Abstract
Objectives
Severe alveolar bone loss affects dental implant placement. Bone augmentation by grafting iliac crest bone rich in osteoprogenitor cells like bone marrow stromal cells (BMSCs) requires a second surgical procedure in non-orofacial bone. Skeletal site-specific osteogenesis indicates maxilla and mandible BMSCs are highly proliferative and exhibit osteogenic properties superior to iliac crest BMSCs. Alveolar bone can be easily obtained during routine dental surgery, but it is unclear if titanium-attached alveolar BMSCs will retain their superior osteogenic properties. This study evaluated and compared in vitro osteogenic properties of titanium-attached maxilla and iliac crest BMSCs in same individuals.
Materials and Methods
Primary culture of maxilla and iliac crest BMSCs from four normal healthy volunteers were expanded in culture. In 24-well plates, first passage BMSCs were seeded directly (1 × 104 cells/well) on oxidized titanium discs (1.27cm diameter and 2mm thickness) or tissue culture plate. Each cell type was assessed for affinity for titanium, post-attachment survival and osteogenic differentiation based on alkaline phosphatase and osteopontin expressions.
Results
There was no difference in the affinity of maxilla and iliac crest BMSCs to titanium. However, titanium-attached maxilla BMSCs were apparently more osteogenically responsive than iliac crest cells based on calcium accumulation and gene expression of alkaline phosphatase and osteopontin. But these differences were not statistically significant in this small patient sample.
Conclusion
Maxilla and iliac crest BMSCs have similar attachment affinity for titanium. This pilot study indicate that titanium-attached maxilla BMSCs were more osteogenically responsive and may be a viable and more readily available donor graft material in implant dentistry.
Introduction
Early tooth loss enhances rapid resorption of alveolar bone. Although dental implants have become the standard of care for replacing missing teeth, inadequate alveolar bone has a major effect on implant prognosis (De Riu, et al. 2007, Sjostrom, et al. 2007). To improve implant osseointegration and clinical outcome, bone augmentation with grafts is often necessary. But this has variable prognosis, delays treatment, increases cost and may be complicated by underlying systemic disorders (Beikler & Flemmig 2003, Hwang & Wang 2007, Moy, et al. 2005). Methods recently tested to improve implant osseointegration include modifying implant surface, addition of growth factors, adhesion molecules and osteoprogenitor cells to the implant site during surgical procedure (Eisenbarth, et al. 2007, Yan, et al. 2005). When exposed to the atmosphere, titanium, a common implant material spontaneously forms a dense amorphous oxide layer with a reported thickness ranging from 1.8 to 17 nm (Sul, et al. 2002). Several treatments have been advocated to enhance the native oxide in order to accelerate the process of osseointegration. Cell attachment studies have shown that titanium oxidation treatments promote osteoblast attachment on titanium (Mante, et al. 2004, Mante, et al. 2003). Implantation studies (Schincaglia, et al. 2007, Schupbach, et al. 2005) have also reported enhanced in vivo osseointegration by oxidized titanium. Heat and several chemical methods have been investigated as titanium oxide modifiers. One of these focused on the formation of amorphous titania by immersion of titanium in a H2O2/HCl mixture at 80°C (Wang, et al. 2001). This amorphous titania gel was transformed to anatase on heating to 400°C. We have earlier described that when titanium is oxidized in a mixture of H2O2 and HNO3 at 80°C with an average roughness of 197 nm, it provided a surface oxide that promoted peptide attachment and subsequent osteoblasts activity (Mante, et al. 2004). To further improve osseointegration, trabecular bone is commonly used as grafts because of the abundance of osteoprogenitor cells like bone marrow stromal cells (BMSCs), a unique postnatal stem cell population with ability to differentiate into multiple tissues that include bone, cartilage, adipose tissue, muscle and neural tissues (Pittenger, et al. 2000). Non-oral sites such as iliac crest are common donor sites for bone grafts but this creates an additional surgical site (Jackson, et al. 1986, Oklund, et al. 1986, Sawin, et al. 1998). Using donor tissues from alveolar bone to bridge alveolar defects may be preferable because the donor and recipient sites are of similar embryological development (Helms & Schneider 2003). The ease of obtaining alveolar bone will eliminate morbidity of a second surgical procedure and enhance graft integration of tissues from similar skeletal sites. Orofacial bone develops from neural crest while axial and appendicular bones develop from mesoderm (Chai, et al. 2000). In same individuals, orofacial BMSCs isolated from maxilla and mandible have superior osteogenic capacities than iliac crest BMSCs (Akintoye, et al. 2006). If titanium-attached orofacial BMSCs can maintain superior osteogenesis over BMSCs from non-oral sites like iliac crest, they will represent a readily available and viable source of graft materials that can be used to promote alveolar bone augmentation and reduce time needed for implant osseointegration. We tested the hypothesis that titanium-attached BMSCs from maxilla display superior osteogenic properties than those of iliac crest in same individuals.
Materials and Methods
Titanium
Cylindrical commercially pure titanium rods (99.7%) of 1.27cm diameter were cut into 2 mm thick disks and sequentially ground on silicon carbide paper from 240 to 600 grit. All disks were rinsed in distilled water, sonicated in acetone for 10 min and air-dried. The titanium disks were oxidized by immersion in a solution of 8.8 M H2O2/0.1M HCl at 80°C for 30 min, and rinsed in distilled water. The disks were transferred to 24-well tissue culture plates (TCP) (Corning Life Sciences, Lowell, MA) and sterilized under ultraviolet radiation for 24 hours.
Bone marrow stromal cell samples
Four healthy normal volunteers who received treatment for 3rd molar surgery were enrolled in an Institutional Review Board-approved protocol at the University of Pennsylvania, Philadelphia PA after obtaining written informed consent. Trabecular bone samples were obtained from maxilla and iliac crest of each volunteer. Primary BMSCs were established in culture from each site and each subject with α-minimum essential medium (α-MEM) supplemented with 20% fetal bovine serum (Equitech Bio Inc, Kerville, TX, USA), 100 U/ml, penicillin, 100 mg/ml, streptomycin sulfate and 2 mM glutamine (BioSource International, Camarillo, CA, USA), incubated at 37 °C in a humidified atmosphere of 5% CO2 and air as previously described (Akintoye, et al. 2006).
Bone marrow stromal cell attachment to titanium surfaces
First passage maxilla and iliac crest human BMSCs were seeded in triplicate wells on oxidized titanium discs at 1 × 104 cells/well in α-MEM growth medium as above. Parallel control experiments were set up in 24-well plates only, without titanium using tissue culture plate (TCP) as baseline. After 24 hours, cells attached to titanium and TCP were detached with Trypsin-EDTA (Invitrogen, Life Technologies, Carlsbad, CA) and counted with a hemocytometer.
Cell proliferation and osteogenesis of titanium-attached bone marrow stromal cells
In triplicate wells of 24-well plates, BMSCs were seeded on titanium and TCP as described above. Attached cells were allowed to proliferate for 48 hours before evaluating for actively metabolizing cells using WST-1 proliferation assay (Roche Applied Science, Indianapolis IN) based on the cleavage of a tetrazolium salt into a colored soluble formazan product. Cells in another set of parallel experiments were kept in culture for 1 week before isolation of total RNA using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA) and real time PCR assessment of the transcripts for alkaline phosphatase and osteopontin as described below.
Real Time PCR analysis of alkaline phosphatase and osteopontin
Using total RNA isolated above, first strand cDNA was prepared with first strand SuperScript™ Double-Stranded cDNA Synthesis Kit (Invitrogen Life Technologies, Carlsbad, CA) using an oligo-dT primer. cDNA corresponding to 50ng input RNA was amplified as previously described (Mante, et al. 2003) to assess transcripts for alkaline phosphatase, osteopontin and GAPDH (internal control) in a SmartCycler (Cepheid, Sunnyvale CA) using a LightCycler–FastStart DNA Master SYBR Green I kit (Roche Applied Science, Indianapolis IN). Primers used were human alkaline phosphatase (NM000478), forward, 5′-ACCATTCCCACGTCTTCACATTTG-3′, reverse, 5′-AGACATTCTCTCGTTCACCGCC-3′; human osteopontin (NM000582), forward, 5′-AGCCAGGACTCCATTGACTCGAAC-3′, reverse, 5′-GTTTCAGCACTCTGGTCATCCAGC-3′; and human GAPDH (M33197), forward: 5′-AGCCGCATCTTCTTTTGCGTC-3′, reverse: 5′-TCATATTTGGCAGGTTTTTCT-3′. To confirm PCR specificity, a final melt curve from 60–95°C was performed.
Calcium accumulation of titanium attached bone marrow stromal cells
To induce calcium accumulation, attached cells were switched to α-MEM containing supplements of 10−8 M dexamethasone, 10−4 M L-ascorbic-2- phosphate and 5 mM β-glycerophosphate for 4 weeks. Cells were then fixed with 70% ethanol and stained with 2% Alizarin Red S (pH 4.2). Unbound and nonspecifically bound stain was removed by copious rinsing while calcium-bound stain was extracted with 0.5 N HCl/5% sodium dodecyl sulfate and measured at an absorbance of 415 nm (Akintoye, et al. 2006).
Statistical Analysis
From triplicate plates, mean values were computed for each subject as outcome variables for specific contrasts between maxilla and iliac crest BMSCs. Outcome measures from titanium-attached cells were compared with matched control cells cultured on TCP and differences were assessed with Kruskal-Wallis one-way analysis of variance (ANOVA) test. Statistical significance was set at P < 0.05.
Results
Bone marrow stromal cell attachment to titanium
BMSCs attached easily to titanium and acquired fibroblast-like morphology within 24 hours (Figure 1a). There was individual variability among the subjects with respect to the number of BMSCs attached to either TCP or oxidized titanium. While fewer cells consistently attached to titanium than TCP (Figure 1b), there was no difference in the affinity of maxilla and iliac crest cells to titanium.
Figure 1.
Affinity of bone marrow stromal cells to titanium. (a) Scanning electron microscopic appearance of a representative iliac crest bone marrow stromal cell within 24 hr of attachment to titanium. Note the characteristic fibroblast morphology (white arrow) displayed on titanium within 24 hrs (similar pattern was displayed by both iliac crest and maxilla cells). Energy of the electron beam was 20 kV and magnification was × 1500. (b) Attached and actively metabolizing bone marrow stromal cells were assessed using WST-1 cell proliferation assay. There was a slight reduction in number of both iliac crest and maxilla bone marrow stromal cells attached to titanium compared with tissue culture plate. There was no statistically significant difference in affinities of both cell types for titanium.
Osteogenesis of titanium-attached cells
The ability to induce osteogenesis after titanium-attachment was compared based on gene expression of alkaline phosphatase and osteopontin. Titanium-attached maxilla BMSCs were more osteogenically responsive than iliac crest cells as they induced higher gene expression of both alkaline phosphatase and osteopontin (Figure 2) in maxilla BMSCs. These differences were not statistically significant due to the small sample size. Similarly, when BMSCs were kept in culture for 4 weeks in mineralization medium, titanium attached cells stained deeper with Alizarin Red S dye than those on TCP (Figures 3a and 3b). However, the amount of calcium-bound stain was not statistically different between the two cell types whether cultured on TCP or oxidized titanium (Figure 3c).
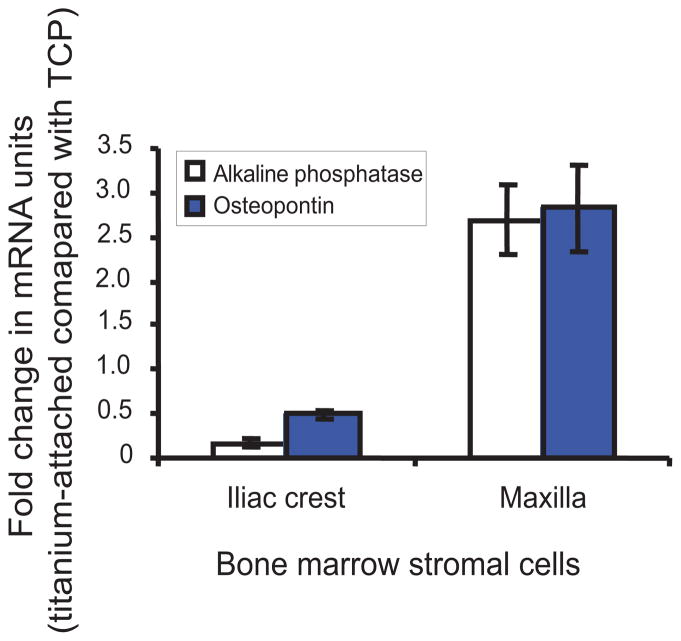
Figure 2.
Osteogenic differentiation of titanium attached bone marrow stromal cells. Total RNA was isolated and mRNA of alkaline phosphatase and osteopontin were amplified and quantified with real time PCR. Compared with cells attached to tissue culture plate, both cell types showed a decrease in alkaline phosphatase but an increase in osteopontin after attaching to titanium. Titanium-attached maxilla bone marrow stromal cells responded osteogenically more than iliac crest cells based on gene expression levels of both alkaline phosphatase and osteopontin.
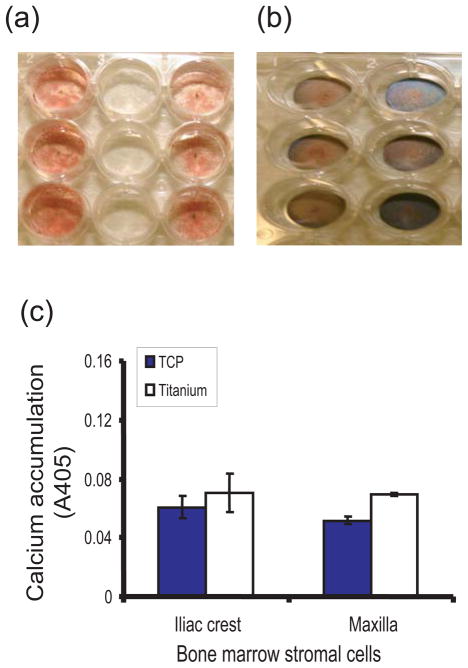
Figure 3.
Mineralization ability of titanium-attached bone marrow stromal cells. Staining for calcium accumulation with Alizarin Red S dye was lower on tissue culture plate (a) than titanium (b). When unbound stain was excluded followed by spectrophotometric evaluation of calcium-bound titanium, the amount of calcium-bound stain was not statistically different between the two cell types whether attached to tissue culture plate or titanium (c).
Discussion
The need to enhance implant osseointegration continues to define improvements to implant materials and bone augmentation (Filho Cerruti, et al. 2007). Emphasis has been shifting to the use of autologous graft materials such as postnatal stem cells to regenerate new bone (Nakamura, et al. 2005). We used oxidized titanium discs as culture surfaces and compared attachment, survival and osteogenesis of BMSCs from two embryologically different skeletal sites, maxilla (orofacial bone) and iliac crest (axial bone) a common graft donor site in tissue transplantation. BMSCs from 4 subjects were tested based on prior studies that showed a sample size of 4 was sufficient to clarify osteogenic differences between orofacial and axial BMSCs (Akintoye, et al. 2006, Stefanik, et al. 2007, von Knoch, et al. 2005). Although there was individual variability among the samples (data not shown), maxilla BMSCs attached comparatively well to titanium as iliac crest cells. Both cell types demonstrated equal survival abilities on both titanium and TCP. BMSCs are known to attach to TCP as early as 4 hours after seeding (Kuznetsov & Gehron Robey 1996), it appears that titanium similarly promoted BMSCs attachment as well as TCP (Figure 1). Some studies have proposed other modifications of titanium surface such as peptide attachment (Mante, et al. 2004) and growth factors (Yan, et al. 2005) to promote cell attachment. In this study, we focused on oxidized titanium surface without additional surface modifications to allow evaluation of direct effects of titanium on BMSCs. Interestingly, the differences between the two cell types based on alkaline phosphatase and osteopontin gene transcripts were better defined than their ability to induce calcium accumulation in a culture dish. Despite higher in vitro calcium accumulation by titanium-attached cells than TCP, the disparate osteogenic response between maxilla and iliac crest BMSCs was better clarified by gene expression of osteogenic markers (Figure 2) than calcium accumulation (Figure 3). Future experiments that assess amount of biological hydroxyapatite in the culture dish may define more clearly these potential skeletal site-specific differences. Titanium-attached maxilla BMSCs were more osteogenically responsive than iliac crest cells because they expressed more of an early osteogenic marker (alkaline phosphatase) and a late marker (osteopontin). Consistent with previous report, (Akintoye, et al. 2006) superior osteogenic response of maxilla over iliac crest BMSCs (data not shown) was also displayed by cells attached to TCP only. Similar superior osteogenic pattern was displayed by titanium-attached maxilla over iliac crest BMSCs despite a reduced number of attached and proliferating cells on titanium compared to TCP (Figure 1b). Therefore, titanium-attachment preserved the previously reported superior osteogenic response of maxilla (orofacial bone) BMSCs over iliac crest (axial bone) cells (Akintoye, et al. 2006). This previous study showed that maxilla and mandible BMSCs can quickly form osteogenic cells much earlier than iliac crest BMSCs. They also formed more abundant bone in vivo than iliac crest cells when transplanted into the subcutis of immunocompromised mice (Akintoye, et al. 2006). Higher osteogenic response of titanium-attached maxilla BMSCs is impressive considering that BMSCs in this study were not cultured in osteogenic medium that usually contains glucocorticoids (Leboy, et al. 1991). If the response of non-osteogenically stimulated cells is considered baseline response, it will be interesting to further evaluate the potential for a more dramatic response of osteogenically stimulated cells especially in a larger patient sample.
In summary, this pilot study indicate that maxilla BMSCs have similar titanium-attachment affinity as iliac crest cells. Survival of both cell types decreased after attaching to titanium, but the superior osteogenic capacity of maxilla BMSCs was apparently preserved. These pilot data suggest that using graft materials from skeletally-similar orofacial bones to augment alveolar bone may be a viable and more readily available alternative source of graft materials in implant dentistry. It is therefore imperative to confirm these findings in a larger patient population.
Acknowledgments
The authors acknowledge the support of Dr. Marian L. Knechel, Department of Chemistry, Ursinus College, Collegeville PA for useful comments and assistance at the early stages of this project. This investigation was supported by Lance Armstrong Foundation and USPHS research grant 1K08CA120875-01 from the National Cancer Institute, National Institute of Health, Bethesda, MD 20892.
References
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. Implants in the medically compromised patient. Crit Rev Oral Biol Med. 2003;14:305–316. doi: 10.1177/154411130301400407. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- De Riu G, De Riu N, Spano G, Pizzigallo A, Petrone G, Tullio A. Histology and stability study of cortical bone graft influence on titanium implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e1–7. doi: 10.1016/j.tripleo.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Eisenbarth E, Velten D, Breme J. Biomimetic implant coatings. Biomol Eng. 2007;24:27–32. doi: 10.1016/j.bioeng.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Filho Cerruti H, Kerkis I, Kerkis A, Tatsui NH, da Costa Neves A, Bueno DF, da Silva MC. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: Clinical case reports. Artif Organs. 2007;31:268–273. doi: 10.1111/j.1525-1594.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- Hwang D, Wang HL. Medical contraindications to implant therapy: Part ii: Relative contraindications. Implant Dent. 2007;16:13–23. doi: 10.1097/ID.0b013e31803276c8. [DOI] [PubMed] [Google Scholar]
- Jackson IT, Helden G, Marx R. Skull bone grafts in maxillofacial and craniofacial surgery. J Oral Maxillofac Surg. 1986;44:949–955. doi: 10.1016/s0278-2391(86)80048-9. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S, Gehron Robey P. Species differences in growth requirements for bone marrow stromal fibroblast colony formation in vitro. Calcif Tissue Int. 1996;59:265–270. doi: 10.1007/s002239900121. [DOI] [PubMed] [Google Scholar]
- Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mrnas in rat marrow stromal cell cultures. J Cell Physiol. 1991;146:370–378. doi: 10.1002/jcp.1041460306. [DOI] [PubMed] [Google Scholar]
- Mante FK, Little K, Mante MO, Rawle C, Baran GR. Oxidation of titanium, rgd peptide attachment, and matrix mineralization rat bone marrow stromal cells. J Oral Implantol. 2004;30:343–349. doi: 10.1563/0.667.1. [DOI] [PubMed] [Google Scholar]
- Mante M, Daniels B, Golden E, Diefenderfer D, Reilly G, Leboy PS. Attachment of human marrow stromal cells to titanium surfaces. J Oral Implantol. 2003;29:66–72. doi: 10.1563/1548-1336(2003)029<0066:AOHMSC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- Nakamura H, Saruwatari L, Aita H, Takeuchi K, Ogawa T. Molecular and biomechanical characterization of mineralized tissue by dental pulp cells on titanium. J Dent Res. 2005;84:515–520. doi: 10.1177/154405910508400606. [DOI] [PubMed] [Google Scholar]
- Oklund SA, Prolo DJ, Gutierrez RV, King SE. Quantitative comparisons of healing in cranial fresh autografts, frozen autografts and processed autografts, and allografts in canine skull defects. Clin Orthop Relat Res. 1986:269–291. [PubMed] [Google Scholar]
- Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: Progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- Schincaglia GP, Marzola R, Scapoli C, Scotti R. Immediate loading of dental implants supporting fixed partial dentures in the posterior mandible: A randomized controlled split-mouth study--machined versus titanium oxide implant surface. Int J Oral Maxillofac Implants. 2007;22:35–46. [PubMed] [Google Scholar]
- Schupbach P, Glauser R, Rocci A, Martignoni M, Sennerby L, Lundgren A, Gottlow J. The human bone-oxidized titanium implant interface: A light microscopic, scanning electron microscopic, back-scatter scanning electron microscopic, and energy-dispersive x-ray study of clinically retrieved dental implants. Clin Implant Dent Relat Res. 2005;7(Suppl 1):S36–43. doi: 10.1111/j.1708-8208.2005.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Sennerby L, Nilson H, Lundgren S. Reconstruction of the atrophic edentulous maxilla with free iliac crest grafts and implants: A 3-year report of a prospective clinical study. Clin Implant Dent Relat Res. 2007;9:46–59. doi: 10.1111/j.1708-8208.2007.00034.x. [DOI] [PubMed] [Google Scholar]
- Stefanik D, Sarin J, Lam T, Levin L, Leboy PS, Akintoye SO. Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Dis. 2007 doi: 10.1111/j.1601-0825.2007.01402.x. Early online: 24-Sep-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul YT, Johansson CB, Petronis S, Krozer A, Jeong Y, Wennerberg A, Albrektsson T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: The oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials. 2002;23:491–501. doi: 10.1016/s0142-9612(01)00131-4. [DOI] [PubMed] [Google Scholar]
- von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–6949. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Wang XX, Hayakawa S, Tsuru K, Osaka A. A comparative study of in vitro apatite deposition on heat-, h(2)o(2)-, and naoh-treated titanium surfaces. J Biomed Mater Res. 2001;54:172–178. doi: 10.1002/1097-4636(200102)54:2<172::aid-jbm3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yan MN, Tang TT, Zhu ZA, Zhou XS, Jia QW, Yu CF, Lou JR, Dai KR. Effects of bone morphogenetic protein-2 gene therapy on the bone-implant interface: An experimental study with dogs. Zhonghua Yi Xue Za Zhi. 2005;85:1521–1525. [PubMed] [Google Scholar]