localization14. However, immunocytochemistry studies of CTL synapses suggest that unlike formins, IQGAP is cleared from the IS as the MTOC approaches19. These discrepancies may reflect differences in the cell types or time points examined. Alternatively, it is possible that MTOC reorientation proceeds in defined stages that have not yet been rigorously defined. It has been argued that the MTOC is brought into close contact with the plasma membrane to facilitate the secretion of lytic factors19. It is possible that the reorientation of the MTOC to face the IS and the subsequent close apposition of the MTOC with the membrane are two distinct processes.
Other vexing issues remain. For example, although it is known that MTOC polarization requires higher intracellular calcium concentrations10, the specific mediators of the relevant calcium-signaling cascades are unknown. Notably, IQGAP binds to calcium-calmodulin, which provides a possible entry point for calcium signals17. Another issue concerns the respective functions of the Rho family GTPases. FMNL1 and Dia1 are directly regulated by Rho and Rac, and IQGAP binds to Cdc42 and Rac15,17. Although this indicates that Rho GTPases are involved in MTOC polarization, the precise nature of this involvement has been difficult to define. An early overexpression study suggested that Cdc42 is required for MTOC reorientation20. However, subsequent siRNA knockdown experiments have indicated that Cdc42 is dispensable and have assigned the dominant function to Rac14. Finally, it remains unclear precisely how all these molecules work together in the context of a dynamic polarization response. MTOC reorientation is typically studied by immunocytochemistry of fixed conjugates. This methodology has been sufficient to identify the candidates described above, in addition to several other molecules (Table 1). However, it is difficult to use this approach to delineate the process in detail sufficient to understand the mechanisms involved. Such studies will require live-imaging approaches that will allow monitoring of the MTOC polarization response with enhanced spatial and temporal resolution.
Synaptic secretion of lytic factors and cytokines
After MTOC polarization, both lytic factors and cytokines are secreted into the IS (Fig. 1). Different mechanisms are used, however, to transport each type of cargo. To a large extent, these differences reflect the kinetics of cargo production and the distinct organelles used for trafficking.
As CD8+ T cells mature into CTLs, cytolytic factors such as perforin and granzyme are compartmentalized into acidified secretory lysosomes commonly known as ‘lytic granules’. These granules are attached to the microtubule cytoskeleton and can be imaged in live cells with lysotracker dyes21. The recognition by a CTL of antigen on the surface of a target cell induces the formation of an IS and concomitant polarization of the MTOC toward the target. Almost immediately, lytic granules in the CTL begin to traffic toward the MTOC at the IS21. This phenomenon depends on ‘negatively’ directed microtubule motors19, although the specific motors involved remain to be defined.
Confocal imaging of fixed CTL–target cell conjugates has shown that individual lytic granules are extruded in a specific domain at the very center of the IS21. This secretory domain is located in the peripheral integrin ring and is adjacent to the central cluster of TCR molecules. The release of lytic granules into the synaptic cleft allows specific killing of the target cell by limiting the exposure of surrounding cells to cytotoxic granule contents. The entire process, which has been called the ‘kiss of death’22, is completed within minutes of TCR stimulation and typically does not exhaust the entire complement of lytic granules. Thus, the CTL can undergo repeated cycles of antigen recognition, polarization and cytolysis.
Cytokines, unlike lytic factors, are newly produced after TCR stimulation by either upregulation of gene transcription or translation of preexisting cytokine-encoding mRNA transcripts. As a result, the release of cytokines takes place on a time scale of hours rather than minutes. In T helper cells, synaptic secretion is facilitated by the positioning of the MTOC and Golgi apparatus at the IS. Immunocytochemistry of T helper–APC conjugates has shown that cytokines destined for release at the IS, including interleukin 2 (IL-2), interferon-γ (IFN-γ) and IL-10, accumulate exclusively in compartments tightly associated with the MTOC5,6,23. Polarization of intracellular IL-2 and IFN-γ to the IS of T helper cells has also been reported in vivo24,25.
In activated T helper cells, cytokine staining partially overlaps with the cis and medial Golgi marker GM130, suggestive of transit through the Golgi and into synaptically targeted vesicular compartments23. The precise identity of these compartments is unknown. A particularly intriguing possibility is that MTOC polarization enables the direct fusion of the trans-Golgi network with the synaptic membrane. In this case, cytokines would be released into the synapse by default and would require active sorting for secretion elsewhere.
Multidirectional secretion of cytokines and chemokines
Synaptic secretion from T cells is a likely idea because it provides a way to maintain antigen specificity during intracellular communication. For example, synaptically secreted IL-2 would ‘preferentially’ stimulate, by an autocrine mechanism, the proliferation of a T cell that had encountered cognate peptide–major histocompatibility complex on an APC. Similarly, synaptic IFN-γ and IL-10 would regulate the activities of macrophages and other ‘professional’ APCs in an antigen-specific way. However, there are circumstances in which unpolarized, multidirectional release of a soluble message (a ‘shout’) would be preferable to the private ‘whisper’ of synaptic secretion. For example, both the stimulation of a local inflammatory response and the recruitment of other cells at a distance would benefit from broad distribution of the relevant cytokine.
Data have shown that T cells are indeed capable of this sort of multidirectional secretion23,26 (Fig. 1). Whereas nascent IL-2 and IFN-γ accumulate in compartments closely associated with the IS in activated T helper cells, the inflammatory factor tumor necrosis factor (TNF) is distributed throughout the T helper cell cytoplasm in distinct vesicular structures23. Given the fact that TNF protein is synthesized anew after Golgi polarization to the IS, its appearance in the cytoplasm suggests that it is actively transported away from the synapse for secretion elsewhere. This idea has been confirmed by live imaging of TNF secretion23. TNF is synthesized as a transmembrane trimer that is released from the plasma membrane by digestion with metalloproteases. In the presence of metalloprotease inhibitors, TNF is retained on the cell surface and can be detected in live cells with fluorescence-conjugated antibodies. Imaging experiments using this approach have demonstrated that TNF is secreted multidirectionally from T cells with no synaptic bias. Indeed, additional experiments with functional cellular arrays have suggested that TNF secretion may even be excluded from the IS in some cases26.
That same multidirectional pathway is also used in T helper cells for the secretion of chemokines such as MIP1-α and RANTES23. This makes sense, given that chemokines act to recruit cells at a distance. Indeed, it seems likely that the multidirectional secretion pathway is designed specifically to promote inflammation and chemotaxis. In this context, it is somewhat unexpected that IL-4 also uses this pathway for secretion23. It is possible that multidirectional release of IL-4 in the context of a lymph node is important for reestablishing a cytokine milieu that promotes the differentiation of T helper type 2 effector cells. Further analysis will be required to confirm this hypothesis.
Mechanism of lytic granule release
In eukaryotic cells, the trafficking of vesicular compartments to specific subcellular locations is mediated by distinct families of proteins with evolutionarily conserved functions (Fig. 2). At the heart of this machinery are the ‘SNARE’ proteins (soluble N-ethylmaleimide–sensitive factor accessory protein receptors), which are helical transmembrane proteins found in either target membranes (t-SNARE proteins) or vesicles (v-SNARE proteins)27. In the appropriate circumstances, v-SNARE and t-SNARE proteins form hetero-oligomers of coiled coils that promote vesicle fusion. Different v-SNARE and t-SNARE proteins localize to distinct subcellular compartments, and there is evidence that they interact with a fair amount of specificity28. SNARE interactions are regulated by a diverse group of accessory proteins, which impart an additional layer of specificity to the trafficking process. In neurons, for example, the SNARE-binding protein Munc13-1 facilitates the interaction of v-SNARE and t-SNARE proteins at the pre-synaptic membrane29. Vesicle fusion in neuronal synapses is also regulated by synaptotagmin, which couples calcium influx to the formation of SNARE complexes30. Particularly important for the specification of intracellular traffic is the Rab family of small GTPases31. Rab proteins associate with many vesicular structures by virtue of post-translational prenylation at their carboxyl termini. They function by recruiting various effector molecules and tethering complexes that specify the identity, and hence the trafficking activity, of the underlying vesicle. Over 60 Rab proteins are expressed in higher mammals, and each defines a distinct pool of vesicles.
Figure 2.
Secretion of vesicles from cells. The secretion of synaptic vesicles at the neuronal synapse (left) is compared with what is known about the secretion of lytic granules (center) and synaptic and multidirectional cytokines (right) from activated T cells. Fusion of synaptic vesicles at the neuronal synapse is driven by a SNARE complex composed of SNAP25, syntaxin 1 (Synt1) and VAMP. Rab3a participates in vesicle docking, whereas Munc13-1 is important for the priming of syntaxin-1. Synaptotagmin (Syt) couples vesicle fusion to the influx of calcium. In general, far less is known about the mechanisms of secretion from the T cell IS. The presentation here of certain molecules in the IS in positions that are functionally analogous to their structural homologs in the neuronal synapse is speculative and is merely meant to suggest a possible model. The synaptic membrane is located at the bottom of each cell. Light blue, T cell nuclei; black lines, microtubules; black dots, MTOC; dark green, Golgi apparatus.
Studies have identified several trafficking proteins important for the release of lytic granules from CTLs (Table 1). A particularly productive approach has been the analysis of genetic disorders that combine immunodeficiency with albinism. Both CTLs and melanocytes secrete secretory lysosomes (lytic granules and melanosomes, respectively), and at least some of the trafficking machinery is probably conserved. Griscelli syndrome, which is characterized by albinism and defects in the cytotoxicity of CTLs and natural killer cells, is caused by the functional loss of Rab27a32. In T cells derived from Rab27a-deficient mice, lytic granules polarize toward the IS in response to TCR stimulation but fail to dock33. Consistent with involvement in docking, the amount of Rab27a associated with lytic granules seems to increase as they approach the IS. Notably, in CTLs missing the geranylgeranyl transferase required for Rab prenylation, lytic granules fail to polarize, which suggests that other Rab proteins are involved in earlier phases of granule trafficking33. Hermansky-Pudlak syndrome type 2 also combines immunodeficiency with albinism and results from mutations in the gene encoding adaptor protein 3 (AP-3)34. AP-3 has been linked to the sorting of lysosomal cargo in many cell types35. AP-3-deficient CTLs have enlarged lytic granules that do not polarize to the IS, which suggests involvement of AP-3 in directional trafficking34. Of note, a similar defect in granule polarization has been reported in CTLs lacking protein kinase C-δ36 and also in natural killer cells deficient in the cytoskeletal adaptor protein WIP37. Whether protein kinase C-δ and WIP work in concert with AP-3 or are involved in a distinct step during translocation remains to be seen.
Familial hemophagocytic lymphohistiocytosis (FHL) represents another set of genetic disorders associated with secretory defects in CTLs but not melanocytes38. Killer lymphocytes from patients with FHL are deficient in their ability to deliver perforin to the IS. FHL type 2 is caused by a loss of perforin itself38. FHL type 3, in contrast, results from mutations in the gene encoding Munc13-4, a homolog of Munc13-1 (ref. 39). In CTLs derived from patients with FHL type 3, lytic granules dock at the IS but fail to fuse. Munc 13-4 interacts with Rab27a and localizes to lytic granules39,40. These data collectively suggest that Munc13-4 acts ‘down-stream’ of Rab27a in the granule secretion process. FHL type 4 (FHL4), which is clinically similar to but less severe than FHL type 3, is caused by defects in syntaxin-11 (ref. 41), a SNARE protein. This deficiency in syntaxin-11 impairs granule exocytosis without affecting polarization, which suggests an important function ‘downstream’ of the recruitment of granules to the IS.
The functional relevance of each of those proteins for cytolysis is now well established. Determining how they contribute mechanistically to the trafficking and secretion of lytic granules will be the next important challenge. It can be speculated that proteins such as Rab27a and Munc13-4 function at positions in the secretory pathway analogous to those of their structural homologs in neurons (Fig. 2). However, it remains to be determined whether this is indeed the case. In vitro systems able to distinguish the many component steps of granule exocytosis will be needed to resolve this issue. The identification of interacting proteins and additional required molecules will also be instrumental. Of note, a study has found that Rab27a interacts with the synaptotagmin-like proteins Slp1 and Slp2, which could both be involved in the docking of granules at the IS42. Another important area of investigation is the regulation of granule trafficking by intracellular signaling pathways. In T cells, it is known that both calcium signaling and activity of the kinase Erk are required for efficient degranulation43,44. The calcium-regulated proteins required for the secretion of lytic granules have not been defined. For the most part, the relevant Erk substrates also remain elusive. A good candidate is paxillin, a cytoskeletal scaffolding protein that has been studied as a component of focal adhesions45. Paxillin is associated with the MTOC in resting T cells, but after antigen recognition, it undergoes Erk-dependent phosphorylation and transits to the IS43. Further studies will be needed to determine the functional importance of this activity.
The molecular basis of cytokine secretion
The molecular mechanisms that govern the secretion of cytokines from T cells are for the most part poorly understood. However, several laboratories have begun to investigate the distribution of various trafficking proteins in activated T helper cells; this has provided a list of potential effectors for both synaptic and multidirectional secretion (Table 1 and Fig. 2). Rab3d and Rab19 both localize together with intracellular IL-2 at the IS, whereas the SNARE protein syntaxin-6 associates with cytoplasmic vesicles containing TNF23. Notably, neither IL-2 nor TNF localizes together with Rab27a23. These results collectively indicate that the synaptic and multidirectional secretion pathways for cytokines are molecularly distinct from each other and also from the lytic granule–secretion pathway in CTLs. In addition, it has been shown that syntaxin-4 and SNAP-23, two plasma membrane t-SNARE proteins, accumulate at the T cell–APC interface, which provides a potential basis for selective vesicle fusion at the IS46.
Although the intracellular distribution of each of those proteins is intriguing, their functional relevance still must be assessed through loss-of-function experiments. Rab3d, SNAP-23 and syntaxin-4 are homologs of Rab3a, SNAP-25 and syntaxin-1, which mediate the release of neurotransmitters at neuronal synapses27. Notably, syntaxin-4 specifically ‘marks’ the basolateral plasma membrane in epithelial cells, which is suggestive of a function in polarized secretion47. Syntaxin-6 is involved in post-Golgi trafficking in many cell types, including neutrophils and PC12 cells, a rat neuronal-line cell line48.
In macrophages, TNF is secreted by a pathway through recycling endosomes that requires syntaxin-6, syntaxin-4 and SNAP-23 (refs. 49–51). The extent to which this pathway is conserved in T cells remains to be determined. The fact that syntaxin-4 and SNAP-23 are localized to the T cell IS, whereas TNF is released multidirectionally, suggests an alternative mechanism23,46. However, intracellular TNF partially localizes together with the endosomal marker transferrin receptor in T cells, which suggests that certain elements of the macrophage pathway may be conserved23.
In addition to trafficking proteins, cytoskeletal regulators undoubtedly are important in polarized cytokine secretion. It has been shown that T cells lacking the Wiskott-Aldrich syndrome protein are unable to secrete IFN-γ but remain able to release chemokines52. Immunocytochemical analysis of these cells has shown that IFN-γ is trapped inside intracellular compartments that are poorly localized to the synapse. These results suggest that the synaptic and multidirectional cytokine-secretion pathways place different demands on the cytoskeleton, which further reinforces the idea that they are molecularly distinct processes.
Future directions
It is now apparent that T cells have many directionally and molecularly distinct secretory pathways, similar to those in more permanently polarized cells types such as neurons. Although the identification of some of the required signaling molecules and trafficking proteins has set the stage for enhanced mechanistic understanding of these processes, there is much left to discover. For example, the precise functions of individual Rab and SNARE molecules in the specification of directional traffic remain unclear. Furthermore, the molecules that drive vesicular transport along microtubules are for the most part unknown. The identification of relevant Rab effectors, tethering proteins and molecular motors will no doubt contribute to progress in these areas, as will improved methodology for the imaging of vesicle dynamics in live T cells.
It will also be important to determine how cytokines are sorted into distinct trafficking compartments. Studies of model secretory systems have identified a variety of sorting mechanisms53. Most involve the recognition of some sort of targeting feature, such as an amino- or carboxy-terminal peptide motif, a signal ‘patch’ in the tertiary structure of the protein or a specific glycosyl modification. Identifying these molecular determinants contained in the individual cytokine molecules, as well as the proteins that mediate their recognition, will probably be a challenging task.
The problem is further complicated by emerging evidence that cytokines and other soluble factors can be targeted in different ways by different lymphocyte cell types. For example, a study has suggested that RANTES, which is released multidirectionally by T helper cells, is secreted synaptically by CTLs54. Furthermore, experiments with functional cellular arrays have shown that IL-10, which is secreted synaptically by T helper cells, is released multidirectionally by B cells. Similarly, IFN-γ, which is targeted to the IS in T helper cells, is released both synaptically and multidirectionally by natural killer cells (Z. Chai and M.M.D., unpublished data). Indeed, it is possible that the directionality of secretion even in the same cell type is modulated by the developmental stage and the strength of stimulation. Future studies will no doubt address these issues.
Improved molecular understanding of polarized secretion would almost certainly facilitate in vivo experiments designed to probe the relevance of these processes for immune function. Proteins required for synaptic or multidirectional secretion could be altered to selectively ablate each pathway or perhaps even to change their polarity. In this way, the value of directional secretion in a specific biological context could be assessed directly. It would be useful to determine, for example, whether synaptic secretion actually maintains antigen specificity during adaptive immune responses and whether multidirectional secretion actually facilitates chemokine function.
Extending the study of polarized secretion in vivo will require the development of methodology to image protein release from T cells in target organs. It will be particularly important to be able to visualize extracellular pools of secreted protein. For example, immunohistochemistry has been used to visualize chemokine gradients in lymph nodes55. This sort of analysis will be instrumental for defining the scope of action of a specific secreted molecule as well as the effects of trafficking perturbations. Knowledge of the extracellular distribution of a secreted factor will probably have therapeutic implications as well. The ability to target a secreted molecule with drugs is probably strongly influenced by its in vivo accessibility. Consistent with that idea, TNF, a cytokine that is released multidirectionally, can be neutralized effectively with antibody-based therapy56.
Polarized secretion adds another layer of complexity to intercellular communication between cells of the immune system. Secretory responses are not merely a function of which molecules are made but also a function of how those molecules are secreted. The coming years should bring further insights into how the immune system uses these different modes of communication and how this flexibility modulates immune function.
ACKNOWLEDGMENTS
We thank Z. Chai for discussions. Supported by the National Institutes of Health (AI057229 to M.M.D.) and the Howard Hughes Medical Institute (M.M.D.).
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Bromley SK, et al. The immunological synapse. Annu. Rev. Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 2.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 3.Boisvert J, Edmondson S, Krummel MF. Immunological synapse formation licenses CD40–CD40L accumulations at T-APC contact sites. J. Immunol. 2004;173:3647–3652. doi: 10.4049/jimmunol.173.6.3647. [DOI] [PubMed] [Google Scholar]
- 4.Poo WJ, Conrad L, Janeway CA., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988;332:378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- 5.Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl. Acad. Sci. USA. 1991;88:775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupfer H, Monks CR, Kupfer A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J. Exp. Med. 1994;179:1507–1515. doi: 10.1084/jem.179.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J. Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc. Natl. Acad. Sci. USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedwick CE, et al. TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J. Immunol. 1999;162:1367–1375. [PubMed] [Google Scholar]
- 10.Kuhne MR, et al. Linker for activation of T cells, ζ-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J. Immunol. 2003;171:860–866. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Cofreces NB, et al. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J. Immunol. 2006;176:4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- 12.Ardouin L, et al. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur. J. Immunol. 2003;33:790–797. doi: 10.1002/eji.200323858. [DOI] [PubMed] [Google Scholar]
- 13.Combs J, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl. Acad. Sci. USA. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez TS, et al. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faix J, Grosse R. Staying in shape with formins. Dev. Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Wen Y, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 17.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandt DT, et al. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 20.Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl. Acad. Sci. USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 22.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 23.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 24.Barcia C, et al. In vivo polarization of IFN-gamma at Kupfer and non-Kupfer immunological synapses during the clearance of virally infected brain cells. J. Immunol. 2008;180:1344–1352. doi: 10.4049/jimmunol.180.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichert P, Reinhardt RL, Ingulli E, Jenkins MK. Cutting edge: in vivo identification of TCR redistribution and polarized IL-2 production by naive CD4 T cells. J. Immunol. 2001;166:4278–4281. doi: 10.4049/jimmunol.166.7.4278. [DOI] [PubMed] [Google Scholar]
- 26.Chen DS, et al. Marked differences in human melanoma antigen-specific T cell responsiveness after vaccination using a functional microarray. PLoS Med. 2005;2:e265. doi: 10.1371/journal.pmed.0020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 28.Parlati F, et al. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 30.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 31.Zerial M, McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 32.Menasche G, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 33.Stinchcombe JC, et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark RH, et al. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat. Immunol. 2003;4:1111–1120. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 35.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 36.Ma JS, et al. Protein kinase Cδ regulates antigen receptor-induced lytic granule polarization in mouse CD8+ CTL. J. Immunol. 2007;178:7814–7821. doi: 10.4049/jimmunol.178.12.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krzewski K, Chen X, Strominger JL. WIP is essential for lytic granule polarization and NK cell cytotoxicity. Proc. Natl. Acad. Sci. 2008;105:2568–2573. doi: 10.1073/pnas.0711593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 39.Feldmann J, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 40.Neeft M, et al. Munc13-4 is an effector of Rab27a and controls secretion of lysosomes in hematopoietic cells. Mol. Biol. Cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryceson YT, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holt O, et al. Slp1 and Slp2-a localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic. 2008;9:446–457. doi: 10.1111/j.1600-0854.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson LK, Mireau LR, Ostergaard HL. A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J. Immunol. 2005;175:8138–8145. doi: 10.4049/jimmunol.175.12.8138. [DOI] [PubMed] [Google Scholar]
- 44.Takayama H, Sitkovsky MV. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J. Exp. Med. 1987;166:725–743. doi: 10.1084/jem.166.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 46.Das V, et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 47.Kreitzer G, et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat. Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- 48.Wendler F, Tooze S. Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic. 2001;2:606–611. doi: 10.1034/j.1600-0854.2001.20903.x. [DOI] [PubMed] [Google Scholar]
- 49.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 50.Murray RZ, Wylie FG, Khromykh T, Hume DA, Stow JL. Syntaxin 6 and Vti1b form a novel SNARE complex, which is up-regulated in activated macrophages to facilitate exocytosis of tumor necrosis factor-α. J. Biol. Chem. 2005;280:10478–10483. doi: 10.1074/jbc.M414420200. [DOI] [PubMed] [Google Scholar]
- 51.Pagan JK, et al. The t-SNARE syntaxin 4 is regulated during macrophage activation to function in membrane traffic and cytokine secretion. Curr. Biol. 2003;13:156–160. doi: 10.1016/s0960-9822(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 52.Morales-Tirado V, et al. Cutting edge: selective requirement for the Wiskott-Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J. Immunol. 2004;173:726–730. doi: 10.4049/jimmunol.173.2.726. [DOI] [PubMed] [Google Scholar]
- 53.Alberts B, et al. Molecular Biology of the Cell. 5th edn. New York: Taylor and Francis; 2007. pp. 711–767. [Google Scholar]
- 54.Catalfamo M, et al. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TCR stimulation. Immunity. 2004;20:219–230. doi: 10.1016/s1074-7613(04)00027-5. [DOI] [PubMed] [Google Scholar]
- 55.Okada T, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005 doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor PC, Williams RO, Feldmann M. Tumour necrosis factor α as a therapeutic target for immune-mediated inflammatory diseases. Curr. Opin. Biotechnol. 2004;15:557–563. doi: 10.1016/j.copbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Sancho D, et al. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J. Cell Biol. 2000;149:1249–1262. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X, et al. CD28-stimulated ER K2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc. Natl. Acad. Sci. USA. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serrador JM, et al. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20:417–428. doi: 10.1016/s1074-7613(04)00078-0. [DOI] [PubMed] [Google Scholar]
- 60.Huse M, et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]