Abstract
The accepted target for organophosphorus agent (OP) binding to enzymes is the active site serine in the consensus sequence Gly × Ser × Gly. New motifs have been identified by using mass spectrometry to fragment OP-labeled peptides. It has been found that OP can make covalent bonds with tyrosine and lysine in proteins that have no active site serine. The OP-tyrosine bond is stable, and does not undergo the decay seen with OP-serine. Information on OP-binding to tyrosine has been applied to diagnosis of OP exposure, through the use of mass spectrometry to detect OP-labeled albumin in human and animal plasma. It is expected that the new OP binding motif will aid in the search for a mechanism of low dose OP toxicity. It is hypothesized that proteins involved in axonal transport, especially proteins whose function depends on reversible phosphorylation, are prime candidates for a role in OP-induced neurodegeneration. Treatment of neurodegenerative disorders could be developed by identifying methods to reverse OP binding to tyrosine.
Keywords: Pesticides, nerve agents, organophosphorus agents, tyrosine, lysine
1. Introduction
Historical perspective on OP binding to the active site serine in the serine hydrolase superfamily
In 1953, scientists knew that organophosphorus agents irreversibly inhibit chymotrypsin, trypsin, acetylcholinesterase, and butyrylcholinesterase (pseudocholinesterase). It was argued that the OP binding site had to be histidine. However, Norwood Schaffer isolated L-serine phosphoric acid from diisopropylfluorophosphate-inhibited chymotrypsin, thus providing the first evidence that the label was on serine [1]. This result was unacceptable to biochemists because they argued that serine could not ionize at physiological pH and therefore could not be directly involved in a reaction with OP. It was suggested that histidine was the original reactive group and that the OP was transferred from histidine to serine [2]. Labeling experiments with eel acetylcholinesterase [3], trypsin [4], horse serum butyrylcholinesterase [5], and horse liver aliesterase [6] confirmed that the OP-labeled residue in these proteins was serine. OP-labeled histidine was never found in these proteins. Determination of the crystal structure of chymotrypsin by Blow et al., led to the understanding that ionization of the active site serine was promoted by interaction with histidine and aspartic acid [7]. Thus, the concept of the catalytic triad for serine proteases and esterases was born. A catalytic triad of serine, glutamic acid, and histidine has been found in the crystal structures of acetylcholinesterase [8] and butyrylcholinesterase [9]. Today it is generally accepted that serine, in the consensus sequence GXSXG, is the active site nucleophile and that this serine makes a covalent bond with organophosphorus agents directly. Covalent binding to the active site serine results in irreversible inhibition of enzymes in the serine hydrolase superfamily. It is generally accepted that the acute toxicity of OP is due to inhibition of acetylcholinesterase in nerve synapses.
2. OP binding to histidine
A 60 kDa glycosylated carboxylesterase isolated from rabbit liver was treated with [H3]-diisopropylfluorophosphate and digested with proteases [10,11]. Peptides were purified by HPLC and sequenced. Radiolabeled diisopropylphosphate was found on the active site serine as well as on the histidine of the catalytic triad. The covalent bond to histidine was stable in 88% formic acid, used during peptide purification. Covalent binding of OP to histidine had long been hypothesized but has never before or since been demonstrated to occur in a protein.
3. OP binding to proteins without inhibiting activity
A single tyrosine residue was covalently labeled by treatment of papain, egg-white lysozyme, and Taka-amylase A with diisopropylfluorophosphate [12–14]. The OP-modified enzymes retained full activity with casein, glycol chitin, and amylose [12]. Up to 4 tyrosines were labeled in stem bromelain by treatment with a 400-fold molar excess of diisopropylfluorophosphate in pH 8.2 buffer [15]. Activity toward casein was unaffected. At pH 11 one tyrosine and 3 additional residues, that were not tyrosine, were modified by treatment of egg-white lysozyme with a 300-fold molar excess of diisopropylfluorophosphate [14]. At pH 9.5 only one residue was labeled, and that residue was tyrosine. The organophosphorylated lysozyme retained full activity on glycol chitin and p-nitrophenyl acetate, but showed 25% decrease in lytic activity on Micrococcus lysodeikticus cells.
4. OP binding to tyrosine
Low dose toxicity
Low doses of OP that do not inhibit acetylcholinesterase nevertheless cause cognitive deficits in some people. Epidemiologists have linked chronic low dose OP exposure to Parkinson’s disease, neurologic dysfunction, Gulf War Illness [16], and depression [16–19].
Bronchoconstriction is caused by low doses of organophosphorus pesticides that inhibit M2 muscarinic receptor function without inhibiting acetylcholinesterase activity [20]. M2 muscarinic receptors covalently bind chlorpyrifos oxon [21] at an unknown site. The M2 muscarinic receptor has no active site serine. Therefore it is a clear example of a protein outside the serine hydrolase superfamily for which reaction with low doses of OP disrupts function.
Chlorpyrifos oxon, at concentrations far below those required to inhibit acetylcholinesterase, reduces neurite outgrowth in primary cultures of sympathetic neurons derived from the embryonic rat superior cervical ganglia. This is an example of disruption of neuron morphogenesis by doses of OP too low to bind to the classical active site serine [22].
Proteins important for axonal transport have been implicated in neurodegenerative diseases [23] and are prime candidates for proteins responsible for OP low dose neurotoxicity. Neurons rely heavily on phosphorylation-dependent intracellular signaling mechanisms [23], suggesting that irreversible OP binding to a site that is normally reversibly phosphorylated will result in neuron dysfunction. If multiple proteins are involved, it follows that different OP will react differently with the different targets, and that the identity of the OP target will depend on the identity of the OP.
Albumin
Our search for additional OP targets was begun by treating live mice with a biotin-labeled OP called FP-biotin [24]. Doses of FP-biotin that did not inhibit acetylcholinesterase were found to label albumin. Mass spectral analysis of pure human albumin, pure mouse albumin, and human plasma identified tyrosine 411 as the amino acid that is labeled by soman, sarin, chlorpyrifos oxon, dichlorvos, diisopropylfluorophosphate, and FP-biotin [25,26]. Additional tyrosines as well as serines are labeled when the experimental conditions are maximized, but tyrosine 411 is always the most reactive residue in human albumin [27,28].
Human plasma treated ex vivo with OP was found to have OP-labeled albumin [25]. Human plasma from individuals who attempted suicide by drinking dichlorvos had dichlorvos-labeled albumin in their blood; the OP label was on tyrosine 411 (Li et al. unpublished). Live guinea pigs treated with nerve agents had OP-labeled tyrosine in plasma digests, the tyrosine presumably originating from albumin [29,30].
OP-albumin adducts on tyrosine have several advantages over OP-cholinesterase as biomarkers of OP exposure. The OP-tyrosine adduct on albumin is more stable than the OP-serine adduct on butyrylcholinesterase as shown by the finding that OP-tyrosine is detectable in guinea pigs 24 days after treatment with nerve agents, at a time when OP-butyrylcholinesterase is undetectable [29]. In vitro studies have shown that the half-life of the tyrosine 411 adduct with soman is 20 days at pH 7.4 and 22° C [26]. The chlorpyrifos oxon adduct on tyrosine 411 of human albumin loses only 25% of the OP after 8 months incubation at pH 7.4 and 22° C [28]. The stability of the OP-tyrosine adduct makes OP-albumin a suitable biomarker of exposure to OP nerve agents and OP pesticides.
Another characteristic of OP-tyrosine adducts is that they do not age [25,26,30–32]. For example, the pinacolyl group of soman is retained in the soman-tyrosine adduct [26]. This contrasts with soman-serine adducts on cholinesterases which lose the pinacolyl group about 2 min after covalent binding to the active site serine. Thus, the character of the OP-albumin adduct is more closely akin to that of the original OP than is the OP-cholinesterase adduct. This makes identification of the original OP more reliable.
Oxime therapy displaces the OP from serine, but not from tyrosine [29]. Therefore, identification of the OP to which a patient was exposed can be made after the patient has been treated with oxime.
Tubulin
OP-labeling of albumin is not an explanation for low dose toxicity. However, covalent binding of OP to tyrosine in albumin suggests that proteins other than the serine hydrolases could be involved in low dose OP toxicity. Tubulin is a candidate protein for this role. Tubulin polymerizes to microtubules. Microtubules play an integral part in axonal transport, and behavioral studies correlate chronic low dose chlorpyrifos exposure with cognitive dysfunction and with disruptions in axonal transport [33,34]. Microtubules are essential for the architecture of neuronal cells, as well as for transport of cell organelles from the cell body to the axon. Drugs such as taxol that target microtubule structure result in cell death.
Studies with pure bovine tubulin have shown that tubulin is readily labeled by chlorpyrifos oxon, soman, sarin, dichlorvos, and FP-biotin [35,36]. The labeled residues are tyrosine and lysine. Tubulin function is disrupted by OP binding as visualized by atomic force microscopy nanoimages of microtubule structure, which show that in the presence of 0.005 mM chlorpyrifos oxon, tubulin polymerizes to abnormally short microtubules [37]. Mice treated with chlorpyrifos at a dose that does not inhibit acetylcholinesterase have diethoxyphosphorylated tubulin in the brain [38].
A broader view of low dose toxicity suggests that OP may be modifying many proteins associated with tubulin in addition to tubulin itself, and that OP modification of these axonal transport proteins results in loss of synaptic function, alterations in axonal connectivity, and finally in cell death [23,39,40].
OP binding to tyrosine as a general phenomenon
The question arose whether OP binding to albumin and tubulin was a special case or whether other proteins could also make a covalent bond with OP. Purified proteins were treated with diisopropylfluorophosphate, chlorpyrifos oxon, FP-biotin, soman, or sarin and the labeled peptide and labeled amino acid were identified by mass spectrometry. Table 1 summarizes the proteins and the OP-modified tyrosines in proteins that have been identified to date. Only the most reactive tyrosine, Tyr 411, is listed in Table 1 for human albumin. Additional OP-labeled tyrosines in human albumin can be found in Ding et al. [28]. A more complete list of proteins labeled by OP on tyrosine and the sequences of OP-labeled peptides is found in Schopfer et al. [41]. It is concluded that OP binding to tyrosine is a general phenomenon. Many proteins can be labeled on tyrosine by OP.
Table 1.
Proteins labeled by OP on tyrosine.
| protein | accession number | OP-labeled tyrosine | reference |
|---|---|---|---|
| human albumin | gi 28592 | NALLVRY411TKKVPQ | [25,27] |
| bovine albumin | gi 30794280 | NALIVRY410TRKVPQ | [45] |
| guinea pig albumin | gi 33518896 | NALAVRY411TQKAPQ | [29] |
| mouse albumin | gi 3647327 | NAILVRY411TQKAPQ | present report |
| mouse albumin | gi 3647327 | YEKLGEY401GFQNAI | present report |
| bovine tubulin alpha | gi 73586894 | EVRTGTY83RQLFHP | [36] |
| bovine tubulin beta | gi 75773583 | EATGGKY59VPRAVL | [36] |
| bovine tubulin beta | gi 75773583 | SRGSQQY281RALTVP | [36] |
| bovine tubulin beta | gi 75773583 | SKIREEY159PDRIMN | [36] |
| mouse tubulin beta | gi 21746161 | LERINVY50Y51NEATGN | [41] |
| human transferrin | gi 136191 | RKPVDEY257KDCHLA | [46] |
| human transferrin | gi 136191 | RKPVEEY593ANCHLA | [46] |
| human kinesin 3C motor domain | gi 160286524 | YLVRASY157LEIYQE | [47] |
| mouse transferrin | gi 21363012 | RKPVDQY257EDCY261LARIPS | [46] |
| mouse transferrin | gi 21363012 | RMDYRLY333LGHNY338VTAIRN | [46] |
| mouse transferrin | gi 21363012 | GIFPKGY448Y449AVAVVK | [46] |
| mouse transferrin | gi 21363012 | QGCAPGY510EKNSTL | [46] |
| mouse transferrin | gi 21363012 | PNNKEEY534NGY537TGAFRC | [46] |
| mouse ATP synthase | gi 20455479 | QKILQDY431KSLQDI | [47] |
| papain | gi 129614 | NEGALLY256SIANQP | [13] |
Numbering for residues in albumin is for mature albumin minus the signal peptide. Numbering for other proteins uses Met in the signal peptide as residue 1. Accession numbers are from the NCBI nonredundant protein database.
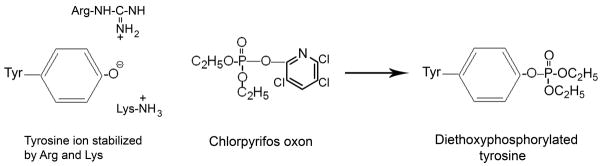
Not all tyrosines in a protein are labeled by OP. The most reactive tyrosines are accessible to solvent and are presumably in an environment that stabilizes the ionized form of the phenolic group of tyrosine. Table 1 summarizes the amino acid sequences around the OP-labeled tyrosines. There is no consensus sequence for the reactive tyrosines, but a positively charged group (Arg, Lys, His) is usually present within 5 residues of the reactive tyrosine. The nearby positively charged residue could stabilize the tyrosinate form of the tyrosine making it a better nucleophile in reactions with OP (Figure 1).
Figure 1.
Reaction of tyrosinate anion with chlorpyrifos oxon to yield diethoxyphosphorylated tyrosine. The anion of tyrosine is stabilized by interaction with positively charged arginine and lysine side chains. The tyrosine anion reacts with chlorpyrifos oxon to make a covalent bond with diethoxyphosphate while displacing 3,5,6-trichloro-2-pyridinol.
The proteins in Table 1 react slowly with OP compared to the rate of reaction of OP with the cholinesterases. The fact that a particular tyrosine in albumin is more reactive than other tyrosines suggests that the reactivity of tyrosine is enhanced by its environment. This further suggests that proteins with even higher reactivity than albumin may exist. If these proteins are in low abundance but have an essential physiological function, then OP binding to such proteins could contribute to low dose toxicity.
OP binding to free tyrosine and to tyrosine in short peptides
Free tyrosine in pH 7 buffer is labeled by DFP, but is not labeled by paraoxon (E600) [42]. Short peptides containing 4 to 5 amino acids including tyrosine are readily labeled by OP when the peptide is treated with a 10-fold molar excess of OP in 10 mM TrisCl pH 8.0 or in 10 mM ammonium bicarbonate pH 8.3. For example a 1 mg/ml solution of peptide RYGRK (1.47 mM) treated with 14.7 mM soman for two days at 37° C labels up to 80% of the tyrosine in that peptide.
5. OP binding to lysine
Mass spectrometry analysis of purified proteins treated with chlorpyrifos oxon or diisopropylfluorophosphate identified OP-labeled lysine. OP-labeled lysines were found in human albumin, human keratins 1 and 10, bovine tubulin alpha and beta, bovine actin, and mouse transferrin [43]. These same proteins also had OP-labeled tyrosine.
Lysine acetylation contributes to RNA splicing, regulation of the cell cycle, chromatin remodeling, DNA replication, transcription, actin cytoskeleton remodeling, nuclear transport, and the function of chaperone proteins [44]. Binding of OP to lysine could interfere with acetylation of lysine and therefore with the function of proteins involved in biological processes.
6. Potential applications and significance of the new OP binding motif
Biomarker of OP exposure
The recognition of tyrosine 411 in albumin as a binding site for OP has led to the development of mass spectrometry assays for detection of OP-labeled albumin in animals and humans exposed to OP. Advantages of using albumin as a biomarker of exposure are 1) OP-labeled albumin is stable. 2) OP-labeled albumin does not age. 3) Albumin is present in saliva, suggesting that an assay using saliva could be developed.
Antibodies for detection of OP exposure
Antibodies for detection of OP-tyrosine and OP-lysine adducts could be developed. Antibodies against OP-tyrosine adducts are more likely to be useful than antibodies against OP-serine adducts because OP-tyrosine adducts are stable, whereas OP-serine adducts undergo spontaneous decay to dehydroalanine as well as to dealkylated “aged” adducts. Decay of the OP-serine adducts complicates their use as antigens for the production of OP-specific antibodies. Decay of OP-serine adducts formed in vivo reduces their potential to be identified.
Peptide scavengers for decontamination
Short peptides could be developed for use as decontaminants of nerve agent spills. These short peptides would include tyrosine and positively charged amino acids to activate the tyrosine for reaction with OP.
Understanding low dose toxicity
The molecular mechanism by which low dose OP exposure results in cognitive dysfunction is not yet understood. Advances in this area are slowed, because it is not yet known which proteins in the brain are involved. Progress in understanding the relationship between low dose OP exposure and disease will be accelerated once the relevant OP-modified proteins have been identified. The new OP binding motifs to tyrosine and lysine suggest that almost any protein should be considered when searching for proteins that could be involved in low dose, OP-induced cognitive dysfunction.
Treatment of low dose toxicity
Oximes do not remove the OP from binding to tyrosine. A search for methods to reverse OP binding to tyrosine could lead to a treatment for cognitive dysfunction from low dose exposure to OP.
7. Conclusion
Mass spectrometry has provided conclusive proof that proteins with no active site serine covalently bind organophosphorus agents on tyrosine and lysine. This result suggests new directions to search for mechanisms of low dose OP toxicity.
Acknowledgments
Supported by the U.S. Army Medical Research and Materiel Command W81XWH-07-2-0034, the National Institutes of Health U01 NS058056 and P30CA36727.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schaffer NK, May SC, Jr, Summerson WH. Serine phosphoric acid from diisopropylphosphoryl chymotrypsin. J Biol Chem. 1953;202:67–76. [PubMed] [Google Scholar]
- 2.Bergmann F. Fine structure of the active surface of cholinesterases and the mechanism of enzymatic ester hydrolysis. Disc Faraday Soc. 1955;20:126–134. [Google Scholar]
- 3.Schaffer NK, May SC, Jr, Summerson WH. Serine phosphoric acid from diisopropylphosphoryl derivative of eel cholinesterase. J Biol Chem. 1954;206:201–207. [PubMed] [Google Scholar]
- 4.Dixon GH, Kauffman DL, Neurath H. Amino acid sequence in the region of diisopropyl phosphoryl binding in DIP-trypsin. J Am Chem Soc. 1958;80:1260–1261. [Google Scholar]
- 5.Jansz HS, Brons D, Warringa MG. Chemical nature of the DFP-binding site of pseudocholinesterase. Biochim Biophys Acta. 1959;34:573–575. doi: 10.1016/0006-3002(59)90320-8. [DOI] [PubMed] [Google Scholar]
- 6.Jansz HS, Posthumus CH, Cohen JA. On the active site of horse-liver ali esterase. II. Amino acid sequence in the DFP-binding site of the enzyme. Biochim Biophys Acta. 1959;33:396–403. doi: 10.1016/0006-3002(59)90129-5. [DOI] [PubMed] [Google Scholar]
- 7.Blow DM, Birktoft JJ, Hartley BS. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969;221:337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- 8.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 9.Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 10.Korza G, Ozols J. Complete covalent structure of 60-kDa esterase isolated from 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced rabbit liver microsomes. J Biol Chem. 1988;263:3486–3495. [PubMed] [Google Scholar]
- 11.Ozols J. Isolation and characterization of a 60-kilodalton glycoprotein esterase from liver microsomal membranes. J Biol Chem. 1987;262:15316–15321. [PubMed] [Google Scholar]
- 12.Murachi T. A general reaction of diisopropylphosphorofluoridate with proteins without direct effect on enzymic activities. Biochim Biophys Acta. 1963;71:239–241. doi: 10.1016/0006-3002(63)91021-7. [DOI] [PubMed] [Google Scholar]
- 13.Chaiken IM, Smith EL. Reaction of a specific tyrosine residue of papain with diisopropylfluorophosphate. J Biol Chem. 1969;244:4247–4250. [PubMed] [Google Scholar]
- 14.Murachi T, Miyake T, Yamasaki N. Alkylphosphorylation of hen egg-white lysozyme by diisopropylphosphorofluoridate. J Biochem. 1970;68:239–244. doi: 10.1093/oxfordjournals.jbchem.a129351. [DOI] [PubMed] [Google Scholar]
- 15.Murachi T, Inagami T, Yasui M. Evidence for alkylphosphorylation of tyrosyl residues of stem bromelain by diisopropylphosphorofluoridate. Biochemistry. 1965;4:2815–2825. doi: 10.1021/bi00888a036. [DOI] [PubMed] [Google Scholar]
- 16.Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, Lyons MJ, Henderson WG, Kang HK, Eisen SA, Murphy FM. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J Int Neuropsychol Soc. 2009;15:717–729. doi: 10.1017/S1355617709990294. [DOI] [PubMed] [Google Scholar]
- 17.Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- 18.Beseler CL, Stallones L, Hoppin JA, Alavanja MC, Blair A, Keefe T, Kamel F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ Health Perspect. 2008;116:1713–1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci. 2005;83:166–176. doi: 10.1093/toxsci/kfi001. [DOI] [PubMed] [Google Scholar]
- 21.Bomser JA, Casida JE. Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro. Toxicol Lett. 2001;119:21–26. doi: 10.1016/s0378-4274(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 22.Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Schopfer LM, Hinrichs SH, Masson P, Lockridge O. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay for organophosphorus toxicants bound to human albumin at Tyr411. Anal Biochem. 2007;361:263–272. doi: 10.1016/j.ab.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Nachon F, Froment MT, Verdier L, Debouzy JC, Brasme B, Gillon E, Schopfer LM, Lockridge O, Masson P. Binding and hydrolysis of soman by human serum albumin. Chem Res Toxicol. 2008;21:421–431. doi: 10.1021/tx700339m. [DOI] [PubMed] [Google Scholar]
- 27.Means GE, Wu HL. The reactive tyrosine residue of human serum albumin: characterization of its reaction with diisopropylfluorophosphate. Arch Biochem Biophys. 1979;194:526–530. doi: 10.1016/0003-9861(79)90647-7. [DOI] [PubMed] [Google Scholar]
- 28.Ding SJ, Carr J, Carlson JE, Tong L, Xue W, Li Y, Schopfer LM, Li B, Nachon F, Asojo O, Thompson CM, Hinrichs SH, Masson P, Lockridge O. Five tyrosines and two serines in human albumin are labeled by the organophosphorus agent FP-biotin. Chem Res Toxicol. 2008;21:1787–1794. doi: 10.1021/tx800144z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read RW, Riches JR, Stevens JA, Stubbs SJ, Black RM. Biomarkers of organophosphorus nerve agent exposure: comparison of phosphylated butyrylcholinesterase and phosphylated albumin after oxime therapy. Arch Toxicol. 2010;84:25–36. doi: 10.1007/s00204-009-0473-4. [DOI] [PubMed] [Google Scholar]
- 30.Williams NH, Harrison JM, Read RW, Black RM. Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch Toxicol. 2007;81:627–639. doi: 10.1007/s00204-007-0191-8. [DOI] [PubMed] [Google Scholar]
- 31.Black RM, Harrison JM, Read RW. The interaction of sarin and soman with plasma proteins: the identification of a novel phosphonylation site. Arch Toxicol. 1999;73:123–126. doi: 10.1007/s002040050596. [DOI] [PubMed] [Google Scholar]
- 32.Adams TK, Capacio BR, Smith JR, Whalley CE, Korte WD. The application of the fluoride reactivation process to the detection of sarin and soman nerve agent exposures in biological samples. Drug Chem Toxicol. 2004;27:77–91. doi: 10.1081/dct-120027901. [DOI] [PubMed] [Google Scholar]
- 33.Terry AV, Jr, Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther. 2007;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- 34.Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther. 2003;305:375–384. doi: 10.1124/jpet.102.041897. [DOI] [PubMed] [Google Scholar]
- 35.Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O. Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol Appl Pharmacol. 2009;240:149–158. doi: 10.1016/j.taap.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents. Chem Biol Interact. 2008;175:180–186. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigoryan H, Lockridge O. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: Implications for neurotoxicity. Toxicol Appl Pharmacol. 2009;240:143–148. doi: 10.1016/j.taap.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. 2010 doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146:330–339. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schopfer LM, Grigoryan H, Li B, Nachon F, Masson P, Lockridge O. Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: Characteristic mass fragments and a new binding motif for organophosphates. J Chromatogr B Analyt Technol Biomed Life Sci. 2010 doi: 10.1016/j.jchromb.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashbolt RF, Rydon HN. The action of diisopropyl phosphorofluoridate and other anticholinesterases on amino acids. Biochem J. 1957;66:237–242. doi: 10.1042/bj0660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grigoryan H, Li B, Xue W, Grigoryan M, Schopfer LM, Lockridge O. Mass spectral characterization of organophosphate-labeled lysine in peptides. Anal Biochem. 2009;394:92–100. doi: 10.1016/j.ab.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 45.Schopfer LM, Champion MM, Tamblyn N, Thompson CM, Lockridge O. Characteristic mass spectral fragments of the organophosphorus agent FP-biotin and FP-biotinylated peptides from trypsin and bovine albumin (Tyr410) Anal Biochem. 2005;345:122–132. doi: 10.1016/j.ab.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Li B, Schopfer LM, Grigoryan H, Thompson CM, Hinrichs SH, Masson P, Lockridge O. Tyrosines of human and mouse transferrin covalently labeled by organophosphorus agents: a new motif for binding to proteins that have no active site serine. Toxicol Sci. 2009;107:144–155. doi: 10.1093/toxsci/kfn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grigoryan H, Li B, Anderson EK, Xue W, Nachon F, Lockridge O, Schopfer LM. Covalent binding of the organophosphorus agent FP-biotin to tyrosine in eight proteins that have no active site serine. Chem Biol Interact. 2009;180:492–498. doi: 10.1016/j.cbi.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]