Abstract
OBJECTIVE
Long-term administration of intravenous bisphosphonates like pamidronate is associated with jaw osteonecrosis but axial and appendicular bones are unaffected. Pathogenesis of bisphosphonate-associated jaw osteonecrosis may relate to skeletal-site specific effects of bisphosphonates on osteogenic differentiation of bone marrow stromal cells (BMSCs) of orofacial and axial/appendicular bones. This study evaluated and compared skeletal site-specific osteogenic response of mandible (orofacial bone) and iliac crest (axial bone) human BMSCs to pamidronate.
MATERIALS AND METHODS
Mandible and iliac crest BMSCs from six normal healthy volunteers were established in culture and tested with pamidronate to evaluate and compare cell survival, osteogenic marker alkaline phosphatase, osteoclast differentiation in co-cultures with CD34+ hematopoietic stem cells, gene expression of receptor activator of NFκB ligand (RANKL) and osteoprotegerin, and in vivo bone regeneration.
BRESULTS
Mandible BMSCs were more susceptible to pamidronate than iliac crest BMSCs based on decreased cell survival, lower alkaline phosphatase production and structurally less organized in vivo bone regeneration. Pamidronate promoted higher RANKL gene expression and osteoclast recruitment by mandible BMSCs.
CONCLUSION
Mandible and iliac crest BMSC survival and osteogenic differentiation are disparately affected by pamidronate to favor dysregulated mandible bone homeostasis.
Keywords: Bisphosphonate, Osteonecrosis, Jaw, Stem cells, Bone
Introduction
Two nitrogen-containing bisphosphonates, pamidronate (Aredia ™) and zoledronic acid (Zometa™) used to treat bone-associated cancer metastasis cause osteonecrosis of the jaws. Bisphosphonate-associated jaw osteonecrosis (BJON) is characterized by tissue dehiscence, chronic bone devitalization, hypocellularity, lytic radiographic features and it is usually refractory to therapy (Marx et al., 2005; Migliorati et al., 2005; Migliorati et al., 2006; Ruggiero et al., 2004). BJON tends to occur in craniofacial bones (maxilla, mandible and an isolated case in external auditory canal (Polizzotto et al., 2006) indicating that it favors craniofacial bones over other skeletal sites. Why this occurs is still unclear. While bisphosphonates are widely used to treat osteoporosis, pamidronate and zoledronic acid are administered intravenously at high doses to treat hypercalcemia of malignancy, Paget’s disease, and osteolytic lesions of multiple myeloma and cancer metastasis.
Bisphosphonates are pyrophosphates analogues with carbon replacing oxygen in the pyrophosphate bond. They have high affinity for hydroxyapatite and remain unmetabolized for long periods of time because of their resistance to enzymatic hydrolysis, heat and most chemicals. In addition, bisphosphonates prevent hydroxyapatite crystal formation, aggregation and dissolution (Fleisch, 2000). Bisphosphonates directly inhibit osteoclast function when internalized during bone resorption (Russell, 2006). Nitrogen-containing bisphosphonates like pamidronate inhibit farnesyl pyrophosphate synthase, an enzyme responsible for the formation of farnesyl pyrophosphate (Roelofs et al., 2006) Inhibition of this pathway results in disruption of protein prenylation, which is vital for the function of essential signaling proteins (GTPases).The inhibitory action of bisphosphonates on farnesyl pyrophosphate synthase, and consequently osteoclasts, as well as high binding affinities to hydroxyapatite both contribute to their high in vivo antiresorptive potency and long duration of action (Michael J. Rogers, 2000; Russell, 2006). Bisphosphonates also inhibit osteoclastic activity indirectly through osteoblasts. Interaction of osteoblast cell surface receptor activator of NFκB ligand (RANKL) with RANK on hematopoietic osteoclast precursor cells enhances osteoclast recruitment and activation. However osteoblasts also secrete osteoprotegerin (OPG), a soluble receptor that competes with RANKL for RANK to inhibit osteoclast recruitment and control osteoblast-osteoclast balance (Kostenuik, 2005; Lacey et al., 1998). RANKL is also expressed on the surface of bone marrow stromal cells (BMSCs), a putative population of postnatal stem cells within the bone marrow organ that can differentiate into multiple cell types including pre-osteoblasts and osteoblasts. Thus BMSCs indirectly modulate osteoblast-osteoclast balance.
It is unclear why BJON is limited to the craniofacial bones. One possibility is the skeletal site-specific osteogenic properties of BMSCs in maxilla and mandible (orofacial bones) compared with those in axial and appendicular bone based on their different embryological origins (Akintoye et al., 2006). Maxilla, mandible including alveolar bone, dentine, pulp and periodontal ligament are formed exclusively by neural crest cells while axial and appendicular bones develop from mesoderm (Chai et al., 2000; Charbord et al., 1996). In same individuals, orofacial BMSCs (from maxilla and mandible) have more proliferative and osteogenic capacities than iliac crest BMSCs (Akintoye et al., 2006). Since BJON is jaw-specific, and orofacial BMSCs are developmentally and phenotypically different from those of iliac crest, we hypothesized that pamidronate modulates in vitro and in vivo osteogenic differentiation of human BMSCs in a skeletal site-specific pattern; a property that may relate to jaw-specific pathogenesis of BJON.
Material and Methods
Subjects and cell culture
After written informed consent, 6 healthy normal, unmedicated volunteers who needed 3rd molar surgery were enrolled in an Institutional Review Board-approved protocol at the University of Pennsylvania, Philadelphia PA. Sample size was based on previous studies that showed 4 subjects were sufficient to demonstrate skeletal site-specific differences at a power of 0.8 and α level of 0.05 (Akintoye et al., 2006; von Knoch et al., 2005). During 3rd molar surgery, trabecular bone with marrow from mandible (orofacial skeletal site) and bone marrow aspirate from iliac crest (axial skeletal site) were obtained simultaneously from each individual. From the two skeletal sites, nucleated cells were isolated. Primary culture of human BMSCs were established with growth medium of α-modified minimum essential medium (α-MEM) supplemented with 20% fetal bovine serum (FBS) (Equitech Bio Inc, Kerville TX), 100 U/ml penicillin, 100 mg/ml streptomycin sulfate and 2 mM glutamine (BioSource International Camarillo CA), incubated at 37 °C in a humidified atmosphere of 5% CO2 and air as previously described (Akintoye et al., 2006).
Dose response and cell proliferation
Dose response of each BMSC type to pamidronate was compared by sub-culturing early passage (passage 2) cells in triplicate wells of 24-well plates at a seeding density of 104 cells/cm2 in growth medium. After 24 hours, growth medium was replaced with fresh ‘modified growth medium’ in which FBS was replaced with 1% ITS+ premix (BD Biosciences, San Jose CA) and different doses of pamidronate (Novartis Pharmaceuticals, East Hanover, NJ) at 0 (control), 2.5, 5, 10 and 25 μg/ml. After 4 days exposure to pamidronate, metabolically active viable cells were assessed using colorimetric WST-1 cell proliferation assay (Roche Applied Science, Indianapolis, IN). In separate plates of parallel experiments, cells were stained with 2 μg/ml DAPI to assess viable BMSCs. Subsequently, 2.5 μg/ml of pamidronate was tested in experiments described below.
Alkaline phosphatase assay
Early passage (passage 2) BMSCs seeded at 104 cells/cm2 were cultured as above in 24-well plates for 4 days before switching to modified growth medium containing 2.5 μg/ml of pamidronate for another 4 days. Alkaline phosphatase activity was assessed on day 8 using the 4-nitrophenyl phosphate reaction as previously described (Akintoye et al., 2006). Alkaline phosphatase activity was normalized to the total protein content of cell lysate. To assess possible recovery from pamidronate effect, pamidronate-treated cells in separate parallel plates were replenished with growth medium without pamidronate on day 9 for additional four days before assaying for alkaline phosphatase on day 12.
Co-culture of human hematopoietic stem cells (HSCs) and bone marrow stromal cells
Human CD34+ HSCs (Cambrex Corporation, East Rutherford, NJ) were added at 5 × 104 cells/cm2 to triplicate wells of 24-well plates containing monolayer of BMSCs cultured with 2.5 μg/ml pamidronate-containing modified medium. The co-culture was maintained at 37°C, in a humidified atmosphere of 5% CO2 and air for 4 weeks. Half of the culture medium was replaced with fresh modified medium containing pamidronate at 3-day intervals.
Tartrate-resistant acid phosphatase (TRAP) staining
After 4 weeks of BMSC and HSC co-culture, modified growth medium was removed, adherent cells were rinsed with PBS pre-warmed to 37°C, fixed with 60% acetone in citrate buffer (pH 5.4) for 1 minute followed by two rinses with warm PBS. Cells were stained for 30 minutes at 37°C with TRAP staining solution (pH 5.0) consisting of 0.05 M acetate buffer, 0.03 M sodium tartrate, 1 mg/ml naphtol AS-MX disodium phosphate, 0.1% Triton X-100 and 0.3 mg/ml Fast Red Violet LB (Mbalaviele et al., 1999). Red staining multinucleated cells with 3 or more nuclei were counted as TRAP+ osteoclast-like cells under a bright field microscope.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Early passage mandible and iliac crest BMSCs were cultured at 104 cells/cm2 in 6-well plates for 24 hours before switching to modified medium containing 2.5 μg/ml pamidronate. Total RNA was isolated from sub-confluent cells using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, California) and first strand cDNA was prepared with First Strand SuperScript™ Double-Stranded cDNA Synthesis Kit (Invitrogen Life Technologies, Carlsbad, California) using an oligo-dT primer. 2 μl of first strand cDNA was added to a total volume of 50 μl PCR buffer containing: 1.5 mM MgCl2, 200 μM dNTP, 2.5U Taq DNA polymerase (Promega, Madison, WI) and 200 nM of each primer set for RANKL (GenBank accession # AB064269.1; forward, 5′-TATGATGGAAGGTTCGTGGCT-3′; reverse, 5′-ATGTTAGAGATCTTGGCCCAG-3′), OPG (GenBank accession # AF134187; forward, 5′-CTT GCC CTG ACC ACT ACT-3′; reverse, 5′-TTG CCG TTT TAT CCT CTC-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, GenBank accession # M33197; forward, 5′-AGCCGCATCTTCTTTTGCGTC-3′; reverse, 5′-TCATATTTGGCAGGTTTTTCT-3′) as internal control. The PCR reaction was carried out in a Perkin Elmer thermal cycler (Perkin Elmer, Boston MA) at 94 °C for 2 minutes for 1 cycle, then 94 °C (1 minute), 55 °C (1 minute), 72 °C (1 minute) for 35 cycles and final extension at 72 °C (10 minutes). 15 μl of the amplified products were separated on 2% agarose gel, stained with ethidium bromide and visualized with Kodak ImageStation 440 (Eastman Kodak, Rochester, NY).
Bone Regeneration
Bone forming ability of pamidronate treated cells was assessed in vivo by transplantation into the subcutis of 8 week-old immunocompromised nude female mice (NIH-III-nu, Charles River Laboratories, Wilmington, MA) following University of Pennsylvania-approved animal protocol. Using both control and pamidronate-treated BMSCs, 2 × 104 cells attached to 40 mg spheroidal hydroxyapatite/tricalcium phosphate (particle size 0.5-1.0 mm, Zimmer, Warsaw IN) were transplanted aseptically into separate subcutaneous pockets (Kuznetsov et al., 1997). Transplants were harvested at 6 weeks, fixed with 4% paraformaldehyde, decalcified in 10% EDTA (pH 8.0) and embedded in paraffin. Hematoxylin/eosin-stained 5 μm sections were evaluated under a brightfield microscope. Bone formation was quantified semi-quantitatively using a previously described and validated bone scoring method that ranged from 0 (no bone evident within the transplant), 1 (minimal bone evident [1 trabecula]), 2 (weak bone formation occupying only a small portion of the transplant), 3 (moderate bone formation occupying a significant portion but less than 50% of the transplant) and 4 (abundant bone formation, occupying more than 50% of the transplant +/− hematopoiesis) (Mankani et al., 2001). To establish human origin of bone within transplants, unstained sections were immunoreacted with rabbit anti-human osteopontin (OPN) polyclonal antibody (Cat # 499265, EMD Biosciences, San Diego, CA) followed by enzymatic staining with broad-spectrum immunoperoxidase AEC kit (Zymed Laboratories, San Francisco, CA) (Denhardt and Noda, 1998).
Statistical Analysis
Results of at least three experiments from all samples were expressed as mean ± standard deviation (SD). Effects of pamidronate on the two cell types were compared either directly or as fold-change over untreated cells. Data analysis was by one-way analysis of variance (ANOVA) followed by post hoc comparisons with the Student’s two-tailed t test. Differences were considered significant at P < 0.05.
Results
Pamidronate reduced mandible BMSCs proliferation in a dose-dependent fashion; however, iliac crest BMSCs proliferation initially increased at 2.5 μg/ml pamidronate before a dose-dependent decrease (Figure 1a). While 2.5 μg/ml dose of pamidronate was sub-lethal to both cell types (Figures 1a and 1b), it significantly increased alkaline phosphatase levels in iliac crest BMSCs with only minor effects on mandible cells (difference significant at p < 0.05), (Figure 1c). These suggest that, iliac crest BMSCs responded to sub-lethal dose of pamidronate with increased alkaline phosphatase production (osteogenic differentiation) and little effect on cell numbers, while mandible BMSCs responded with little increase in alkaline phosphatase and reduced cell numbers (Figures 1a and 1c). After pamidronate-treated cells were re-introduced to normal growth medium without pamidronate, mandible BMSCs recovered from the effect of pamidronate by producing higher level of alkaline phosphatase (Figure 1c), but iliac crest BMSCs did not show the same degree of recovery. Ability of osteoprogenitor cells to increase osteoclast formation is a major mechanism by which bone-forming cells promote bone resorption, therefore both mandible and iliac crest BMSCs induced osteoclast-like cell aggregates when co-cultured with CD34+ HSCs in growth medium without pamidronate (Figures 2a). After addition of pamidronate to the BMSCs-HSC co-culture, the number of osteoclast aggregates was relatively more suppressed in iliac crest than in mandible co-cultures (Figure 2b) but this difference was not statistically significant. RT-PCR analyses of RANKL mRNA levels (Figure 2c) showed that pamidronate increased RANKL expression in mandible BMSCs but not in iliac crest BMSCs. These data provide additional evidence that 2.5 μg/ml pamidronate acted on mandible BMSCs to promote osteoclast differentiation and RANKL gene expression but did not have the same effect on iliac crest BMSCs. OPG mRNA levels between the two cell types were unchanged.
Figure 1.
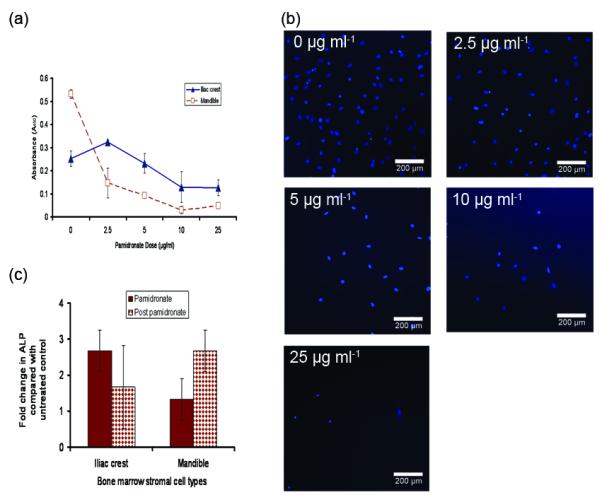
Dose response of mandible and iliac crest bone marrow stromal cells to pamidronate and in vitro osteogenic response. Surviving number of mandible and iliac crest BMSCs after 4 days in medium containing increasing doses of pamidronate show a decrease in number of mandible BMSCs starting from 2.5 μg/ml pamidronate, while cell numbers of iliac crest BMSCs were not significantly affected by pamidronate until the concentration exceeded 5 μg/ml (a). Dose-dependent decrease in cell survival is further demonstrated by a representative DAPI staining of viable mandible BMSCs after 4 days in culture (b). (A similar pattern was displayed by iliac crest BMSCs). 2.5 μg/ml pamidronate increased alkaline phosphatase response of iliac crest BMSCs more than mandible BMSCs (* p< 0.03) which indicate that mandible BMSCs are comparatively less responsive to pamidronate. After replenishing the culture with growth medium without pamidronate for another 4 days, mandible BMSCs responded with enhanced alkaline phosphatase production than iliac crest BMSCs (not significant at p< 0.05) that may indicate a relatively better recovery capacity of mandible BMSCs to short-term exposure to pamidronate (c). (Data presented is mean of n = 6 subjects).
Figure 2.
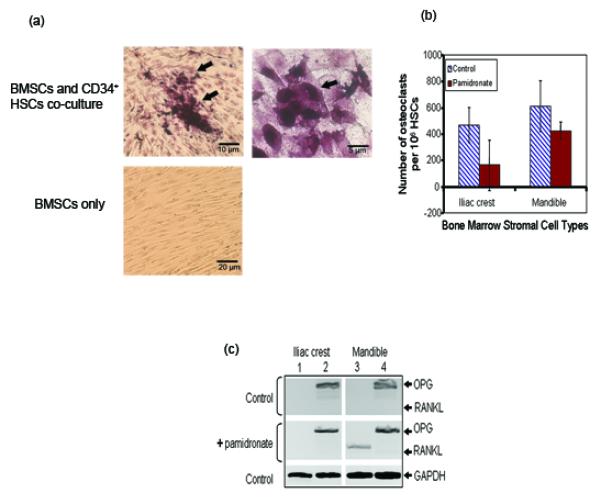
Effect of sub-lethal dose of pamidronate on BMSCs-promoted osteoclast differentiation. Mandible and iliac crest BMSCs co-cultured with CD34+ human HSCs for 4 weeks induced osteoclast-like cells that were positive for tartrate-resistant acid phosphatase. Representative samples are shown at low and high magnifications (a). Pamidronate-treated mandible BMSC-HSC co-culture retained higher osteoclast promoting ability than iliac crest BMSCs based on higher number of CD34+ HSCs that differentiated to osteoclasts (data presented is mean of n = 6 subjects) (b). This is consistent with reverse transcriptase PCR results presented in the representative ethidium bromide-stained agarose gels (c). Note the undetectable expression of receptor activator of NFκB ligand (RANKL) in control cells (lanes 1 and 3; top panel). After pamidronate treatment, there was marked expression of RANKL by mandible BMSCs (lane 3; middle panel) that still remained relatively undetectable in iliac crest cells (lane 1; middle panel). Osteoprotegerin (OPG) level was not different between the two cell types in both control and pamidronate-treated cells (lanes 2 and 4; top and middle panels). (Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control; lower panel).
All pamidronate-treated BMSCs transplants formed ectopic bone, with more histologically observable bone produced by mandible BMSCs (Figure 3b) than iliac crest BMSCs (Figure 3a), but bone laid down by pamidronate-pretreated mandible BMSCs appeared less organized with more hypocellular osteoid-like regions lacking bone-lining cells (Figure 3d) while iliac crest BMSCs formed a fibro-cellular bone matrix with cell-lined surface. Row of lining cells were visible in transplants of untreated control BMSCs (Figures 3a and 3b) and treated iliac crest cells (Figure 3c) but not in bone formed by pamidronate-treated mandible BMSCs (Figure 3d). The ectopic bone was a product of transplanted human BMSCs because bone in the transplants was immunoreactive with rabbit anti-human OPN (Figure 3e). Semi-quantitative assessment of bone formed by control and pamidronate-treated BMSCs showed pamidronate enhanced in vivo bone formation in both control and pamidronate-treated cells (Figure 3f); an indication that pre-treatment of BMSCs with pamidronate did not impair bone-forming ability of either cell type when subsequently transplanted into a pamidronate-free host.
Figure 3.
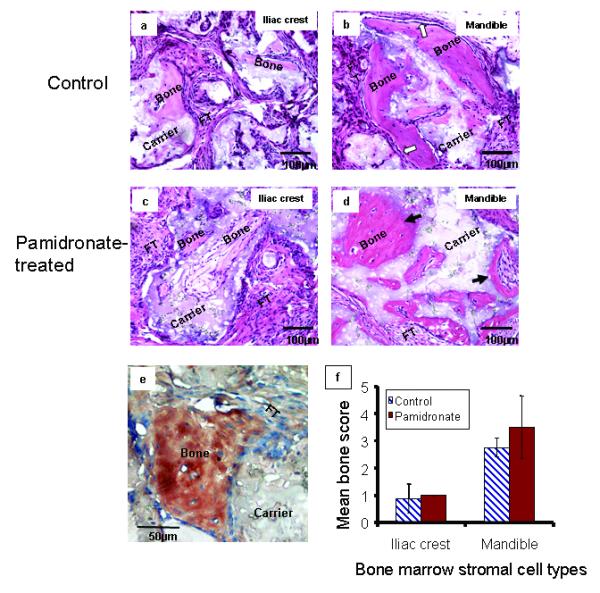
In vivo bone formation by pamidronate-treated mandible and iliac crest BMSCs Hematoxylin/eosin-stained sections of bone formed by transplanted control (a and b) and pamidronate-treated (c and d) BMSCs demonstrated that mandible BMSCs (b and d) formed more histologically observable bone than iliac crest BMSCs (a and c) as previously reported (Akintoye et al., 2006). Note distinct osteoblastic rimming indicative of bone lining cells in mandible control samples (white arrows, b). After pre-treatment with pamidronate followed by transplantation, mandible BMSCs formed structurally less-organized osteoid-like bone with fewer cells and loss of osteoblastic rimming (dark arrows, d) but iliac crest BMSCs formed a more fibrocellular bone matrix with cell-lined surface. Representative immunohistochemical staining with rabbit anti-human osteopontin (mandible control shown) confirms that bone formed by transplanted BMSCs were of human origin and not mice (e). Semi-quantitative analysis of in vivo bone showed that BMSCs pre-treated with pamidronate recovered from effects of pamidronate to form appreciable bone although not significantly higher than their respective controls (f). Higher quantitative bone by both control and pamidronate-treated mandible BMSCs further confirm their better ectopic bone-forming capacity relative to iliac crest BMSCs. (Data presented are representative histology sections and mean of n = 6 subjects).
Discussion
Pamidronate is one of two intravenously administered bisphosphonates highly associated with BJON that prompted several alerts to patients and clinicians (Greenberg, 2004; MedWatch, 2005; Migliorati, 2005; Migliorati et al., 2005; Ruggiero et al., 2004; Woo et al., 2006). Although underlying pathogenesis remains unknown, results presented here indicate pamidronate affected cell survival and osteogenic properties of mandible BMSCs differently from those of iliac crest BMSCs. The pamidronate dose (2.5 μg/ml) tested was sub-lethal to BMSCs, so that actively proliferating and viable cells were compared. This dose was also therapeutically relevant because it lies within 0.73 - 2.61 μg/ml, the range of maximum serum concentration 4 hours after pamidronate infusion (U.S. Package Insert, Novartis Pharmaceuticals, East Hanover, NJ). BMSCs usually exhibit osteogenic properties whether cultured in osteogenic or non-osteogenic medium, and superior osteoblastic differentiation by mandible BMSCs over iliac crest BMSCs has been previously described (Akintoye et al., 2006). In this study, BMSCs were tested under serum-free, non-osteogenic conditions to minimize confounding variables since st culture media contain serum and glucocorticoids. Compared with iliac crest, mandible BMSCs were less osteogenically responsive to pamidronate based on alkaline phosphatase level, but they promoted osteoclast differentiation of HSCs more than iliac crest BMSCs. The more marked osteogenic response of iliac crest BMSCs to pamidronate is consistent with earlier reports that demonstrated increased proliferation and osteogenic differentiation of bone marrow stromal cell lines (Nishida et al., 2005) and iliac crest-derived BMSCs from 4 patients (von Knoch et al., 2005), but BMSCs from orofacial skeletal sites were not evaluated. Interestingly, mandible BMSCs showed better recovery from pamidronate effect by producing more alkaline phosphatase than iliac crest cells after they were re-cultured in growth medium without pamidronate (Figure 1c). Also, the marked differences in RANKL mRNA transcripts and osteoclast aggregates induced by mandible BMSCs (Figure 2b) were noteworthy because osteoclastogenesis in the co-culture system was independent of added growth factors or cytokines. It is likely that mandible BMSCs promoted osteoclast differentiation more than iliac crest BMSCs by expressing higher levels of RANKL to activate RANK on osteoclast progenitors (Figure 2c). Although in vivo bone formed by pamidronate-treated mandible BMSCs appeared less organized structurally (Figure 3d), pamidronate did not disrupt the relatively higher in vivo bone regenerative capacity of mandible BMSCs (Figure 3f). A higher dose or continuous exposure to pamidronate may be needed to demonstrate more site-specific in vivo osteogenic differences. Despite equal cell plating density, control (untreated) mandible BMSCs have consistently demonstrated higher proliferative capacity than iliac crest BMSCs (Akintoye et al., 2006); this may account for their susceptibility to pamidronate since highly proliferating cells easily succumb to external chemical and physical insults (Baran, 1996; Hayflick and Moorhead, 1961). The recovery effect demonstrated in vitro by BMSCs in this study is noteworthy because transplanted pamidronate-treated mandible BMSCs also recovered to form appreciable ectopic bone in host tissue. Comparatively, the reduced cell proliferation and osteogenic differentiation coupled with enhanced RANKL expression and osteoclastogenesis by mandible BMSCs favor disruption of bone homeostasis in the mandible more than iliac crest.
It has been proposed that bisphosphonates effects may be concentrated in the jaws because of higher blood supply and bone turnover than other bones due to constant daily activity, presence of teeth and periodontal ligament (Marx et al., 2005). These make jaw bones more susceptible to the anti-angiogenic actions of bisphosphonates (Fournier et al., 2002; Santini et al., 2002; Watts et al., 1990) and dysregulated osteoblast-osteoclast equilibrium. The average lifespan of osteoblast is 3 months compared to 2 weeks for osteoclasts (Manolagas, 1999), but both function together in the same basic multicellular unit (BMU) during bone turnover to maintain equilibrium. It is possible that BMU balance may be disrupted in mandible by pamidronate suppression of BMSCs osteoblastic differentiation while enhancing osteoclast differentiation and recruitment. Inadequate number of viable osteoblasts in the BMU coupled with constant forces of jaw motion, speech and teeth occlusion will make bisphosphonate-compromised jaw bones susceptible to microfractures. Subsequent tracking of oral microbes from oral cavity through periodontal tissues can lead to subclinical infections and tissue necrosis that may eventually cause BJON. Clinically, it is possible that a combination of bisphosphonate-associated cellular stress on maxilla and mandible BMSCs due to long-term use, as well as cancer related co-morbid factors, uncoupling of osteoblast-osteoclast equilibrium, reduced vasculature, bone microfractures and tracking of oral microbes through the periodontium may act in concert to produce a ‘bandwagon effect’ that lowers susceptibility threshold in favor BJON (Sarin et al., 2007). Furthermore, paucity of bone lining cells at sites where pamidronate-treated mandible BMSCs formed ectopic bone suggests that future bone repair might be impaired.
There are limitations of the present study. The sample size of 6 is small, although power analysis indicates sample size of 4 will show a difference between mandible and iliac crest BMSCs. It is necessary to reconfirm these results in a larger population especially individuals taking bisphosphonates. Also, BMSCs from multiple craniofacial, axial and appendicular skeletal sites need to be compared and tested with several bisphosphonate formulations to conclusively define skeletal site-specificity of BJON. The BMSCs were cultured in pamidronate for only 4 days whereas patients usually take bisphosphonates for months and years to control skeletal lesions. Clinically, there are other co-morbid factors that may promote BJON, because cancer patients on bisphosphonates are usually taking multiple medications including chemotherapy (Migliorati et al., 2006; Woo et al., 2006). While defining the details of pamidronate effect on orofacial BMSCs will require further examination, it seems likely that site-specificity of BJON may be attributed to skeletal-site specific osteogenic differentiation of human BMSCs and osteoclast population.
Acknowledgements
The authors thank Dr. Elliot Hersh, Ms. Bridget Gallagher and Ms. Stacy Secreto for assistance with subject enrollment and clinical care. This investigation was supported by Lance Armstrong Foundation and USPHS research grant 1K08CA120875-01 from the National Cancer Institute, National Institute of Health, Bethesda, MD 20892.
References
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–68. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Baran I. Calcium and cell cycle progression: possible effects of external perturbations on cell proliferation. Biophys J. 1996;70:1198–213. doi: 10.1016/S0006-3495(96)79679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr., Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996;87:4109–19. [PubMed] [Google Scholar]
- Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl. 1998;30-31:92–102. [PubMed] [Google Scholar]
- Fleisch H. Bisphosphonates in bone disease: From the laboratory to the patient. 4th/Ed Academic Press; San Diego: 2000. [Google Scholar]
- Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, Clezardin P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–44. [PubMed] [Google Scholar]
- Greenberg MS. Intravenous bisphosphonates and osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:259–60. doi: 10.1016/j.tripleo.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–25. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey PG. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Mankani MH, Kuznetsov SA, Fowler B, Kingman A, Robey PG. In vivo bone formation by human bone marrow stromal cells: effect of carrier particle size and shape. Biotechnol Bioeng. 2001;72:96–107. doi: 10.1002/1097-0290(20010105)72:1<96::aid-bit13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. Cell number versus cell vigor--what really matters to a regenerating skeleton? Endocrinology. 1999;140:4377–81. doi: 10.1210/endo.140.10.7129. [DOI] [PubMed] [Google Scholar]
- Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–75. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Jaiswal N, Meng A, Cheng L, Van Den Bos C, Thiede M. Human mesenchymal stem cells promote human osteoclast differentiation from CD34+ bone marrow hematopoietic progenitors. Endocrinology. 1999;140:3736–43. doi: 10.1210/endo.140.8.6880. [DOI] [PubMed] [Google Scholar]
- MedWatch . Safety Information. U.S Food and Drug Administration; 2005. http://www.fda.gov/medwatch/SAFETY/2005/zometa_deardentite_5-5-05.pdf. [Google Scholar]
- Rogers Michael J., SGHLBFPCSPLJMJCF Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Migliorati CA. Bisphosphonate-associated oral osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:135. doi: 10.1016/j.tripleo.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136:1658–68. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508–14. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- Nishida S, Tsubaki M, Hoshino M, Namimatsu A, Uji H, Yoshioka S, Tanimori Y, Yanae M, Iwaki M, Irimajiri K. Nitrogen-containing bisphosphonate, YM529/ONO-5920 (a novel minodronic acid), inhibits RANKL expression in a cultured bone marrow stromal cell line ST2. Biochem Biophys Res Commun. 2005;328:91–7. doi: 10.1016/j.bbrc.2004.12.145. [DOI] [PubMed] [Google Scholar]
- Polizzotto MN, Cousins V, Schwarer AP. Bisphosphonate-associated osteonecrosis of the auditory canal. Br J Haematol. 2006;132:114. doi: 10.1111/j.1365-2141.2005.05833.x. [DOI] [PubMed] [Google Scholar]
- Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Russell RG. Ibandronate: pharmacology and preclinical studies. Bone. 2006;38:S7–12. doi: 10.1016/j.bone.2006.01.151. [DOI] [PubMed] [Google Scholar]
- Santini D, Vincenzi B, Avvisati G, Dicuonzo G, Battistoni F, Gavasci M, Salerno A, Denaro V, Tonini G. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res. 2002;8:1080–4. [PubMed] [Google Scholar]
- Sarin J, DeRossi SS, Akintoye SO. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Diseases. 2007 doi: 10.1111/j.1601-0825.2007.01381.x. In press. [DOI] [PubMed] [Google Scholar]
- von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–9. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, Licata AA, Ross P, Woodson GC, 3rd, Yanover MJ, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323:73–9. doi: 10.1056/NEJM199007123230201. [DOI] [PubMed] [Google Scholar]
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]


