Abstract
Genetic susceptibility to antisocial behavior may increase fetal sensitivity to prenatal exposure to cigarette smoke. Testing putative Gene × Exposure mechanisms requires precise measurement of exposure and outcomes. We tested whether a functional polymorphism in the gene encoding the enzyme monoamine oxidase A (MAOA) interacts with exposure to predict pathways to adolescent antisocial behavior. We assessed both clinical and information-processing outcomes. 176 adolescents and their mothers participated in a follow-up of a pregnancy cohort with well-characterized exposure. A sex-specific pattern of gene × exposure interaction was detected. Exposed boys with the low activity MAOA 5’ untranslated region variable number of tandem repeats (uVNTR) genotype were at increased risk for Conduct Disorder (CD) symptoms. In contrast, exposed girls with the high activity MAOA uVNTR genotype were at increased risk for both CD symptoms and hostile attribution bias on a face-processing task. There was no evidence of a gene-environment correlation (rGE). Findings suggest that the MAOA uVNTR genotype, prenatal exposure to cigarettes, and sex interact to predict antisocial behavior and related information-processing patterns. Future research to replicate and extend these findings may focus on elucidating how gene × exposure interactions may shape behavior via associated changes in brain function.
Keywords: prenatal smoking, prenatal smoking, MAOA, gene × environment interaction, developmental psychopathology
Considerable evidence links prenatal exposure to cigarettes (“exposure ”) to antisocial behavior 1, 2. Recent work has focused on mechanisms 3, 4 through precise phenotypic specification 5 and, the intersection of exposure with other risks 6, 7. In particular, these investigations have focused on examining the gene-exposure interface in an effort to disentangle the extent to which exposure serves as a risk marker versus exerting a direct teratologic effect 8, 9.
Advances in molecular biology and systems neuroscience provide particularly useful strategies in developmental contexts 9–12. This work has begun to show that genetic variation modifies the effects of prenatal exposure to cigarettes on both perinatal and clinical outcomes 13–17. As a rule, however, examinations of gene × prenatal exposure to cigarettes (G × Epec) interactions have not focused specifically on antisocial behavior, the clinical phenotype where exposure-behavior associations appear strongest.
MAOA degrades monoamines and thus critically regulates behavior 18, 19. The monoamine oxidase A untranslated variable number of tandem repeat markers (uVNTR; heretofore “MAOA genotype”) is the most well-established susceptibility variant for human aggression and related phenotypes in other species 18, 19. MAOA genotype appears to influence the development of antisocial behavior by altering vulnerability to the effects of early adverse environments 18, 20, 21. In particular, there is robust evidence of the interaction of MAOA and early maltreatment (GMAOA × Emalt ) in predicting antisocial behavior 21. Maltreatment is associated with increased risk of antisocial behavior for males with the low-expression MAOA (MAOA-L) allele but not for males with the high-expression MAOA allele (MAOA-H) 22. Recent evidence also links MAOA-L and low brain MAOA with trait aggression and neural hypersensitivity to social cues 23–25. While the relation of the interaction between MAOA and adverse environments to intermediate phenotypes has been hypothesized 18, it has not yet been tested.
Within the context of examining MAOA’s potential modulating effects on the impact of early environmental experience, prenatal exposure to cigarettes is a particularly intriguing adverse environment to study because it directly impacts fetal brain development. Nicotine is the primary teratogenic compound in tobacco, with specific nicotinic acetylcholine receptors (nAChRs) mediating its effects27. Fetal nAChRs sit on dopaminergic neurons lying in pathways critical for regulation of attention and emotion. Serotonergic systems are similarly affected27. Studies with rodents indicate that exposure results in improper reactivity to neuronal stimuli during key developmental periods, with long-term effects on neural systems27.
Most GMAOA × Emalt interaction studies and, many studies of exposure and antisocial behavior, have been in males 2, 21. However, MAOA is an X-linked gene (with men carrying only one allele and women carrying two), suggesting the possibility of sex differences in epigenetic regulation 27. Recent work examining sex differences in the effects of MAOA has reported conflicting findings. Some studies find sex differences in effects on brain structure and information-processing 18, 28 whereas others do not 24. Of particular relevance, the few studies of female patterns in relation to GMAOA × Emalt interactions are inconsistent: two studies identified MAOA-L as the risk allele for females 29, 30, one study identified MAOA-H as the risk allele for females 27 and one study failed to find a significant interaction GMAOA × Emalt interaction in females 31. Thus, sex differences in patterns of interaction of MAOA and adverse early environmental exposures are a critical area of inquiry. The importance of this line of investigation is bolstered by evidence of sex differences in exposure-related patterns from both basic 32–34 and clinical 2, 35 studies.
We tested for sex-specific GMAOA × Epec interactions in predicting antisocial behavior and an information-processing intermediate phenotype i.e., a direct assessment of face-processing errors related to hostile attribution bias. Hostile attribution bias is an information-processing substrate linked to reactive aggression 36. Further, brain imaging work demonstrates an association between MAOA genotype and neural circuits engaged by face-emotion processing 25.
The present paper also discriminates between a true G × Epec and a passive G × Epec correlation (rGE). rGE is a correlation between genotype and environment 3, 37. When rGE is present, exposure to an adverse environment and clinical outcomes may appear to be causally linked, when in fact, their association results from the correlation between the environmental exposure and genotype. This is of particular concern in the case of prenatal smoking, because women with a history of antisocial behavior are substantially more likely to persist in smoking during pregnancy and to have antisocial partners 7, 38. Thus, in addition to the prenatal exposure, pregnant smokers are also more likely to transmit genetic susceptibility to antisocial behavior and to provide a harsh parenting environment. As a result, controlling for parental antisocial behavior and parenting environment is important for establishing independent effects of prenatal smoking. A more direct test of rGE is examining whether women with a risk allele for antisocial behavior are more likely to persist in pregnancy smoking than women without this risk allele. Here we controlled for parental antisocial history and harsh parenting and also directly tested whether prenatal smoking status varied by maternal MAOA genotype.
Most prior work in this area has used large population-based samples 3, 4, 39. While large sample sizes enhance statistical power, power may not be fully realized in large studies using retrospective, indirect measures of exposure. We use a modest sample in which exposure has been directly measured. Prospective, repeated, assay-based measurement provides greater sensitivity 11, 40–42. Increasing sensitivity in characterization of phenotypes and potential modulators may also increase the likelihood of discovering gene effects, even in relatively small samples such as ours 43. Our data derive from the East Boston Family Study (EBFS), an adolescent follow-up of a pregnancy cohort with high rates of heavy prenatal smoking 44. To our knowledge, this is the first investigation of G × Epec interaction which uses prospective, multi-method assessment of prenatal exposure to cigarettes as it interacts with MAOA in relation to both clinical and information-processing phenotypes.
METHODS
PARTICIPANTS
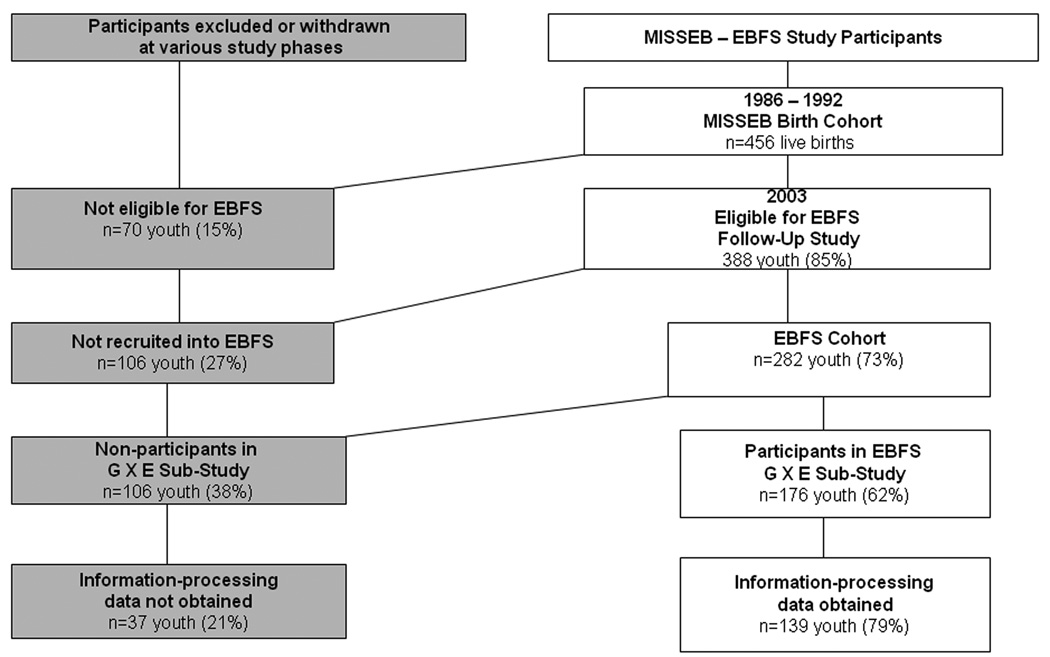
Participants were from a prospective pregnancy cohort of non-Hispanic white women recruited from a neighborhood health clinic in East Boston, enrolled in the Maternal Infant Smoking Study of East Boston (MISSEB) between 1986–1992 44, 45. Women were eligible for MISSEB if they attended the East Boston neighborhood health clinic, were less than 20 weeks pregnant, and were 19 or older. The present study reports on data from the East Boston Family Study (EBFS), an adolescent follow-up of the MISSEB cohort. (Although MISSEB also included Hispanic women, they were not eligible for EBFS because they smoked at very low rates (6.5%), consistent with national trends 48). Figure 1 illustrates the sequence of the study from MISSEB through EBFS and participation at various phases. 85% of the offspring from MISSEB were eligible for EBFS (the remaining 15% had previously withdrawn or had not participated in any postnatal visits). From this pool of eligible youth from MISSEB, 73% were re-ascertained for EBFS (n=282 youth, representing 251 families due to the inclusion of 30 sets of siblings). The EBFS was a longitudinal study with three annual waves, with retention at Waves 2 and 3 of 91% and 92% respectively. CD symptoms were assessed at each wave. Genotype and face-processing data were collected at Wave 2.
Figure 1.
Study Flowchart
The EBFS cohort did not differ from eligible non-participants in terms of maternal smoking status. However, EBFS mothers had higher education levels (75% vs. 62% high school completion, X2=6.0, df=1, p<.01) and were slightly older (mean age at pregnancy= 27 vs. 26 years, t=2.5, df=346, p<.01) than mothers who did not participate. Because of the focus of the present paper on GMAOA × Epec interactions, only youth on whom saliva was obtained for genotyping were included (82%). An additional 2% were excluded because their biological mothers were not participating and 16% with non-White fathers were excluded to minimize problems from population substructure. Two additional youth were excluded because they lacked data on CD symptoms. Thus, the primary analytic sample for this G × E sub-study is comprised of 176 non-Hispanic white youth (mean age=15 (SD=1.8), 56% girls, 23 sibling pairs) and their biologic mothers. Mothers were predominantly working class (median income $40-50,000/year) and high school educated (72%). The G × E sub-sample did not differ from the full EBFS sample in terms of exposure status, demographic factors, parental antisocial behavior or harsh parenting. Seventy-nine percent of this G × E sub-sample (n=139) also completed the information-processing task via laptop computer at Wave 2. Missing information-processing data were due to Wave 2 attrition and lack of availability for in-person assessments. Youth with information-processing data did not differ from those without it in terms of exposure status, family demographics or parental antisociality. However, youth with information-processing data were slightly younger than those in the larger G × E sub-sample (mean of 14.9 vs. 15.6 years at baseline, p<.05).
MEASURES
Exposure
At each prenatal visit, women reported current smoking. Blood and urine were also obtained for cotinine assays, which were conducted via radioimmunoassay 46. For the present study, serum cotinine level was used in combination with self-reported smoking to develop a “best-estimate” measure of exposure 47. This “best-estimate” measure was generated using all available data on each woman, employing statistical methods developed for this study 47. Hierarchical (subject-specific) modeling of cotinine metabolism was used to generate a cotinine-based correction factor that accounted for inaccuracy due to nondisclosure or underreporting. This correction factor was based on the woman-specific exponential-decay of cotinine in blood, as a function of time since last cigarette and self-reported smoking pattern. The deviations from the exponential-decay model according to the “average” cotinine concentration per cigarette in a pregnant population were then used to indicate accuracy and adjust maternal report accordingly. Small adjustments to exposure level (<1 cigarette/day) likely reflect differences in smoking topography and metabolism, whereas larger adjustments likely reflect underreporting or nondisclosure.
This best-estimate method led to a mean adjustment of 1.04±2.46 cigarette/day for the sample as a whole. Within the sub-group of self-reported non-smokers, 10% required an adjustment (mean adjustment=.37±1.59 cigarettes): 50% of these were apparent non-disclosers (range of adjustment for this group=3.96–9.24 cigarettes). In contrast, exposure values for 89% of the self-reported smokers required adjustment (mean adjustment=1.85 ±3.03cigarettes). Of this group, 43% appeared to significantly underreport amount smoked with adjustments of >1 cigarette per day (range 1.24–9.24). Based on these serum-cotinine corrected values, 51% of the EBFS youth were classified as exposed. The mean number of cigarettes per day in the smoking group was 12.8±8.2, with 61% of these exposed to ½ pk/day or more. For the present analyses, we used the continuous serum-cotinine corrected measure of average cigarettes per day across the pregnancy.
MAOA 5’ UTR Genotyping
Saliva samples were collected from the youth and their mothers using DNA Genotek Oragene self-collection kits. After extraction, DNA was quantitated with a fluorescent Quant-iT™ PicoGreen® dsDNA Assay (Invitrogen, Carlsbad, California) and normalized to a concentration of 10ng/uL. DNA was amplified with PET™ forward primer 5'-PET-ACAGCCTGACCGTGGAGAAG-3' (Applied Biosystems, Foster City, CA) and reverse primer (with pigtail sequence within parentheses 5'-(GTTTCTT)GAACGGACGCTCCATTCGGA-3' using HotStarTaq DNA Polymerase (Qiagen Inc. USA, Valencia, California) with an initial denaturation step at 95 ° C for 15 min followed by 40 cycles of 1min at 94 ° C, 1min at 58.2 ° C and 2 min. at 72 ° C. Products were separated on a 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA) in the UIC Research Resources Center DNA Services Facility. Alleles were called blind to phenotype data using Genemapper v 3.7.
Genotype frequencies for boys and girls are reported in Table 1. Because MAOA is X-linked, boys have only one allele and thus can either be MAOA-L or MAOA-H. However, because girls have two alleles, they may be either homozygous or heterozygous. In order to test Hardy Weinberg equilibrium (HWE), MAOA was considered multi-allelic with four possible alleles of 3, 3.5, 4, 5 repeats and only tested in unrelated females (p= 0.918). There is agreement across studies that 3 repeats should be classified as MAOA-L and 4 repeats as MAOA-H, but there is some discrepancy in regard to classification of 5 repeats 19, 49. Consistent with the approach of Sabol and Kim-Cohen 19, 21, we classified the 5 repeats as MAOA-L. Because females have two copies of MAOA, studies have taken varying approaches to classifying heterozygous females. Based on prior findings that patterns for heterozygous females are similar to females homozygous for the low activity allele 27, for primary analyses we classified female subjects homozygous for 4 repeats, homozygous for 3.5 repeats, or heterozygous with both high activity genotypes (3.5 and 4 repeats) as high activity and female subjects with all other genotypes as lower activity. We also examined this assumption in supplementary analyses using a 3-level classification for females.
Table 1.
Distribution of MAOA Genotypes for Girls and Boys
| MAOA Genotype | Percent (Frequency) Girls | Percent (Frequency) Boys |
|---|---|---|
| 3/3 | 12 (12) | 39 (30) |
| 3/4 | 42 (42) | 0 (0) |
| 3/5 | 2 (2) | 0 (0) |
| 3.5/3.5 | 0 (0) | 4 (3) |
| 3.5/4 | 1 (1) | 0 (0) |
| 4/4 | 41 (41) | 53 (41) |
| 4/5 | 1 (1) | 0 (0) |
| 5/5 | 0 (0) | 4 (3) |
Youth Antisocial Behavior
At each of the three annual waves, lifetime Conduct Disorder (CD) symptoms were assessed based on parent and youth report using the Diagnostic Interview for Children (C-DISC-IV)50. We used continuous symptoms scores as relatively few youth in this community sample met criteria for CD across the three waves of the study (7% of girls and 22% of boys). Because youth and parent reports are only moderately correlated and youth often underreport conduct symptoms 51, and, consistent with previous investigations of G × E pec 16, 52, we used parent report as the primary indicator of symptoms, with secondary analyses conducted based on youth report.
Youth Hostile Attribution Bias
The Diagnostic Analysis of Nonverbal Accuracy-2 (DANVA-2) 53 was used to assess receptive knowledge of adult happy, sad, angry and fearful facial emotions at Wave 2. Accuracy of emotion identification was scored automatically by DANVA2 software. A DANVA hostile attribution bias score was generated by calculating the number of happy, sad and fearful faces misidentified as angry. We also tested whether impaired sensitivity in emotion identification problems was unique to anger by creating discrimination indices for each of the emotions 54.
Covariates
Covariates considered for inclusion in the models were family income, teen age, prenatal alcohol use, maternal and paternal antisocial behavior and harsh parenting. Mean imputation was used for missing data (ranging from 1–15%).
Prenatal alcohol exposure
To establish that the association of prenatal smoking to youth outcomes was not confounded with other substance use during pregnancy, maternal prenatal alcohol use was controlled. Alcohol use during pregnancy was assessed at each prenatal interview and coded as two dichotomous indicators which were entered simultaneously into the regression models: (1) drank at low levels (0=never drank during pregnancy; 1=drank during pregnancy but never 2+ drinks at a sitting) and, (2) had significant alcohol consumption on at least one occasion (0=never had 2+ drinks at a sitting; 1=had 2+ drinks at a sitting on one or more occasion).
Parental Antisocial Phenotype
To account for the possible spuriousness of exposure to parental antisocial behavior, we controlled for both parental antisocial history and harsh parenting environment. Mothers reported on their own antisocial behavior using the Antisocial Behavior Checklist 55, a widely used 45-item measure that assesses a range of delinquent behaviors that occurred during childhood/adolescence and as an adult. Because mothers were reporting about the fathers’ antisocial behavior, we used a measure specifically validated for use by maternal report, with strong cross-informant agreement reported when comparing maternal reports of fathers’ antisocial behavior to fathers’ self reports 56, 57. This 44- item measure uses the Achenbach family of adult report instruments to assess lifetime history of aggressive and delinquent behavior. Parental harsh discipline was assessed via combined maternal and youth report on the Punitive Discipline sub-scale of the Hetherington Discipline Scale58 . Using Mplus, factor scores representing harsh parenting were created from three standardized measures: youth report of maternal and paternal discipline and maternal report of own discipline. Higher factor scores represent more punitive discipline.
Descriptive characteristics of the sample and for these covariates are provided in Table 2.
Table 2.
Sample Characteristics and Descriptives (n=176)1
| Variables | Mean (standard deviation) or Percent |
Range |
|---|---|---|
| Predictor | ||
| Maternal Smoking during Pregnancy (Mean cigs/day) | 6.56 (8.70) | 0 – 40.3 |
| Outcomes | ||
| Average CD Symptoms (Parent-report 3 waves) | 2.25 (2.99) | 0–15.7 |
| Average CD Symptoms (Youth-report 3 waves) | 3.88 (3.78) | 0–17.7 |
| Hostile Attribution Score | 1.38 (1.18) | 0–6 |
| Covariates | ||
| Harsh Parenting | −0.01 (0.16) | −0.25–0.58 |
| Maternal Antisocial Behavior | 12.87 (10.46) | 0–66 |
| Paternal Antisocial Behavior | 18.32 (17.60) | 0–66 |
| Teen Age (years) | 14.5 (1.77) | 11–18 |
| Prenatal Alcohol Exposure: | ||
| % drank but < than 2 drinks/ sitting | 32% | |
| % drank 2 or > drinks/ sitting | 25% |
Participants in EBFS G × E sub-study. 85% of the original MISSEB cohort were eligible for the EBFS follow-up study (based on participation in at least one MISSEB postnatal visit and/or not having previously withdrawn from MISSEB). Of those eligible, 73% were re-ascertained for EBFS. Of EBFS participants, 62% participated in the G × E sub-study based on availability of full genotype data on biologic mother and youth, CD symptom data and, both parents being white (to minimize problems with population substructure).
Note: Hostile attribution score=number of non-angry faces misidentified as angry on the DANVA; Harsh parenting factor score based on maternal self-report and youth report of maternal and paternal parenting; Prenatal alcohol exposure measured with two dichotomous indicators: (a) mother drank during pregnancy but less than 2 or > drinks per sitting (0=no; 1=yes) and (b) mother drank 2 or > drinks per sitting during pregnancy (0=no; 1=yes); MAOA Genotype (0=MAOA-L; 1=MAOA-H).
DATA ANALYSIS
Because MAOA is X-linked, primary analyses were conducted separately for girls and boys, consistent with prior research 30. Linear mixed models were used to predict CD symptoms from Waves 1–3, with wave as a time-dependent covariate, using unstructured covariance matrices. Linear models were used to predict the hostile attribution score. Permutation methods 59, 60 were used were to test for GMAOA × Epec interactions. In order to account for family structure, the dependent variable was permuted across the families with one teen and separately permuted across the families with siblings. Reported standard errors of the effect sizes and p-values were determined empirically. This approach was selected because permutation tests allow relaxing of distributional assumptions and are more appropriate for skewed distributions and situations with complex dependencies, such as those found in family data 60. Because of their theoretical importance, paternal and maternal antisocial behavior were included as covariates in all primary regression models. Backwards elimination was conducted for selection of all other covariates in the full analytic sample. Analyses were conducted with SAS version 9.1.
RESULTS
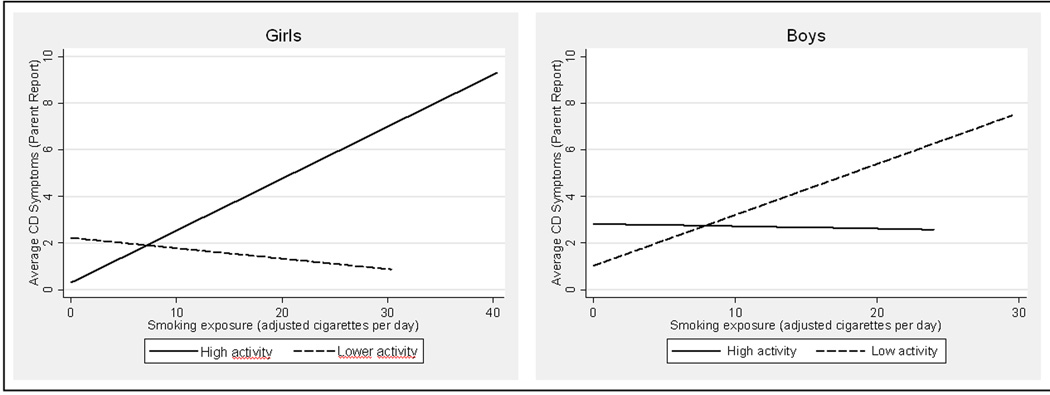
Parent-reported CD symptoms were regressed on MAOA genotype, a continuous measure of exposure, and the GMAOA × Epec interaction, with interview wave, youth age, family income, harsh parenting, prenatal alcohol exposure, and maternal and paternal antisocial behavior as covariates (see Table 3). The GMAOA × Epec interaction was significant for both girls and boys (b=.25, p=0.002 and b=−.18, p=0.03, respectively). However, the direction of these effects differed (Figure 2). Consistent with previous research, increased exposure was associated with increased CD symptoms for boys with MAOA-L. In contrast, increased exposure was associated with increased CD symptoms for girls with MAOA-H. Supplementary analyses using a 3-level genotype classification for girls, demonstrated similar patterns for homozygous low activity girls and heterozygous girls, supporting our approach of classifying these together as a lower activity group (see supplementary Figure 3S). Inspection of the data in the girls’ primary regression model revealed that two sisters might be considered multivariate outliers: both were exposed to more than 30 cigarettes per day and had high CD symptoms. We conducted a sensitivity analysis, excluding these siblings. In this conservative model, the 2-way GMAOA × Epec interaction remained significant (p=0.02). The GMAOA × Epec interaction was also significant for girls’ self-reported CD symptoms, but not boys (reported in supplementary materials, Table 3S).
Table 3.
Interaction of Exposure × MAOA Genotype in Predicting Parent-Reported CD Symptoms for Girls and Boys (n=175)1
| Predictors | Beta (STE) Girls | P value | Beta (STE) | P value |
|---|---|---|---|---|
| Girls | Boys | Boys | ||
| Teen MAOA | −1.876 (0.823) | 0.005 | 1.221 (0.906) | 0.110 |
| Prenatal Exposure to Cigarettes | −0.066 (0.034) | 0.048 | 0.213 (0.069) | 0.004 |
| Harsh Parenting | 1.931 (1.641) | 0.174 | 1.505 (2.424) | 0.436 |
| Maternal Antisocial Behavior | 0.068 (0.034) | 0.014 | 0.020 (0.043) | 0.565 |
| Paternal Antisocial Behavior | 0.020 (0.018) | 0.147 | −0.004 (0.025) | 0.838 |
| Teen Age (years) | 0.267 (0.189) | 0.089 | 0.619 (0.199) | 0.002 |
| Prenatal Exposure to Alcohol | 1.256 (0.753) | 0.041 | 0.546 (0.923) | 0.469 |
| MAOA × Prenatal Exposure to | 0.245 (0.076) | 0.002 | −0.178 (0.089) | 0.032 |
| Cigarettes |
One youth was missing parent-report on the DISC.
Note: MAOA Genotype (0=MAOA-L; 1=MAOA-H); Prenatal alcohol exposure (0=did not consume 2 or > drinks/sitting, 1=consumed 2 or > drinks/sitting); Other covariates were included as continuous predictors.
Figure 2.
Interaction of MAOA X Exposure in Prediction of Youth Antisocial Behavior
2a. Girls
2b. Boys
We next tested whether the GMAOA × Epec interaction predicted our information-processing phenotype, i.e., the DANVA hostile attribution bias score. Backward elimination resulted in youth age, prenatal alcohol use and family income dropping from the model. As Table 4 shows, the GMAOA × Epec interaction was a significant predictor of hostile attribution bias for girls (b=.06, p=0.04) but not boys (b=-.05, p=0.22). Similar to the pattern for girls’ CD symptoms, for girls with MAOA-H, increased levels of exposure were associated with a greater tendency towards hostile attribution bias (see supplementary Figure 4S). In post-hoc analyses, we tested whether this pattern was unique to angry faces. The MAOA-H group was poorer at anger discrimination (p=.05) but there were no differences in discrimination of other emotions.
Table 4.
Interaction of Exposure × MAOA Genotype in Predicting DANVA Hostile Attribution Bias Score for Girls and Boys (n=139)
| Predictors | Beta (STE) | P value | Beta (STE) | P value |
|---|---|---|---|---|
| Girls | Girls | Boys | Boys | |
| Teen MAOA | −0.453 (0.386) | 0.258 | 0.648 (0.396) | 0.110 |
| Prenatal Exposure to | −0.025 (0.019) | 0.207 | 0.016 (0.031) | 0.628 |
| Cigarettes | ||||
| Harsh Parenting | 1.365 (0.903) | 0.143 | 1.381 (1.115) | 0.224 |
| Maternal Antisocial | 0.001 (0.017) | 0.953 | −0.002 (0.017) | 0.894 |
| Behavior | ||||
| Paternal Antisocial | −0.010 (0.009) | 0.239 | 0.003 (0.010) | 0.794 |
| Behavior | ||||
| MAOA × Prenatal | 0.062 (0.029) | 0.037 | −0.046 (0.038) | 0.217 |
| Exposure to | ||||
| Cigarettes |
Note Hostile attribution score=number of non-angry faces misidentified as angry on the DANVA; MAOA Genotype (0=MAOA-L; 1=MAOA-H)
In order to formally test for the 3-way interaction of GMAOA × Epec × youth sex, we also conducted an additional set of regression analyses on the full sample. These analyses yielded significant 3-way interactions for prediction of both CD symptoms (parent-report, p=0.001; youth report, p=0.04), and the hostile attribution bias score (p=0.02). We also conducted a sensitivity analysis using the uncorrected self-reported measure of exposure. The use of this uncorrected measure of exposure did not substantially alter the relation of predictors to outcome, with the 3-way interaction remaining significant for parent report (p=0.001), trending towards significance for youth report (p=0.07) and remaining significant for hostile attribution bias (p=0.04).
Finally, to examine whether these findings might result from a passive gene-environment correlation (rGE), we used Mantel Haenszel chi-square tests to examine whether mothers’ prenatal smoking status varied as a function of their MAOA genotype. These analyses did not support presence of an rGE. The distribution was as follows: 40% of mothers with the MAOA-H genotype smoked during pregnancy compared to 33% of women who did not smoke during pregnancy and, 48% of pregnancy smokers had the MAOA-L genotype compared to 52% of women who did not smoke during pregnancy (X2=.77, p=0.38).
DISCUSSION
Prenatal exposure to cigarettes is a well-established risk factor for offspring antisocial behavior. Using data from a pregnancy cohort with prospective, repeated measures of exposure, we tested whether exposure interacts with MAOA genotype to predict both antisocial behavior and hostile attribution bias in male and female adolescents. Central strengths of the study include: (a) precision of exposure measurement; (b) combining molecular G × E pec and rGE approaches to examine the interplay of exposure and a monoamine-related genotype previously shown to modify environmental effects on antisocial behavior; (c) testing associations with both clinical and information-processing phenotypes; (d) robust control for parental antisocial history and environment and; (e) examining sex-specific effects. While nearly half a dozen studies have demonstrated G × E pec interactions, these studies focus primarily on dopaminergic genotypes as moderators of ADHD. To our knowledge, the present study is the first to examine the interaction of MAOA genotype and exposure in prediction of antisocial behavior. Findings are also novel in demonstrating links between exposure and a theoretically-derived index of information-processing, which is a well-established substrate of antisocial behavior.
We also found sex-specific GMAOA × Epec patterns. Consistent with previously reported patterns for males 21, we found that the MAOA-L genotype conferred increased risk of CD symptoms in exposed boys. Consistent with findings of Sjoberg 27, we found a contrasting pattern in girls, i.e., the MAOA-H genotype conferred increased risk in exposed girls. This pattern for girls was also found in relation to directly-assessed hostile attribution bias. This sex-specific pattern is especially intriguing given the failure of most prior studies to consider females 2, 21. Recent studies in both model organisms and humans also show that females exhibit unique exposure-related perturbations in brain structure and function 32–34.
The current findings are broadly consistent with previous work on the role of MAOA genotype in moderating the effect of adverse early environmental exposures 21. This parallel pattern of findings for the interaction of MAOA with two very different types of “environments” in predicting antisocial pathways is particularly striking. Previous work has examined MAOA’s interaction with social aspects of the environment. In contrast, prenatal exposure involves delivery of a chemical directly to the fetal brain. Clearly, an exposure that directly acts on the brain is different from one in which effects on the brain are transduced through complex social events 61.
Variations in MAOA have been linked to the substrates of reactive, impulsive aggression including hostile personality traits and deficits in face emotion processing 24, 25, 62. Buckholtz and Meyer-Lindenberg have theorized that this is due to the effects of MAOA genotype on the neural circuitry underlying social decision-making 18. Of relevance, work by Blair and colleagues establishes that the orbital frontal cortex (OFC) plays a critical role in the modulation of aggression 63, 64. Moreover, the association between OFC dysfunction and human aggression is thought to be at least partly modulated by the OFC’s role in the detection of, and response to, anger cues 65. This work shows that individuals with OFC dysfunction manifest biased perceptions of hostility 64. Of special salience, a recent structural MRI study by Toro et al., demonstrates that exposure predicts reduced thickness of the OFC with concomitant decrements in social concern and connectedness in girls 34. Thus our findings that exposed girls with the high activity MAOA genotype have an increased tendency to misidentify emotions as angry are consistent with prior theoretical and empirical work.
Sex differences in patterns of GMAOA × Epec interaction may reflect differences in how MAOA genotype interacts with sex-specific patterns of prenatal brain development 27. The current findings, when integrated with findings from the laboratories of Blair, Toro and Buchkholtz & Meyer-Lindenberg 18, 34, 64, suggest an important specific direction for future research in which functional MRI studies can be used to detect similar signs of sex-specific GMAOA × Epec interactions, manifest on measures of OFC function and face-emotion identification.
To our knowledge, the present study is the first to combine examination of the interaction of measured genotype and prenatal exposure with testing for passive gene-environment correlations. Data from two large cohorts using kinship designs suggest the possibility that the exposure: antisocial behavior association reflects an rGE 3, 37. In addition to controlling for maternal and paternal antisocial behavior and an environmental correlate of parental antisociality, i.e., harsh parenting, we used molecular approaches to directly test whether exposure status differed as a function of maternal MAOA genotype. Using these methods, we do not find evidence of rGE in the present sample. However, we have measured only one genotype and thus, cannot address the broader question of the contribution of rGE to these pathways.
A primary limitation of the present study is its relatively small sample size. Nevertheless, although small sample sizes reduce power, the current study had sufficient power to detect most hypothesized differences. Still, while the GMAOA × Epec interaction was consistently found for girls regardless of informant (parent vs. youth) or the nature of the phenotype (clinical vs. information-processing), it was only significant for boys’ parent-reported CD symptoms. Replication and further examination of these issues in larger samples using multi-level phenotypic assessments and sufficient numbers of girls and boys to test sex-specific effects are warranted.
Another limitation of this study is the classification of MAOA alleles into high activity genotypes on the basis of prior work 19 that used an in vitro transfection study rather than direct study of genotype effects on expression. The only study examining relevant gene-brain relationships showed no difference in [11C]-clorgyline-measured MAOA protein expression related to genotype 66. The relevant relationship between the marker and gene expression and ultimately neurotransmitter regulation would need to be studied in utero and throughout development. Such an investigation has not yet been conducted. Other limitations relate to the focus on one MAOA variant, as opposed to full interrogation of all MAOA variants, and the failure to consider transmission of genotypes, leaving open the possibility of population-stratification effects.
Previous studies have often minimized the importance of accurate exposure measurement because brief, retrospective recall was the standard in initial investigations. In the present study, sophisticated statistical modeling incorporating direct and indirect measures of exposure led to adjustments in exposure level for most smokers. Although this adjusted measure did not demonstrate substantially enhanced sensitivity in the present analyses, we believe that the reductions in error and reporting bias that is likely to result from this mutli-method approach will be crucial for more fine grained analyses of exposure effects such as establishing dose-response and threshold effects. The importance of measurement accuracy is particularly important in studies that go beyond presence or absence of exposure to examine discordance in exposure intensity (exact amount smoked) as a “test” for genetically mediated effects 3, 37. Thus, precision of exposure measurement is key.
Convergence of findings for clinical and information-processing patterns for girls in this study suggests the importance of modeling “exposure effects” on both behaviors and brain-based measures for elucidating mechanisms. Specifically, a neuroscientific perspective via application of information-processing methods may provide a window on how the early neurobiologic insult of prenatal exposure to cigarettes shapes individual differences in behavior in a manner that increases risk of antisocial pathways 18, 67. The identification of specific neurodevelopmental substrates also enables the examination of these patterns as they unfold, prior to the onset of frank clinical patterns 5.
There is increasing evidence that prenatal factors have long-term effects on health and behavior 68. The present findings provide a first examination of the potential role of MAOA in modulating effects of prenatal exposure to cigarettes. Together with the recent work of others 3, 4, 69, they highlight the complex interplay of prenatal exposure to cigarettes and genetic susceptibility in pathways to antisocial behavior. Resolution of whether prenatal exposure to cigarettes plays a causal role in these pathways requires translational approaches that move beyond a scientific perspective juxtaposing teratologic, genetic and contextual processes as mutually exclusive explanations to one that examines them in concert to explicate their relative contributions and mutual influences during the critical prenatal period. This work may provide a unique window on the genesis of developmental psychopathology. Future research that combines genetically- informed designs with theoretically-derived, well-specified measurement of exposure, putative mechanisms and component phenotypes is the critical next step for advancing this line of investigation.
Supplementary Material
Acknowledgements
This work was supported by NIDA grant DA15223 to Dr. Wakschlag, including support to Drs. Pickett, Cook, Dukic, Wright and Leventhal. Drs. Wakschlag, Leventhal and Cook were also supported by the Walden & Jean Young Shaw and Children’s Brain Research Foundations and Dr. Pickett was supported by a UK National Institute for Health Research Career Scientist Award. Very special thanks to our colleagues, Margaret Briggs-Gowan, Kimberly Espy, David Henry, Brian Mustanski and Patrick Tolan, whose ongoing critical feedback and consultation on this work has been invaluable. We thank Ira Tager, MISSEB’s founder, for his enthusiasm about the EBFS follow-up and facilitating access to this cohort. We gratefully acknowledge Neal Benowitz’s contribution to exposure measurement. Vincent Smeriglio’s steadfast commitment to this program of research is deeply appreciated. We thank Nora Volkow for guidance on the scientific approach taken in this study. Finally, we thank the EBFS research staff whose thoughtful efforts were vital to study completion, particularly Marian Parker’s work on re-ascertainment, Phil Schumm & Ted Pollari’s work on electronic data transmission, and Kathy Hennessy and Greg Moy of the IJR Laboratory of Developmental Neuroscience for their work on genotyping. Portions of this paper presented at the Meetings of the American College of Neuropsychopharmacology. Society for the Study of Addiction and the Society for Research in Child Development.
References
- 1.Ernst M, Moolchan E, Robinson M. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–642. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Wakschlag L, Pickett K, Cook E, Benowitz N, Leventhal B. Maternal smoking during pregnancy and severe antisocial behavior in offspring: A review. Am J Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Onofrio B, Van Hulle C, Waldman I, et al. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maughan B, Taylor A, Caspi A, Moffitt T. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Arch Gen Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- 5.Wakschlag L, Leventhal B, Pine D, Pickett K, Carter A. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Dev. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 6.Button T, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Hum Dev. 2007;83:727–737. doi: 10.1016/j.earlhumdev.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huijbregts S, Seguin J, Zoccolillo M, Boivin M, Tremblay R. Maternal prenatal smoking, parental antisocial behavior and early childhood physical aggression. Dev Psychopathol. 2008;20:437–453. doi: 10.1017/S0954579408000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraemer H, Kadzin A, Offord D, Kessler R, Jensen P, Kupfer D. Coming to terms with the terms of risk. Arch Gen Psychiatry. 1997;54:337–343. doi: 10.1001/archpsyc.1997.01830160065009. [DOI] [PubMed] [Google Scholar]
- 9.Thapar A, Harold G, Rice F, Langley K, O'Donovan M. The contribution of gene-environment interaction to psychopathology. Dev Psychopathol. 2007;19:989–1004. doi: 10.1017/S0954579407000491. [DOI] [PubMed] [Google Scholar]
- 10.Langley K, Turic D, Rice F, et al. Testing for gene × environment interaction effects in attention deficit hyperactivity disorder and associated antisocial behavior. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:49–53. doi: 10.1002/ajmg.b.30571. [DOI] [PubMed] [Google Scholar]
- 11.Moffitt T, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 12.Rutter M. Genes and Behavior: Nature-nurture interplay explained. Oxford: Blackwell: 2006. [Google Scholar]
- 13.Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of Dopamine Transporter Genotype with Prenatal Smoke Exposure on ADHD Symptoms. J Pediatr. 2008;152:263. doi: 10.1016/j.jpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Kahn R, Khoury M, Nichols T, Lanphear B. Role of Dopamine Transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. J Pediatr. 2003;143:104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 15.Infante-Rivard C, Weinberg C, Guiguet M. Xeniobiotic metabolizing genes and small for gestational age births: Interaction with maternal smoking. Epidemiology. 2006;17:38–46. doi: 10.1097/01.ede.0000187669.34003.b1. [DOI] [PubMed] [Google Scholar]
- 16.Neuman R, Lobos E, Reich W, Henderson C, Sun L, Todd R. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry. 2007;61:1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. Journal of the American Medical Academy. 2002 Jan 9;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Buckholtz J, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Sabol S, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 20.Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 21.Kim-Cohen J, Caspi A, Taylor A, et al. MAOA, maltreatment and gene-environment interaction predicting children's mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 22.Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Dev Psychopathol. 2008;19:1029–1038. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- 23.Alia-Klein N, Goldstein R, Kriplani A, et al. Brain Monoamine Oxidase A activity predicts trait aggression. J Neurosci. 2008 May 7;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberger N, Way B, Taylor S, Welch W, Lieberman M. Understanding genetic risk for aggression: Clues from the brain's response to social exclusion. Biol Psychiatry. 2007;61:1100–1108. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006 Apr 18;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slotkin T. Fetal nicotine or cocaine exposure: Which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 27.Sjoberg R, Nilsson K, Wargelius H, Leppert J, Lindstrom L, Oreland L. Adolescent girls and criminal activity: role of MAOA-LPR genotype and psychosocial factors. Am J Med Genet (Neuropsychiatric Genetics) 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- 28.Rommelse N, Altink M, Arias-Vasquez A, et al. Differential association between MAOA, ADHD and neuropsychological functioning in boys and girls. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008 doi: 10.1002/ajmg.b.30845. [DOI] [PubMed] [Google Scholar]
- 29.Ducci F, Enoch MA, Hodgkinson C, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2007;123:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- 30.Widom C, Brzustowicz L. MAOA and the 'cycle of violence': Childhood abuse and neglect, MAOA genotype and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Cate S, Battistuzzi C, Oquendo M, Brend T, Mann J. An association between a functional polymorphism in the monomaine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen L, Slotkin T, Mencl E, Frost S, Pug K. Gender-Specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- 33.Slotkin T, MacKillop E, Rudder C, Ryde I, Tate C, Seidler F. Permanent, sex-selective effects of prenatal or adolescent nicotine, exposure, separately or sequentially in rat brain regions: Indices of cholinergic and serotenergic synaptic function, cell signaling and neural cell numbre and size at six months of age. Neuropsychopharmacology. 2007;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- 34.Toro R, Leonard G, Lerner J, et al. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33:1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- 35.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Dodge K. Translational science in action: Hostile attributional style and the development of aggressive behavior problems. Dev Psychopathol. 2006;18:791–814. doi: 10.1017/s0954579406060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilman S, Gardener H, Buka S. Maternal smoking during pregnancy and children's cognitive and physical development: A causal risk factor? Am J Epidemiol. 2008;168:522–531. doi: 10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middlecamp Kodl M, Wakschlag L. Does a childhood history of externalizing problems predict smoking during pregnancy? Addict Behav. 2004;29:273–279. doi: 10.1016/j.addbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Thapar A, Fowler T, Rice F, et al. Maternal smoking during pregnancy and Attention Deficit Hyperactivity Disorder symptoms in offspring. Am J Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- 40.Benowitz N. SRNT Sub-Committee on Biochemical Verification. Verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–160. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 41.Pickett K, Rathouz P, Kasza K, Wakschlag L, Wright R. Self-reported smoking, cotinine levels and patterns of smoking during pregnancy. Paediatr Perinat Epidemiol. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 42.Rice F, Lewis A, Harold G, et al. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: The influence of maternal and child characteristics. Early Hum Dev. 2007;83:497. doi: 10.1016/j.earlhumdev.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Wojczynski M, Tiwari H. Definition of phenotype. In: Rao D, Gu CC, editors. Genetic dissection of complex traits. Vol 60. NY: Elsevier; 2008. [Google Scholar]
- 44.Hanrahan J, Tager I, Segal M, et al. The effect of maternal smoking during pregnancy on early infant lung function. American Review of Respiratory Disease. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 45.Tager I, Ngo L, Hanrahan J. Maternal smoking during pregnancy: Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med. 1995;152:977–983. doi: 10.1164/ajrccm.152.3.7663813. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Tager I, Van Vunakis H, Sperizer F, Hanrahan J. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. a prospective cohort study. Int J Epidemiol. 1997;26:978–987. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- 47.Dukic V, Niessner M, Benowitz N, Hans S, Wakschlag L. Modeling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: A deterministic approach. Nicotine and Tobacco Research. 2007;9:453–466. doi: 10.1080/14622200701239530. [DOI] [PubMed] [Google Scholar]
- 48.Mathews J. Smoking during pregnancy in the 1990s. Hyattsville, MD: National Center for Health Statistics; 2001. [PubMed] [Google Scholar]
- 49.Deckert J, Catalano M, Syagailo Y, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- 50.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Loeber R, Green S, Lahey B, Stouthamer-Loeber M. Optimal informants on childhood disruptive behavior. Dev Psychopathol. 1989;1:317–338. [Google Scholar]
- 52.Langley K, Holmans P, van den Bree M, Thapar A. Effects of low birthweight, maternal smoking during pregnancy and social class on the phenotypic manifestations of Attention Deficit Hyperactivity Disorder and associated antisocial behaviour: Investigation in a clinical sample. BMC Psychiatry. 2007;7 doi: 10.1186/1471-244X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nowicki S, Duke MP. Individual differences in the nonverbal communication of affect: The Diagnostic Analysis of Nonverbal Accuracy Scale. Journal of Nonverbal Behavior. 1994;18:9–35. [Google Scholar]
- 54.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 55.Zucker R, Noll R, Ham H, Fitzgerald H, Sullivan L. Assessing antisociality wtih the Antisocial Behavior Checklist: Reliability and validity studies: University of Michigan & Michigan State University. 1994 [Google Scholar]
- 56.Achenbach TM. Manual for the Young Adult Self-Report and Young Adult Behavior Checklist. Burlington: University of Vermont Department of Psychiatry; 1997. [Google Scholar]
- 57.Caspi A, Taylor A, Smart M, Jackson J, Tagami S, Moffitt T. Can women provide reliable information about their children's fathers? Cross-informant agreement about men's lifetime antisocial behaviour. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2001;42:915–920. doi: 10.1111/1469-7610.00787. [DOI] [PubMed] [Google Scholar]
- 58.Hetherington EM, Clingempeel WG, Anderson ER, et al. Coping with marital transitions: A family systems perspective. Monographs of the Society for Research in Child Dev. 1992;57(2/3) [Google Scholar]
- 59.Fisher R. The design and analysis of experiments. Edinburgh: Oliver & Boyd; 1935. [Google Scholar]
- 60.Neuhaus G, Zhu L. Permutation tests for multivariate location problems. Journal of Multivariate Analysis. 1999;69:167–192. [Google Scholar]
- 61.Meyer-Lindenberg A, Weinberger D. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Neuroscience. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 62.Buckholtz J, Callicott J, Kolachana B et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2007:1–12. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- 63.Blair R. Neurocognitive markers of aggression, the antisocial personality disorders and psychopathy. J Neurol Neurosurg Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 65.Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev Psychopathol. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- 66.Fowler J, Alia-Klein N, Kriplani A et al. Evidence that brain MAOA activity does not correspond to MAOA genotype in healthy male subjects. Biol Psychiatry. 2007;62:355–358. doi: 10.1016/j.biopsych.2006.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pine D. Research Review: A neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 68.Thapar A, Harold G, Rice F et al. Do intrauterine or genetic influences explain the foetal origins of chronic disease? A novel experimental method for disentangling effects. BMC Medical Research Methodology. 2007;7:25. doi: 10.1186/1471-2288-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Button T, Thapar A, McGuffin P. Relationship between antisocial behavior, attention-deficit hyperactivity disorder and maternal prenatal smoking. Br J Psychiatry. 2005;187:155–160. doi: 10.1192/bjp.187.2.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.