Abstract
Background
Rapid growth in prescription drug costs has compelled insurers to require increased patient cost-sharing.
Objective
The aim of this study was to compare the effects of 2 recent cost-sharing policies on emergency hospitalizations due to chronic obstructive pulmonary disease, asthma, or emphysema (CAE), and on physician visits.
Methods
We analyzed data from a large-scale natural experiment in British Columbia (BC), Canada. The cost-sharing policies were a fixed copayment policy (fixed copay policy) and an income-based deductible (IBD) policy with 25% coinsurance (IBD policy). Prescription, physician billing, and hospitalization records were obtained from the BC Ministry of Health. From the total population of BC residents ≥65 years of age, we extracted data from all patients dispensed an inhaled corticosteroid, β2-agonist, or anticholinergic from June 30, 1997, to April 30, 2004. Poisson regression was used to evaluate the impact of the policies in a cohort of patients receiving long-term inhaler treatment. An identically defined historical control group unaffected by the policy changes was used for comparison.
Results
The study population included 37,320 users of long-term inhaled medications from the BC population of 576,000 persons ≥65 years of age. During the IBD period but not the fixed copay period, emergency hospitalizations for CAE increased 41% (95% CI for adjusted rate ratio [RR], 1.24–1.60) in patients ≥65 years of age. There was also a significant increase in physician visits of 3% (95% CI for adjusted RR, 1.01–1.05). No significant increases were observed during the fixed copay period. In a secondary analysis using a concurrent control group, we estimated a smaller but significant increase in emergency CAE hospitalizations of 29% (95% CI for adjusted RR, 1.09–1.52). This analysis also showed increases in physician visits (fixed copay period RR, 1.03 [95% CI for adjusted RR, 1.01–1.05]; IBD period RR, 1.07 [95% CI for adjusted RR, 1.05–1.08]).
Conclusion
The results suggest that the IBD policy was likely associated with an increased risk for emergency hospitalization and physician visits in these users of inhaled medications who were aged ≥65 years.
Keywords: inhaled medications, asthma, COPD, health care utilization, health outcomes, drug benefit plans, health services research, pharmacoeconomics
INTRODUCTION
Drug benefit plans have developed a variety of policies, such as tiered copayments and deductibles, to encourage more efficient use of limited drug budgets.1 However, rigorous published evaluations of the clinical effects of these polices are few in number, and there are no available evaluations comparing multiple policies within the same elderly population. Available evidence suggests that inflexible policies, such as prescription caps, high premiums, and deductibles, can lead to undesirable consequences (in some cases, higher rates of hospitalization and long-term care facility admissions).2-4 However, cost-sharing5-8 and delisting9 policies can yield savings without adverse outcomes when there are generous exemptions. The effects of 3-tier copayment policies are debatable.10-12
British Columbia (BC), Canada, has provided a large-scale natural experiment of a sequence of drug benefit policies in an older population (≥65 years of age) within a 5-year period. Prior to January 2002, older residents received full coverage of all prescription ingredient costs. In January 2002, a fixed prescription drug copayment policy (fixed copay policy) for older residents was implemented. In May 2003, the fixed copay policy was replaced with an income-based deductible plus 25% coinsurance policy named Fair PharmaCare (IBD policy).
The shift from full coverage to the fixed copay and IBD policies created a financial incentive for patients to consider cost as a factor in their prescription decisions. Older patients with chronic obstructive pulmonary disease (COPD) who reduced their use of inhalers may have been at increased risk for adverse health outcomes such as hospitalization and exacerbations, a reasonable expectation in light of evidence that use of inhaled anticholinergics reduces the risk for hospitalization, exacerbation, and respiratory-related death in patients with COPD.13 There is also evidence that long-acting β2-agonists (LABAs) and inhaled corticosteroids reduce COPD exacerbations.14-16 The costliness of inhalers, particularly LABAs and corticosteroids, and the potential for short-term adverse outcomes made patients with COPD an ideal high-risk group in which to study the early adverse effects of the policy changes.
In a previous report, we found that older patients decreased their use of inhalers during the fixed copay and IBD policy periods. Overall, inhaled corticosteroid use during the combined fixed copay and IBD policy periods was 12.3% lower than predicted from a linear regression on 5 years of prepolicy data. Inhaled anticholinergic use was 12.2% lower than predicted, and inhaled β2-agonist use (LABA and short-acting β-agonist [SABA]) was lower by 5.8%.17 Virtually none of the decrease in inhaler use could be explained by increases in the use of other drugs, such as leukotriene antagonists. Estimated decreases were greater, but not statistically significantly so, under the IBD policy than the fixed copay policy. In this analysis, we measured the association of the drug policy changes on COPD-related emergency hospitalizations and physician visits in the same population.
The aim of this study was to compare the effects of the 2 recent cost-sharing policies on emergency hospitalizations due to COPD, asthma, or emphysema (CAE), and on physician visits.
MATERIALS AND METHODS
The study protocol was approved by the ethics committees at the Brigham & Women’s Hospital, Boston, Massachusetts, and the University of Victoria, Victoria, British Columbia, Canada. The BC Ministry of Health Services and the BC College of Pharmacists PharmaNet Committee approved data access.
Data Collection
All prescriptions dispensed at community pharmacies in BC were obtained from the PharmaNet database, a province-wide electronic pharmacy network. These records were linked using an encrypted personal health number to the BC Ministry of Health administrative databases on physician services and hospitalizations, which included International Classification of Diseases, Ninth Revision, Clinical Modification18 or Tenth Revision19 (ICD-9-CM or ICD-10) – coded diagnoses (up to 25 discharge diagnoses) with dates of service or admission. The completeness and misclassification of diagnostic coding has been reported similar to other administrative databases.20-24
Exposure
Residents of BC ≥65 years of age had full coverage for prescription ingredient costs until December 31, 2001, for drugs listed on the provincial formulary. We defined 3 exposure levels denoted by the preexisting full coverage policy and 2 subsequent policy changes—fixed copay and IBD. A fixed copayment of Can $25 per prescription for seniors ($10 for seniors with incomes <$22,000/y) started on January 1, 2002, and lasted until April 30, 2003. The IBD policy, implemented on May 1, 2003, had 3 components: (1) family deductible of 0% to 2% based on family income; (2) a coinsurance payment of 25% for prescriptions after passing the deductible; and (3) an out-of-pocket ceiling equal to 1.25%, 2%, or 3% of income. Families were responsible for all drug costs under their deductible but may have had supplemental private insurance. The coinsurance rate was the portion of a prescription a family continued to pay after passing its deductible but before reaching its ceiling. Pharma-Care covered all costs above a family’s ceiling.25 For example, older families with net incomes of $47,500 to $50,000 had a deductible of $500, paid 25% coinsurance on prescriptions from $501 to $1000 in out-of-pocket charges, and then paid zero thereafter. Longterm care facility residents were exempt from both policies and continued to receive full coverage.
Outcomes
The primary outcome was emergency hospitalization with a combined primary reason for admission of COPD (ICD-9-CM codes 491, 492, and 49626; ICD-10 codes J41–44), bronchitis (ICD-9-CM code 490; ICD-10 code J40), asthma (ICD-9-CM code 493; ICD-10 code J45), or emphysema (ICD-9-CM code 492; ICD-10 code J43). The loss of disease specificity from combining COPD, asthma, and emphysema into a single outcome (CAE) was necessary to eliminate bias caused by a switch in diagnostic coding from ICD-9-CM to ICD-10 that occurred in hospital records starting on April 1, 2001. Secondary outcomes were emergency hospital admissions for any reason and number of physician visits. Multiple physician visits by a single patient on the same day were counted as 1 visit.
Study Population
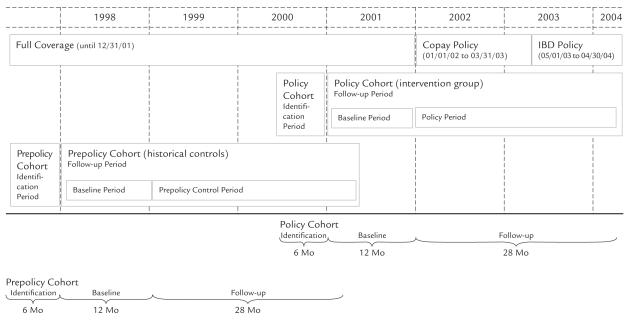
The source population included all BC residents ≥65 years of age and not living in a long-term care facility (576,000 in 2004). We used a cohort design with a historical control group to estimate changes in our primary and secondary outcomes before and after the policy changes. The policy intervention group included long-term inhaler users ≥65 years of age at the study baseline date of January 1, 2001 (1 year before fixed copay policy implementation). We defined longterm inhaler users as those who received ≥3 prescriptions for an inhaler within 6 months prior to baseline, with ≥1 prescription between 4 and 6 months prior to baseline, and ≥1 prescription between 1 and 3 months prior to baseline (Figure 1). Follow-up began on January 1, 2001, and ended on April 30, 2004, the end of medical services coverage, entry into a long-term care facility, or death, whichever came first. The control group was defined historically using an identical cohort definition, but with a baseline date of January 1, 1998, and a follow-up end date of April 30, 2001. The historical controls received full coverage for prescription ingredient costs during the entire follow-up period. The policy intervention group and historical controls together formed the study population.
Figure 1.
Cohort identification and follow-up periods. Prior to January 1, 2002, patients ≥65 years of age received full ingredient cost coverage from the province for eligible drugs. Patients were responsible for dispensing fees. From January 1, 2002, to April 30, 2003, low-income elderly patients paid $10 per prescription for the first 20 prescriptions of the year, and other elderly patients paid $25 per prescription for the first 11 prescriptions. Starting in May 2003, elderly patients had a deductible equal to 0%, 1%, or 2% of their income, after which they paid 25% of all prescription costs until reaching their out-of-pocket ceiling. In the first 8 months of the income-based deductible (IBD) policy, seniors were allowed to carry over out-of-pocket expenditures from the first 4 months of the year, which effectively reduced the IBD that year. Patients started at zero again in January 2004.
Cohort Study of Health Services Outcomes—Primary Analysis
The patient was the unit of analysis. For each calendar month we measured the number of days of follow-up and the number of CAE emergency hospitalizations. The monthly rates of CAE emergency hospitalizations in the intervention and control groups were estimated with Poisson regressions that used generalized estimating equations to adjust SEs for correlations between repeated observations.27 Because of the delay between changes in inhaler use and events, an autoregressive covariance structure with lags of 1 month was adopted.28 One regression compared the fixed copay policy exposure level to the full coverage exposure level. Another regression compared the IBD exposure level to the full coverage level. Monthly rates of all-cause emergency admissions and physician visits were estimated in separate Poisson regressions.
Six models were estimated for the primary analysis, one for each outcome and policy change. Predictors and potential confounders included in the regressions were cohort status (policy or control), sex, income status (household adjusted annual income derived for a limited number of categories defined through premium subsidy information29: high = >$22,000; moderate = $16,000 to $22,000; low = <$16,000); admission to hospital 6 months prior to baseline, quartile of physician use 6 months prior to baseline, and Romano comorbidity score (meant to adjust for confounding by concomitant illnesses by assigning weights to a patient’s ICD-9-CM diagnoses and summing those weights into a single score),30 and the numbers of nonasthma and asthma medications dispensed 6 months prior to baseline. Age grouping and Romano comorbidity score were time-varying covariates updated every 3 months.
Secondary Analysis of Health Services Outcomes (Concurrent Controls)
To determine whether the analysis was robust to bias from the diagnostic coding change in 2000, secular changes in treatment (eg, increased use of combination inhalers and LABAs), and other unknown cointerventions, a second cohort analysis was conducted with concurrent controls. The cohort included patients ≥65 years of age who were long-term inhaler users and a concurrent control group of 23,894 long-term inhaler users 55 to <65 years of age. The younger controls were not adversely affected by the fixed copay policy (because they were too young) or by the IBD policy (because PharmaCare was already paying nothing toward their drug costs). As with the primary analysis, the study design included a baseline period to adjust for baseline differences between the intervention and control groups.
Follow-up for each patient began on the first day of the month immediately following the month he or she first became a long-term inhaler user, according to the same definition used in the primary analysis. Patients were excluded from the control group if they were dispensed >$800 of prescription drugs in the previous calendar year. This restriction was used to ensure that patients in the control group did not have coverage under either policy and were therefore not affected by the policy change.
Poisson regressions were estimated for each of the study outcomes (3 regressions). The regressions included the same covariates as in the primary analysis, except the policy changes were estimated using policy-by-age group interaction variables. These interaction variables were interpreted as the effect of the policy changes adjusted for the other covariates in the regression, and for the baseline difference between the intervention and control groups.
Association Between Discontinuation of Inhalers and Emergency Hospitalization for Chronic Obstructive Pulmonary Disease, Asthma, or Emphysema
We used a nested case–control analysis to analyze the association between discontinuation of inhaler use and emergency CAE admissions. The study was nested in a cohort of long-term users ≥65 years of age. Longterm inhaler use was defined as in the previously mentioned analyses, and follow-up for each patient began on the first day of the month following the date he or she became a long-term user. The study period was January 1, 2000, to April 30, 2004, which included 12 months of full coverage, 16 months of the fixed copay policy coverage, and 12 months of the IBD policy coverage. The cases were cohort members who were admitted to the hospital for a CAE emergency for the first time during follow-up. The controls for each case were cohort members at risk for an emergency CAE admission in the same month and were randomly sampled at a ratio of 10 controls to each case. Cohort members were not eligible to be cases or controls if they had <90 days of prior followup time or a prior hospital admission during followup. Cases were assigned an index date equal to their admission date, and controls were assigned an index date of mid-month.
Patients were classified as stoppers if they discontinued use of their inhaled corticosteroid, β2-agonist, or anticholinergic treatment within 90 days preceding their index date and did not obtain additional medication within 60 days of the estimated date on which their most recent prescription was depleted.17 We used multivariate logistic regression to estimate odds ratios (ORs) and 95% CIs for the association between discontinuation of inhaler use and emergency CAE hospitalization during fixed copay and IBD policy coverage, adjusted for the baseline association between discontinuation and emergency CAE admission during full coverage. Other covariates included in the regression were the same as those used in the primary analysis.
RESULTS
Baseline characteristics of the cohort members in the primary analysis are shown in Table I. The policy group and the historical controls were well balanced on most factors. However, the historical controls were less likely to have used LABAs compared with SABAs.
Table I.
Baseline characteristics of elderly (aged ≥65 years) long-term users of inhaled medications in British Columbia (N = 37,320). Values are no. (%) of patients unless otherwise specified.
| Characteristic | Policy Group Cohort (January 1, 2001) (n = 19,985) |
Prepolicy Group Cohort (January 1, 1998) (n = 17,335) |
|---|---|---|
| Age group | ||
| 66–70 y | 5852 (29) | 5451 (31) |
| >70–75 y | 5349 (27) | 4631 (27) |
| >75–80 y | 4645 (23) | 3758 (22) |
| >80 y | 4139 (21) | 3495 (20) |
| Sex | ||
| Female | 10,566 (53) | 9121 (53) |
| Male | 9419 (47) | 8214 (47) |
| Annual income* | ||
| High (Can >$22,000) | 11,729 (59) | 9703 (56) |
| Moderate (>$16,000–$22,000) | 2922 (15) | 2764 (16) |
| Low ($0–$16,000) | 5334 (27) | 4868 (28) |
| Emergency hospitalizations† | ||
| CAE | ||
| 1 | 649 (3) | 651 (4) |
| 2 | 122 (1) | 107 (1) |
| ≥3 | 36 (0) | 27 (0) |
| Any reason | ||
| 1 | 2268 (11) | 2126 (12) |
| 2 | 558 (3) | 574 (3) |
| ≥3 | 226 (1) | 218 (1) |
| Physician visits† | ||
| 0–4 | 4610 (23) | 4439 (26) |
| ≥5 | 15,375 (77) | 12,896 (74) |
| Romano comorbidity score, mean (SD)† | 0.91 (1.2) | 0.94 (1.2) |
| Respiratory disease grouping*‡ | ||
| COPD | 3553 (18) | 3059 (18) |
| Asthma | 3422 (17) | 3199 (18) |
| Mixed COPD/asthma | 1913 (10) | 1783 (10) |
| Other | 11,097 (56) | 9294 (54) |
| Drug utilization† | ||
| Nonasthma/COPD drugs | 18,977 (95) | 16,255 (94) |
| No. of drugs, mean | 6.5 | 5.9 |
| Asthma/COPD drugs§ | ||
| Inhaled corticosteroids | 15,024 (75) | 12,897 (74) |
| Inhaled SABAs | 14,855 (74) | 13,977 (81) |
| Inhaled anticholinergics | 10,027 (50) | 8322 (48) |
| Inhaled LABAs | 5174 (26) | 1585 (9) |
| Xanthines | 2033 (10) | 2400 (14) |
| Leukotriene antagonists | 819 (4) | 497 (3) |
CAE = chronic obstructive pulmonary disease (COPD), asthma, or emphysema; SABAs = short-acting β2-agonists; LABAs = long-acting β2-agonists.
Percentages may not total 100% due to rounding.
Utilization 6 months prior to follow-up.
To be classified as having CAE, patients were required to have ≥3 physician visits 6 months prior to baseline with a diagnosis of CAE (International Classification of Diseases, Ninth Revision codes 491, 492, 493, 496), with ≥75% of those physician visits being for CAE. Patients with ≥3 visits for CAE but with no disease accounting for ≥75% of visits were classified as mixed COPD/asthma. All remaining patients were classified as “other.”
Monotherapy or combination preparations.
Emergency Hospitalization
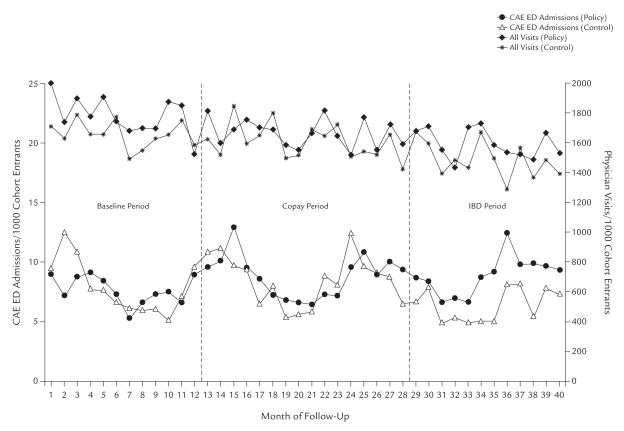
Rates of emergency CAE admissions in the primary analysis cohort are shown in Figure 2. During fixed copay policy coverage, the rate of emergency CAE admissions was not significantly different adjusted for before- versus after-changes in the historical control group (adjusted rate ratio [RR], 1.08; 95% CI, 0.97–1.19) (Table II). A small but statistically significant decrease in emergency admissions for any reason was observed in the primary analysis (adjusted RR, 0.91; 95% CI, 0.86–0.96), but no change was observed in the secondary analysis that used concurrent controls (adjusted RR, 1.04; 95% CI, 0.96–1.11) (Table III).
Figure 2.
Monthly emergency department (ED) admissions for chronic obstructive pulmonary disease, asthma, or emphysema (CAE), and monthly number of physician visits in the policy and prepolicy intervention groups.
Table II.
Emergency hospital admissions and physician visits in older (aged ≥65 years) long-term users of inhaled medications before and after the fixed copay and income-based deductible (IBD) changes (from closed cohort study [n = 37,322] with historical controls).
| Outcome | Baseline Period (Months 1–12) Events/1000 Patient-Years |
Fixed Copay Period (Months 13–28) |
IBD Period (Months 29–40) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Events/1000 Patient-Years |
Crude RR |
Adjusted RR* |
95% CI | Events/1000 Patient-Years |
Crude RR |
Adjusted RR* |
95% CI | ||
| Emergency CAE hospitalization |
1.07 | 1.08 | 0.97–1.19 | 1.42 | 1.41 | 1.24–1.60 | |||
| Policy group | 98 | 122 | 135 | ||||||
| Control group | 101 | 117 | 98 | ||||||
| Emergency hospitalization for any reason |
0.91 | 0.91 | 0.86–0.96 | 1.00 | 0.99 | 0.93–1.05 | |||
| Policy group | 402 | 388 | 399 | ||||||
| Control group | 439 | 465 | 435 | ||||||
| Physician visits | 0.96 | 1.01 | 1.00–1.03 | 0.99 | 1.03 | 1.01–1.05 | |||
| Policy group | 22,913 | 23,142 | 24,470 | ||||||
| Control group | 21,325 | 22,322 | 23,020 | ||||||
RR = rate ratio; CAE = chronic obstructive pulmonary disease, asthma, or emphysema.
RR from a Poisson regression adjusted for age, sex, Romano comorbidity score, income status, number of asthma drugs used 6 months prior to baseline, number of nonasthma drugs used 6 months prior to baseline, whether admitted to hospital within 6 months prior to baseline, and quartile of physician use 6 months prior to baseline. Results are from 6 generalized estimating equation regressions (1 for each outcome and policy).
Table III.
Secondary analysis of emergency hospital admissions and physician visits in long-term users of inhaled medications before and after the fixed copay and income-based deductible (IBD) policy changes (from open cohort study with younger controls; n = 75,628)
| Outcome | Baseline Period (Months 1–12) Events/1000 Patient-Years |
Fixed Copay Period (Months 13–28) |
IBD Period (Months 29–40) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Events/1000 Patient-Years |
Crude RR |
Adjusted RR* |
95% CI | Events/1000 Patient-Years |
Crude RR |
Adjusted RR* |
95% CI | ||
| Emergency CAE hospitalization |
1.10 | 1.13 | 0.97–1.32 | 1.18 | 1.29 | 1.09–1.52 | |||
| Policy group (≥65 y) | 67 | 72 | 79 | ||||||
| Control group (55–<65 y) | 36 | 35 | 36 | ||||||
| Emergency hospitalization for any reason |
1.02 | 1.04 | 0.96–1.11 | 0.95 | 1.08 | 1.00–1.17 | |||
| Policy group (≥65 y) | 330 | 314 | 313 | ||||||
| Control group (55–<65 y) | 158 | 148 | 158 | ||||||
| Physician visits | 1.02 | 1.03 | 1.01–1.04 | 1.06 | 1.07 | 1.05–1.08 | |||
| Policy group (≥65 y) | 22,013 | 22,298 | 19,068 | ||||||
| Control group (55–<65 y) | 17,739 | 17,626 | 14,520 | ||||||
RR = rate ratio; CAE = chronic obstructive pulmonary disease, asthma, or emphysema.
RR from a Poisson regression adjusted for age, sex, Romano comorbidity score, income status, number of asthma drugs used 6 months prior to baseline, number of nonasthma drugs used 6 months prior to baseline, whether admitted to hospital within 6 months prior to baseline, and quartile of physician use 6 months prior to baseline. Results are from 3 generalized estimating equation regressions (1 for each outcome).
During the IBD policy period, emergency CAE admissions increased significantly in the policy intervention group (adjusted RR, 1.41; 95% CI, 1.24–1.60) (Table II). The secondary analysis also found a significant but lower increase in emergency CAE admissions (adjusted RR, 1.29; 95% CI, 1.09–1.52) (Table III). In the case–control analysis, at baseline, the proportion of patients who experienced an emergency CAE admission and discontinued inhaler use was significantly greater during the IBD policy period compared with full coverage (adjusted OR, 1.30; 95% CI, 1.03–1.64), suggesting a direct relationship between discontinuation of inhaler use and CAE admission during the IBD policy period.
Physician Visits
Rates of physician visits from the primary analysis are shown in Figure 2. Visits were unchanged during the fixed copay policy period in the primary analysis (adjusted RR, 1.01; 95% CI, 1.00–1.03) (Table II). In the secondary analysis using concurrent controls, we observed an increase of 3% (adjusted RR, 1.03; 95% CI, 1.01–1.04) (Table III). The primary analysis showed an increase in physician visits of 1.03 (95% CI for adjusted RR, 1.01–1.05) (Table II) during the first year of the IBD policy, and the secondary analysis showed an increase of 1.07 (95% CI for adjusted RR, 1.05–1.08) (Table III).
DISCUSSION
Cost-sharing policies can be made more tolerable by fully covering ≥1 drug in each therapeutically equivalent group and/or by allowing patients to pay deductibles gradually so that they receive some coverage at all times.
The results of the present analysis suggest that the IBD policy, a plan that broadly applied income-based deductibles and coinsurance payments to most drugs for most families, likely increased the risk for adverse health outcomes among older users of inhaled medi- cations compared with full coverage. Increases in emergency CAE admissions and physician visits were observed to be greater under IBD policy coverage than the fixed copay policy coverage. This inference is strengthened by the observation of an apparent direct link between discontinuation of inhaler use and emergency hospital admissions, which increased under IBD coverage. However, we were unable to determine whether there were carryover effects from the fixed copay policy into the IBD period.
To patients using inhaled corticosteroids in BC, the 25% coinsurance rate under the IBD policy cost roughly as much as a $25 copayment because the average cost of an inhaled corticosteroid prescription in 2003 was $110 (ie, a $27.50 coinsurance payment). A 25% coinsurance payment would have typically been <$25 for the less expensive β2-agonist and anticholinergic classes, suggesting that the overall increase in emergency CAE admissions that occurred during IBD policy coverage may have been influenced by the deductible component of the policy, which left patients without any public coverage until they passed the deductible.
The findings from this study are compatible with those from prior analyses that found that users of long-term inhaled corticosteroids were 21% more likely to discontinue inhaled corticosteroid use during IBD policy coverage and that patients newly diagnosed with asthma or COPD were 24% less likely to start using inhaled corticosteroids during the first year of the IBD policy period compared with full coverage.17 The results from the current analysis suggest a direct link between increased discontinuation of inhaler medication use under the IBD policy and an increased risk for CAE emergency admissions adjusted for any such association at baseline. Our strict definition of stopping inhaler use (within 60 days to lapse after the end of medication use without receiving a refill) may have led to misclassification of some stoppers as nonstoppers, possibly resulting in a conservative estimation.
The increased risk for CAE emergency hospitalization observed during IBD policy coverage might be cause for concern because hospitalization is a compelling marker for clinically relevant deterioration in health status, and it exposes patients to greater risk for other adverse outcomes, such as infections. Alternatively, increased hospitalizations could have occurred in some patients in the absence of clinically significant deteriorations in health status. For example, it is possible that, after losing the psychological comfort of their inhalers, some patients with COPD contacted their physicians, who might have chosen to be cautious by sending such patients to the hospital. If true, that scenario would beg the question of what responsibility a drug plan has to meet a patient’s expectations of drug therapy beyond the therapeutic evidence for its use. Randomized controlled trial methodology—the “gold standard” for evaluating drug effects—purposely tests for therapeutic benefits beyond placebo effects, and drugs found to be no more effective than placebo are not covered. Should a parallel be drawn to policy interventions in which policy effects produced by placebo effects are also discounted?
The change in diagnostic coding in hospital records from ICD-9-CM to ICD-10 in April 2001 might have been an alternative explanation for the observed increase in emergency hospitalizations, as the coding change could have caused patients to be classified differently. Fortunately, the diagnostic coding change occurred early in the baseline period, and as a safeguard we combined COPD, asthma, and emphysema into a single disease grouping (CAE) to reduce the likelihood of bias resulting from misclassification. The present cohort design with concurrent controls would have inherently adjusted for a secular change in COPD classification with respect to time.
The clinical importance of increased physician visits is unclear. The modest increase in physician visits might have been the consequence of physicians’ seeing patients more frequently as policy-induced changes were made to their drug regimens31 or patients’ requesting less expensive drug therapies, receiving closer monitoring because of medication changes, receiving assistance coping with more frequent or severe symptoms, or obtaining free drug samples. A previous study of reference pricing in BC concluded that policy-mandated medication switching was associated with an increase in physician visits of 11% (95% CI, 7%–15%).5 BC residents did not have to pay for medically necessary visits to a physician. However, starting in January 2002, the Ministry of Health ceased to pay for chiropractic, massage therapy, naturopathy, physical therapy, and nonsurgical podiatry services in most patients. To ensure that these changes did not affect our analysis, we excluded visits for those services from both the pre- and postpolicy data. Some patients might have had private insurance coverage for prescription drugs and/or supplemental medical services, such as podiatry and optometry. We did not have access to private insurance data. Private insurance might have introduced bias if the controls had different insurance coverage than the policy intervention groups. The primary analysis used an identically defined historical control group, which would have been expected to have avoided such bias. Any bias from private insurance data in these analyses, if it existed, was expected to have been toward the null because these patients would have had more coverage than we observed.
This study found that a combined deductible and coinsurance drug plan was associated with increased adverse health outcomes among these older users of inhaled medications. Increased discontinuation of use of inhaler medications was the apparent causal intermediary to increased emergency CAE hospitalizations. From a health plan’s perspective, a coinsurance policy would be preferable to a copayment policy because patient cost-sharing amounts automatically increase as prices increase. From a patient’s perspective, however, coinsurance payments become less affordable as prices increase, and a deductible that exposes patients to a period of no drug coverage may be particularly risky. Two modifications that could reasonably be expected to make these types of polices better tolerated would be to fully cover ≥1 drug in each essential therapeutic class, similar to reference drug programs,5 and/or allowing enrollees to pay their deductibles in installments so that they receive some coverage at all times during the year. BC has already implemented the latter option. Covering one essential drug in each class is also likely to reduce morbidity and insurance plan costs.32
CONCLUSION
The results of this analysis suggest that IBD policy coverage was associated with increased emergency hospitalization and physician visits in these users of inhaled medications aged ≥65 years.
ACKNOWLEDGMENTS
This research was funded by grants to Dr. Schneeweiss from the National Institute on Aging (R01-AG021950) and the Agency for Healthcare Research and Quality (2-RO1-HS10881), US Department of Health and Human Services, Rockville, Maryland.
All authors substantially contributed to the conception, design, analysis and interpretation of data, drafting, and revising the article for important intellectual content, and provided approval of the final version.
Dr. Dormuth was a recipient of a Canadian Institutes of Health Research doctoral research award (68359), and has been employed by and received consulting fees from the BC Ministry of Health. Dr. Maclure was supported by a Distinguished Scholar Award from the Michael Smith Foundation for Health Research, is employed by the BC Ministry of Health, and has received support from Pfizer Inc., New York, New York. Dr. Glynn has been an independent statistical monitor for AstraZeneca PLC, Wilmington, Delaware. Dr. Neumann has received support from Elan Pharmaceuticals, Inc., Morristown, New Jersey; and has been a member of the advisory board for Schering-Plough Corporation, Kenilworth, New Jersey. Dr. Brookhart has received support from Amgen Inc., Thousand Oaks, California. Dr. Schneeweiss has received support from Pfizer Inc.; Merck & Co., Inc., Whitehouse Station, New Jersey; HealthCore, Inc., Wilmington, Delaware; and I3 Drug Safety, Waltham, Massachusetts.
REFERENCES
- 1.Maclure M. Drug insurance utilization management policies and “reference pricing”: An illustrated commentary on the article by Vittorio Maio and colleagues. Milbank Q. 2005;83:131–147. doi: 10.1111/j.0887-378X.2005.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soumerai SB, Ross-Degnan D, Avorn J, et al. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med. 1991;325:1072–1077. doi: 10.1056/NEJM199110103251505. [DOI] [PubMed] [Google Scholar]
- 3.Tamblyn R, Laprise R, Hanley JA, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA. 2001;285:421–429. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- 4.Kozyrskyj AL, Mustard CA, Cheang MS, Simons FE. Income-based drug benefit policy: Impact on receipt of inhaled corticosteroid prescriptions by Manitoba children with asthma. CMAJ. 2001;165:897–902. [PMC free article] [PubMed] [Google Scholar]
- 5.Schneeweiss S, Walker AM, Glynn RJ, et al. Outcomes of reference pricing for angiotensin-converting-enzyme inhibitors. N Engl J Med. 2002;346:822–829. doi: 10.1056/NEJMsa003087. [DOI] [PubMed] [Google Scholar]
- 6.Grootendorst PV, Dolovich LR, O’Brien BJ, et al. Impact of reference-based pricing of nitrates on the use and costs of anti-anginal drugs. CMAJ. 2001;165:1011–1019. [PMC free article] [PubMed] [Google Scholar]
- 7.Schneeweiss S, Soumerai SB, Maclure M, et al. Clinical and economic consequences of reference pricing for dihy-dropyridine calcium channel blockers. Clin Pharmacol Ther. 2003;74:388–400. doi: 10.1016/S0009-9236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JK, Grootendorst PV, O’Brien BJ, et al. Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia. CMAJ. 2002;166:1655–1662. [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeweiss S, Maclure M, Carleton B, et al. Clinical and economic consequences of a reimbursement restriction of nebulised respiratory therapy in adults: Direct comparison of randomised and observational evaluations. BMJ. 2004;328:560. doi: 10.1136/bmj.38020.698194.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huskamp HA, Deverka PA, Epstein AM, et al. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med. 2003;349:2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 11.Motheral B, Fairman KA. Effect of a three-tier prescription copay on pharmaceutical and other medical utilization. Med Care. 2001;39:1293–1304. doi: 10.1097/00005650-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Briesacher B, Kamal-Bahl S, Hochberg M, et al. Three-tiered-copayment drug coverage and use of nonsteroidal anti-inflammatory drugs. Arch Intern Med. 2004;164:1679–1684. doi: 10.1001/archinte.164.15.1679. [DOI] [PubMed] [Google Scholar]
- 13.Salpeter SR, Buckley NS, Salpeter EE. Meta-analysis: Anti-cholinergics, but not β-agonists, reduce severe exacerbations and respiratory mortality in COPDI. J Gen Intern Med. 2006;21:1011–1019. doi: 10.1111/j.1525-1497.2006.00507.x. [published correction appears in J Gen Intern Med. 2006;21:1131] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleton S, Poole P, Smith B, et al. Long-acting β2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD001104.pub2. CD001104. [DOI] [PubMed] [Google Scholar]
- 15.Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: A systematic review of randomized placebo-controlled trials. Am J Med. 2002;113:59–65. doi: 10.1016/s0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- 16.van Grunsven PM, van Schayck CP, Derenne JP, et al. Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: A meta-analysis. Thorax. 1999;54:7–14. doi: 10.1136/thx.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dormuth CR, Glynn RJ, Neumann P, et al. Impact of two sequential drug cost-sharing policies on the use of inhaled medications in older patients with chronic obstructive pulmonary disease or asthma. Clin Ther. 2006;28:964–978. doi: 10.1016/j.clinthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Clinical Modification. International Classification of Diseases. 2003 9th Revision. [CDC Web site]. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD9-CM/2003/
- 19.World Health Organization (WHO) International Statistical Classification of Diseases and Related Health Problems. 2nd ed. WHO; Geneva, Switzerland: 2004. 10th rev. [Google Scholar]
- 20.Williams JI, Young W. Technical report. Institute for Clinical Evaluative Sciences; Toronto, Ontario, Canada: 1996. Inventory of studies on the accuracy of Canadian health administrative databases. [Google Scholar]
- 21.Fowles JB, Lawthers AG, Weiner JP, et al. Agreement between physicians’ office records and Medicare Part B claims data. Health Care Financ Rev. 1995;16:189–199. [PMC free article] [PubMed] [Google Scholar]
- 22.Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32:81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Glynn RJ, Monane M, Gurwitz JH, et al. Agreement between drug treatment data and a discharge diagnosis of diabetes mellitus in the elderly. Am J Epidemiol. 1999;149:541–549. doi: 10.1093/oxfordjournals.aje.a009850. [DOI] [PubMed] [Google Scholar]
- 24.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: Progress has been made but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. [Accessed April 14, 2008];Pharmacare. Your Fair PharmaCare coverage [Pharmacare Web site] www.healthservices.gov.bc.ca/pharme/plani/planiinfo.html#M.
- 26.Truitt T, Witko J, Halpern M. Leval-buterol compared to racemic al-buterol: Efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128–135. doi: 10.1378/chest.123.1.128. [DOI] [PubMed] [Google Scholar]
- 27.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 28.Kennedy P. A Guide to Econometrics. 4th ed MIT Press; Cambridge, Mass: 1988. pp. 123–125. [Google Scholar]
- 29.Warburton RN. Takeup of income-tested health-care premium subsidies: Evidence and remedies for British Columbia. Can Tax J. 2005;53:1–28. [Google Scholar]
- 30.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9 CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 31.Schneeweiss S, Maclure M, Soumerai SB. Prescription duration after drug copay changes in the elderly: Methodological aspects. J Am Geriatr Soc. 2002;50:521–525. doi: 10.1046/j.1532-5415.2002.50120.x. [DOI] [PubMed] [Google Scholar]
- 32.Choudhry NK, Avorn J, Antman EM, et al. Should patients receive secondary prevention medications for free after a myocardial infarction? An economic analysis. Health Aff. 2007;26:186–194. doi: 10.1377/hlthaff.26.1.186. [DOI] [PubMed] [Google Scholar]