Abstract
Growing evidence indicates that physical and psychosocial stressors, in part acting through the hypothalamic-pituitary-adrenal (HPA) axis, may accelerate the process of Alzheimer’s disease (AD). In this review, we summarize recent research related to the effects of stress and stress hormones on the various disease process elements associated with AD. Specifically, we focus on the relationships among chronic stressors, HPA axis activity, amyloid-β protein, and amyloid-β plaque deposition in mouse models of AD. The potential mechanisms by which stress and stress-related components, especially corticotrophin-releasing factor and its receptors, influence the pathogenesis of AD are discussed.
Keywords: Alzheimer’s disease, amyloid-β, corticotrophin-releasing factor (CRF) and receptors (CRFRs), hypothalamic-pituitary-adrenal (HPA) axis, stress, Tg2576 mice
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the most likely cause of dementia in the elderly. The neuropathological basis of the disease is the presence of amyloid-β (Aβ) deposits, neurofibrillary tangles, and neuronal loss across the neocortex and hippocampus [1, 2]. Although mutations in three genes – amyloid-β precursor protein (AβPP), presenilin1 (PS1), and presenilin2 (PS2) have been found to be the basis of uncommon forms of familial AD, the causes of the far more common sporadic form of AD remain unknown. It is widely believed that sporadic AD is caused by complex interactions between various genetic influences and environmental factors. One of the most powerful indicators of the rate of progression of AD is stage of illness [3, 4]. In addition, environmental factors known to influence the pathogenesis of AD include comorbid medical conditions, such as cerebrovascular disease and diabetes [5–7]. However, more recently, there has been growing speculation that stress may accelerate the progression of AD [8, 9].
Physical and psychosocial stressors are well known to trigger increases in the activity of the hypothalamic-pituitary-adrenal (HPA) axis, which in turn can have deleterious effects on the structure and function of several brain regions, especially the hippocampus [10–14]. The hippocampus contains a high density of glucocorticoid receptors (GRs) and corticotrophin-releasing factor receptors (CRFRs) that regulate the HPA axis in response to stress. Hippocampal damage induced by stress combined with increases in glucocorticoid hormones could promote a repetitive cycle of increasing HPA deregulation and further hippocampal injury. Moreover, increased secretion of stress hormones has been associated with multiple neuropsychiatric disorders, including depression and dementia [14–17]. Since the hippocampus is one of the earliest sites of AD neuropathology and hippocampal degeneration is one of the most prominent characteristics of AD [18–21], determination of the role of HPA axis dysregulation on AD pathogenesis should help shed light on the causal factors of AD and perhaps suggest new strategies for therapeutic interventions to slow disease progression.
STRESS AND NEURONAL STRUCTURE AND FUNCTION
After exposure to a stressor, the HPA axis increases its activity and causes the adrenal glands to increase their secretion of glucocorticoids that affect the entire body, including the brain [22]. The major glucocorticoid hormone is cortisol in humans or corticosterone in rodents, and under ordinary circumstances, secretion of these hormones enables the organism to adapt physically and mentally to the stressor. Stress-mediated effects on brain function can be beneficial or detrimental, depending on the type of stressor, the duration of exposure to the stressor, and the developmental stage of the organism [23]. A large body of evidence indicates that intense, repeated stressors trigger persistent increases in glucocorticoid levels, which modulate function within the brain by changing neuronal structure and function. As noted, the hippocampus is particularly sensitive to the deleterious effects of chronic increases in glucocorticoid secretion [10, 12, 24–27]. For example, stress has been found to induce neuronal loss in the hippocampus [27] and atrophy of neuronal dendrites within the cornu ammonis (CA) of the hippocampus [28, 29]. Also, stress suppresses neurogenesis among dentate gyrus granule neurons [30] and reduces hippocampal brain-derived neurotrophic factor mRNA expression in the rat [31]. Deficits in learning and memory function have been reported in rodents after chronic stress or the administration of corticosterone [32, 33].
The cellular mechanisms underlying the effects of chronic stress on hippocampal structure and function remain under investigation, but it is believed that glucocorticoids play a central role [22, 26]. Moreover, glucocorticoids interact with a variety of neurochemical systems, including serotonin, endogenous opioids, GABA-benzodiazepine receptors, and calcium currents, and finally, excitatory amino acids [26]. Stressors and corticosterone have been observed to increase excitatory amino acid release [34, 35] as well as the expression of NMDA-type glutamate receptors [36–38] in rodents. Thus, glucocorticoid-triggered increases in excitatory amino acid transmission could induce neuronal injury in the brain [22]. In addition, one of the functions of the hippocampus is to inhibit over-activity of the HPA axis [39], and so hippocampal damage induced by stress and glucocorticoids could initiate a repetitive cycle of increasing HPA deregulation and hippocampal injury.
STRESS AND ALZHEIMER’S DISEASE
There is growing evidence that the effects of physical and psychosocial stressors on the HPA axis may be implicated in the onset and progression of AD [8, 40–42]. For instance, increases in plasma cortisol levels have been reported in patients diagnosed with AD [43–46]. Moreover, a correlation has been reported between increases in 24 h cortisol levels and the severity of cognitive deficits in AD patients [47], and between elevations in basal cortisol levels the frequency of dexamethasone non-suppression in AD [48]. Furthermore, postmortem cerebrospinal fluid (CSF) cortisol levels in AD patients were 83% higher than in controls [14]. Finally, changes in the HPA axis in AD do not appear to be secondary to depression. In one study, CSF cortisol levels were found to be significantly higher in AD patients than in controls, while there were no significant differences in AD patients with or without depression [49].
Changes in other elements of the HPA axis in AD patients have been less consistent. For example, reductions in CRF immunoreactive cells have been reported in the frontal, temporal, and occipital cortices and the caudate nucleus in AD patients [50–53]. However, other studies suggest that increases occur in the density of postsynaptic CRFRs [54, 55]. Whether these changes are related (i.e., reciprocal), and share a common mechanism is unknown. Also, CRF concentrations in several cortical regions (such as Brodmann areas 8, 32, 44, and 22) were slightly elevated in AD patients who died with evidence of very mild dementia (i.e., a clinical dementia rating scale (CDR) score of 0.5), but were reduced in AD patients who died with moderate dementia (i.e., a CDR score of 2.0). Such findings suggest that AD may involve a temporary increase in CRF levels early in the course of disease [56].
While there is growing evidence that stress has an important impact on the pathogenesis of AD, the cellular mechanisms that might link stress and AD are not well understood. Moreover, since “healthy” aging probably also involves changes in the HPA axis [27, 57], distinguishing between the effects of stress on the normal aging process and on the pathogenesis of AD is challenging. Age-related increases in corticosterone levels were first reported in rodents more than 25 years ago [58] and were found to be correlated with declines in spatial memory [59, 60]. More recently, evidence for the hypothesis that glucocorticoids might accelerate normal human aging has been reported. Correlations have been documented between increased plasma cortisol levels, memory impairments, and smaller hippocampal volumes in elderly subjects [14, 61, 62]. In another study, Lupien and colleagues [63] reported increases, decreases, and no change in plasma cortisol levels in subgroups of elderly subjects. One possible explanation for this finding is that subjects in these subgroups may have differed with regard to the frequency of preclinical forms of AD or other neuropsychiatric disorders.
STRESS AND THE NEUROPATHOGENSIS OF ALZHEIMER’S DISEASE
The major hallmarks of the neuropathology of AD are extracellular Aβ plaques, intracellular neurofibrillary tangles, neuronal loss and decreased volumes of certain brain regions, especially in the cortex and the hippocampus [64, 65]. Aβ is a 39–43 amino acid peptide generated via the proteolytic processing of the amyloid-β precursor protein (AβPP) by β- and γ-secretases [66–68]. As levels of this peptide increase, deposits of Aβ eventually form in the parenchyma (also known as amyloid plaques or senile plaques) and in the cerebral vasculature under certain circumstances. Aβ, and especially Aβ oligomers, have been considered neurotoxic, destructive to synaptic function, and a plausible basis for memory loss in early AD [69]. While increasing levels of such oligomers and plaque formation have been correlated with cognitive impairments in AD patients [70, 71], the mechanisms by which Aβ may be responsible for the loss of neurons in AD remain largely unknown. Aβ can be found in many forms in the central nervous system, such as soluble non-fibrillar amyloid, diffuse fibrillar peptides, or as larger fibrillar aggregates. Aβ levels in the extracellular space are influenced by factors regulating its production and release from neurons as well as post-secretory events such as transport and clearance. Recent evidence has shown that Aβ production and release from neurons is regulated by neuronal or more specifically, synaptic activity, over minutes to hours [72, 73]. However, whether behavioral manipulations regulate synaptic activity and extracellular Aβ levels is still under investigation.
Few studies have directly tested the hypothesis that stress influences the pathogenesis of AD in humans. While severe constraints are evident in clinical AD research where biological samples are limited to plasma, CSF, and postmortem brain measures, longitudinal clinical studies have provided significant findings. Wilson and colleagues showed that elderly individuals with a high level of distress proneness (score = 24, 90th percentile) were 2.7 times more likely to develop AD than those not prone to distress (score = 6, 10th percentile). Distress proneness was also associated with more rapid cognitive decline. Furthermore, higher levels of chronic psychological distress were associated with an increased incidence of mild cognitive impairment (MCI). Nevertheless, they did not find a correlation between stress and the pattern of AD pathology [74–76]. In other studies, HPA axis changes have been associated with hippocampal atrophy in AD patients [77] and dysregulation of the HPA axis may be responsible for some of the abnormal behavioral patterns exhibited by AD patients [78]. Finally, administration of prednisone to reduce inflammation has been found to accelerate cognitive deterioration in AD patients, while the glucocorticoid antagonist, mifepristone, has been found to slow cognitive decline in AD patients [79, 80]. In a nonhuman primate (Macaca nemestrina) study, reduced insulin-degrading enzyme mRNA and increased Aβ42 levels were detected after chronic glucocorticoid administration [81].
Genetically manipulated mice that imitate at least some of the neuropathology changes of AD provide us with an important opportunity to investigate the impact of environmental factors, such as stress, on the pathogenesis of AD. The Tg2576 mouse is arguably the most well-known mouse model of AD. The Tg2576 mouse contains the Swedish AD mutant gene, hAPP K670N, M671L (Lys670-Asn, Met671-Leu) driven by a hamster prion protein promoter. This mouse model overexpresses human AβPP695 and shows Aβ plaques in the cortex and hippocampus at 9–10 months of age, in association with behavioral deficits, impairments in hippocampal long-term potentiation, and synaptic loss [82–84]. However, these mice do not show neuronal loss until the very late stages of life [85].
In the early 2000s, our laboratory began studying the association between stress and the biological markers of AD neuropathogenesis in Tg2576 mice. We stressed Tg2576 mice by exposing them to extended isolation; that is, at the point of weaning for 3 days, 7 days, 4 months, and 6 months. We found that Aβ plaques appeared more frequently in the cortex and hippocampus of Tg2576 mice that had been isolated for 6 months, which is generally not observed in unstressed Tg2576 mice [86]. Since then, we have conducted a series of experiments to confirm our observations that isolation stress accelerates plaque formation in Tg2576 mice and to attempt to discover the mechanism by which stressors exert such effects. In keeping with our observation of accelerated plaque deposition and the observations made by others that plaque deposition is dependent upon tissue levels of Aβ, we have also observed increases in tissue levels of both forms of Aβ (Aβ1–40 and Aβ1–42) in stressed Tg2576 mice in conjunction with impaired memory-related behaviors [86, 87] (Fig. 1).
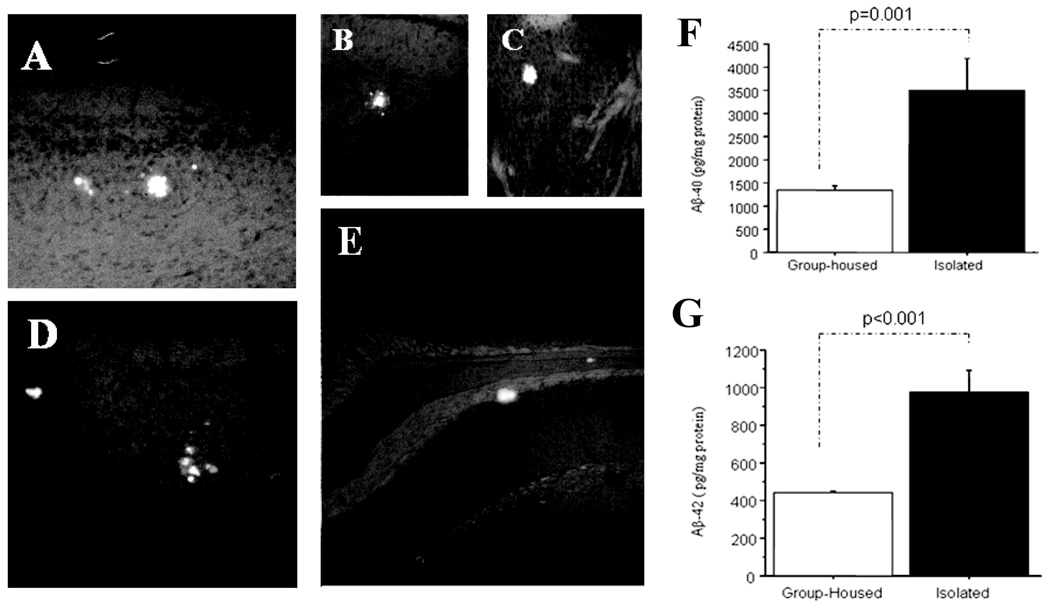
Fig. 1.
Thioflavin S fluorescence stains indicate that relatively smaller sizes of Aβ-plaques were distributed in the cortex (panel A and D), hippocampus (panel B and E), even in the stratum (panel C) [40, 80] after 6 months of isolation stress in the Tg+ mice brain, which were rare in Tg+ mice in age-matched group-housed controls. ELISA analysis indicates Aβ1–40 and Aβ1–42 levels in brain tissue were significantly increased in Aβ1–40 (panel F) and Aβ1–42 (panel G) in the 6 months isolation-stressed Tg+ mice as compared with group-housed Tg+ mice at the same age [87].
Jeong and collaborators have also investigated the effects of long-term stress on the onset and severity of cognitive deficits and pathological changes in APPV717I-CT100 transgenic mice, another animal model of AD. Their results also suggest that long-term stress accelerates the progression of behavioral deficits and extracellular amyloid deposition, as well as intraneuronal Aβ and AβPP-CTFs immunoreactivity, and neurodegeneration in the APPV 717I-CT00 AD model [88]. In addition, a recent study by LaFerla’s group has shown increased brain levels of AβPP, Aβ, amyloid-β precursor protein cleaving enzyme (BACE), and the β-C-terminal fragment (β-CTF) of AβPP after administration of dexamethasone to triple transgenic AβPP/PS1/MAPT mice [89]. Finally, our group has recently shown that acute and chronic behavioral stressors increase interstitial levels of Aβ [9].
Studies addressing the association between stress and stress-related hormones and other elements of AD neuropathology, especially neurofibrillary tangles and neuronal loss, are very limited in these mouse models. However, one study reported that stress and CRFRs have been implicated in stress-induced hippocampal tau phosphorylation (tau-P) in rodents [90]. Again, consistent with this observation, we have also found decreases in hippocampal volumes in stressed Tg2576 mice as compared to unstressed Tg2576 mice [87].
Taken together, the results of these studies suggest that stress and stress hormones may increase production of Aβ and its incorporation into Aβ plaques. Although further study is needed to determine the relative effects of stress on Aβ production, aggregation, and elimination in various mouse models of AD, it is plausible that stress could also enhance Aβ-mediated neuronal toxicity or increase tau accumulation or phosphorylation and tangle formation.
MECHANISMS BY WHICH STRESS ACCELERATES THE PATHOGENESIS OF ALZHEIMER’S DISEASE
Whether stress plays a role in the pathogenesis of AD by its direct effects on a specific molecule such as Aβ or by indirect effects on other downstream targets remains unclear (see above). However, the activity of CRF and the density of CRFRs seem to be likely places where stress could influence the production of Aβ, based on the function of CRF and CRFRs in the brain [9, 87, 91, 92]. Data from a recent publication indicated that repeated restraint stress accelerates amyloid plaque pathogenesis in Tg2576 mice by generating metabolic oxidative stress and these effects were reversed by administration of the CRFR antagonist, NBI27914 [93]. In further studies, we found that extended exposure to isolation stress increased plasma corticosterone levels as well as GR and CRF receptor 1 (CRFR1) expression in the cortex and hippocampus (Fig 2 & Fig 3). Moreover, CRFR1 expression was significantly correlated with tissue levels of Aβ in Tg2576 mice [87]. In a microdialysis study of Tg2576 mice exposed to acute and chronic behavioral stressors, we found that stressors produced significant increases in interstitial fluid (ISF) levels of Aβ [9]. Furthermore, administration of exogenous CRF, but not corticosterone, mimicked the effects of the behavioral stressors. Finally, blockage of endogenous CRFRs and inhibitors of neuronal action potentials blocked the effects of stress (Fig. 4). The results of these studies suggest that behavioral stressors increase CRF release, which increases basal levels of neuronal activity, which then stimulates Aβ release. Since the formation of amyloid plaques is dependent on the concentration of Aβ [94], increases in ISF levels of this peptide brought about by stress-induced increases in CRF levels and neuronal activity is a plausible explanation for our and other original findings of accelerated plaque deposition in AD mouse models exposed to stressors [86–89].
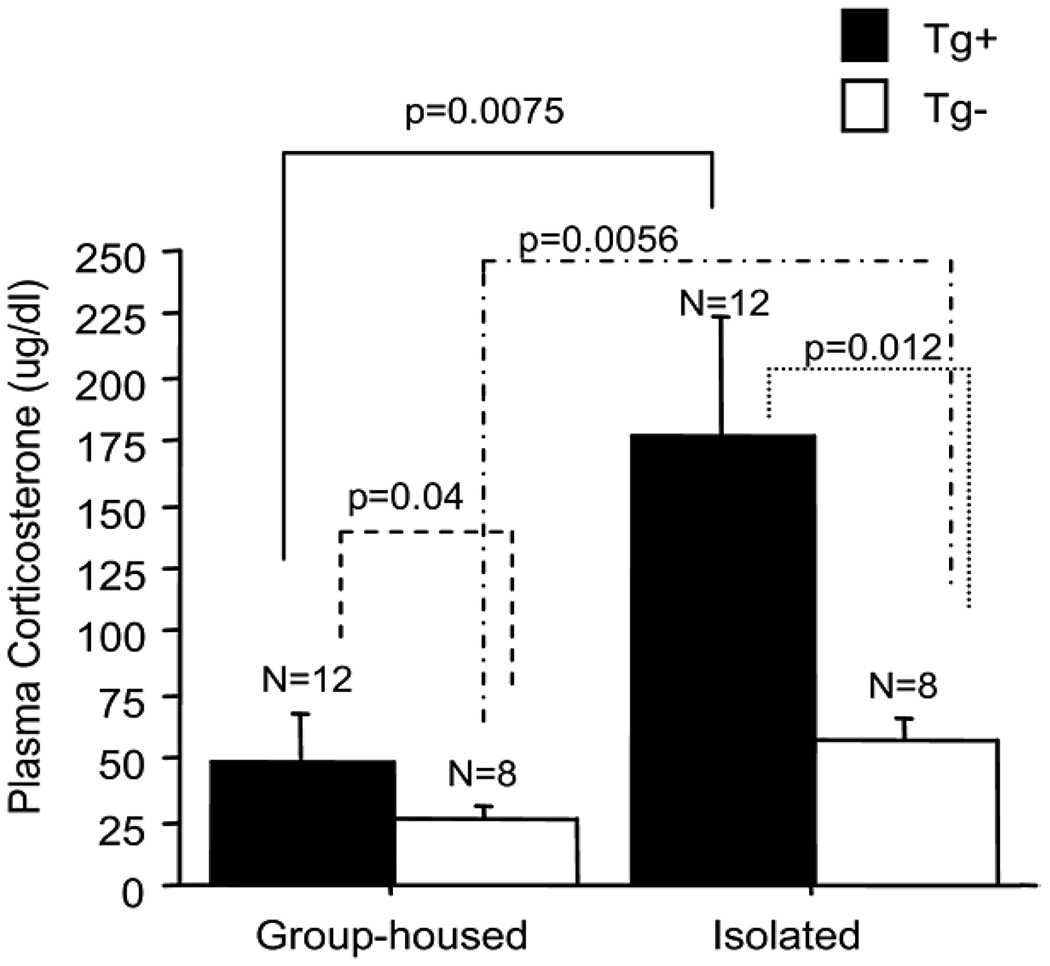
Fig. 2.
Plasma corticosterone levels in Tg+ and Tg− mice. Regardless of housing condition, plasma corticosterone levels were significantly higher in Tg+ mice than Tg− mice. Plasma corticosterone levels were dramatically increased in both Tg+ and Tg− mice after 24 weeks of isolation stress [80].
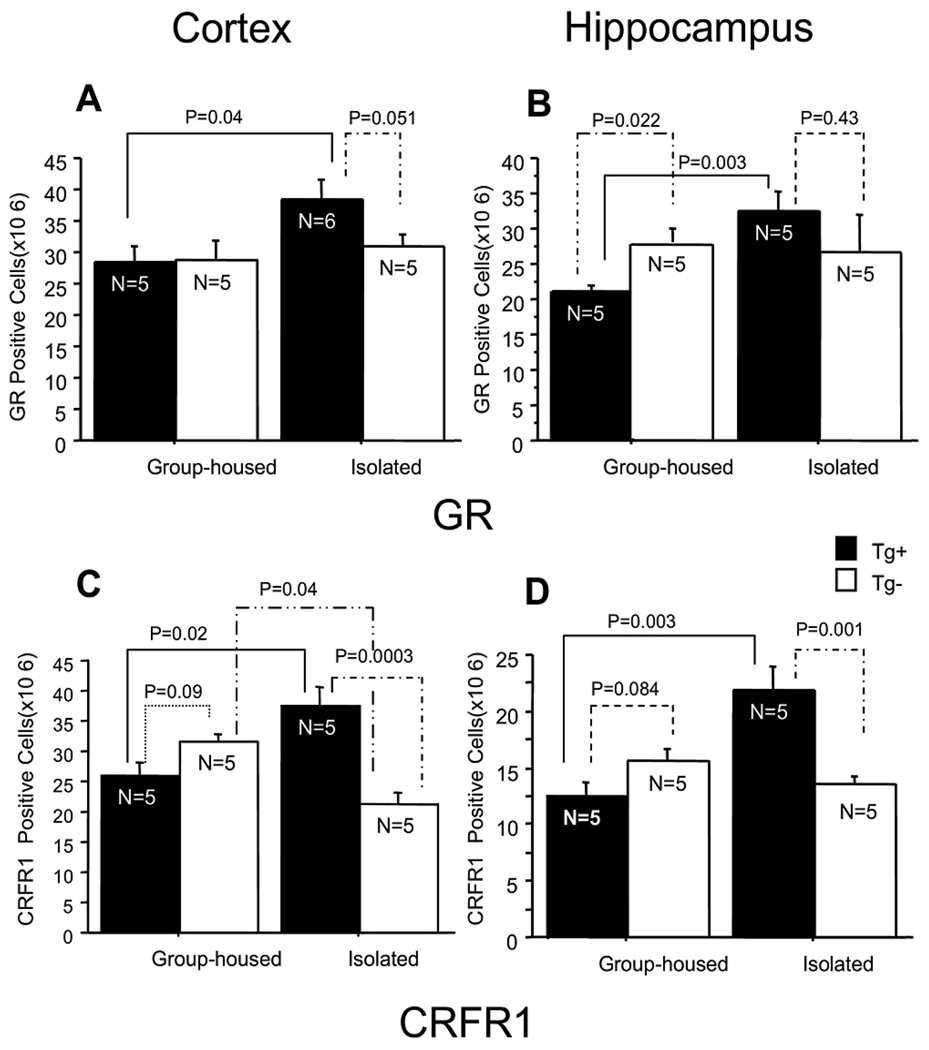
Fig. 3.
Quantitative analysis of GR and CRFR1 expression in Tg+ and Tg− mice. GR-immunoreactive cell density was significantly increased in isolated Tg+ mice as compared with group-housed Tg+ mice in the cortex and the hippocampus (A, B). CRFR1-immunoreactive cell density was significantly increased in the cortex of isolated Tg+ mice as compared with group-housed Tg+ mice and isolated Tg− mice. CRFR1-immunoreactive cell density was significantly decreased in the cortex of isolated Tg− mice as compared with group-housed Tg− mice (C). CRFR1 immunoreactive cell density was significantly increased in the hippocampus of isolated Tg+ mice as compared with group-housed Tg+ mice and isolated Tg− mice, but there was no significant change between group-housed Tg− mice and isolated Tg− mice (D) [87].
Fig. 4.
CRF was administrated by reverse microdialysis in the hippocampus of 3- to 4-month-old Tg2576 mice. (A) 100nM CRF in the microdialysis fluid resulted in an increase ISF Aβ levels at 3h after drug infusion, whereas 200nM CRF increased ISF Aβ levels immediately after drug infusion (n = 5 per group). (B) Both 100 and 200nM CRF increase ISF Aβ levels in a dose-dependent manner, reaching 138.3 = 7.027% and 171.9 = 17.83% of baseline by 12 h, respectively (P < 0.0001 and P < 0.0001, respectively). (C) Three-hour restraint stress increased ISF Aβ levels to 132 ± 6.896% compared with baseline 13h after the beginning of stress initiation (P < 0.003; n = 10 for stress). Treatment with α-helical CRF9–41 (αCRF9–41), a CRF receptor antagonist, given 30 min before restraint stress until the end of the experiment, blocked the stress-induced increase in ISF Aβ levels (P < 0.006; n = 5 for stress + αCRF9–41) [9].
Currently, we are assessing the effects of CRFR1 antagonists on Aβ tissue levels in vitro and in Tg2576 mice, with and without stress manipulation. In a first experiment, primary hippocampal neuron cultures were created, and different doses of CRF and/or the CRFR antagonists antalarmin, R121919.HCL, and LWH-63.HCL were then directly added to the culture. We subsequently measured the Aβ40 and Aβ42 concentration in the media using an enzyme-linked immunosorbent assay (ELISA). In the second experiment, Tg2576 mice were exposed to either isolation or control conditions immediately after weaning for one week. During this time, four subgroups of isolated and group-housed mice were injected daily with one of three CRFR1 antagonists: antalarmin (20 mg/kg), R121919. HCL (10 mg/kg), LWH-63.HCL (20 mg/kg), or vehicle (20% Tween 80). Tissue Aβ levels in the hippocampus and overlying cortex were measured using ELISA. Preliminary results indicate that CRFR1 antagonists, especially antalarmin and R121919.HCl, significantly decreased the Aβ40 and Aβ42 in vivo and in vitro (Dong et al., unpublished data) in both stressed and unstressed conditions. Confirmation of these results will provide a potential therapeutic intervention for the treatment of AD.
CRF has two distinct but interrelated functions in the nervous system. CRF and CRFRs released from the hypothalamus regulate basal and stress-induced glucocorticoid secretion and are critical regulators of HPA axis activity. CRF and its receptors are also extensively distributed throughout the cortex and hippocampus, are synthesized and released at extra-hypothalamic sites, and may thus exert a neuromodulatory effect over neuronal activity involved in stress-sensitive processes, such as memory and anxiety [91, 92, 95]. CRFRs are high affinity, G protein-coupled receptors that stimulate adenylate cyclase activity, thereby increasing intercellular levels of cAMP. The highest level of adenylate cyclase activity is in the cerebral cortex, an area that is profoundly involved in AD [51, 96]. This neuroanatomical specificity supports the hypothesis that CRFRs play a central role in the pathogenesis of AD, although there is no published data indicating that CRFR directly regulates amyloid formation and/or degradation. Our recent studies [9] of stressed Tg2576 mice, as well as other studies [72, 97], demonstrate that neuronal activity, specifically synaptic activity and synaptic vesicle release, may be linked with the release of Aβ from neurons. Moreover, there is growing evidence that one of the glutamate or other excitatory neurotransmitter systems may influence the expression and function of CRF and CRFRs [98, 99]. Glutamate-related excitatory synapses represent more than 80% of synapses. The glutamate system is also thought to be involved with Aβ formation and neurotoxicity [100–103]. Glucocorticoids are also considered to be the key regulators of neuronal activity, particularly under stressful conditions [104, 105]. Thus, it is possible that environmental manipulations, such as behavioral stressors, may affect synaptic activity in brain regions over longer periods of time (e.g., hours to days) and thus have marked effects on the physiological regulation of extracellular brain Aβ levels and potentially the long-term risk for AD.
It should also be kept in mind that besides CRF and its receptors, there may be other mechanisms by which stress and stress-related factors can impact Aβ levels and the neuropathogenesis of AD. In vivo and in vitro studies indicate that treatment of microglia with dexamethasone impedes the degradation of Aβ peptides [106]. It has also been shown that in aged macaques, elevated glucocorticoid levels are associated with the decreased degradation of Aβ through a down-regulation of insulin-degrading enzyme [81]. Conversely, elevated glucocorticoid levels have been shown to increase the susceptibility of cholinergic neurons to Aβ42-mediated toxicity in rats [107]. Finally, behavioral stressors might enhance the generation of free radicals in mitochondria, and by this alternate mechanism create an association between Aβ proteins and the generation of free radicals and oxidative damage that would accelerate neuronal loss in AD.
In summary, it is likely that there are multiple links among stress, stress-induced changes of the HPA axis, neuronal activity, and the pathogenesis of AD. Such changes may also be superimposed on the effects of stress and stress hormones on a multitude of processes related to “healthy” aging. Figure 5 is a diagram depicting some of these links. Determining how environmental manipulations affect synaptic activity and Aβ levels may be important in understanding the vulnerability of specific brain regions to AD-like changes. Further research is needed in both animal models of AD as well as patients with AD to investigate these links.
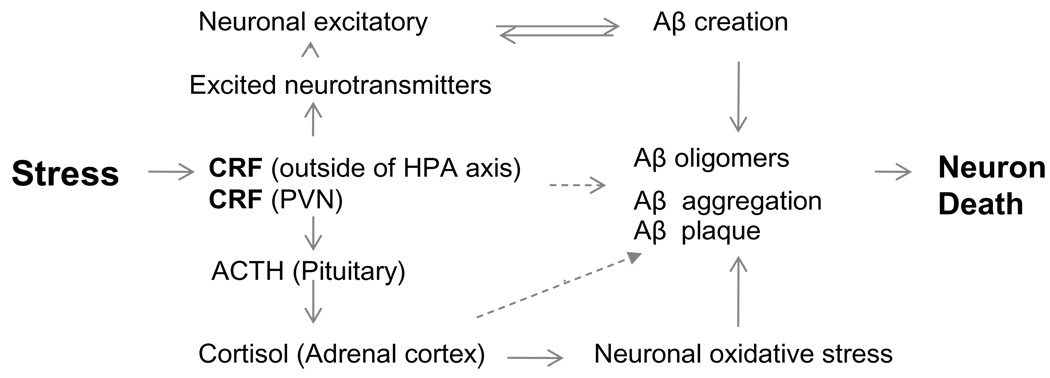
Fig. 5.
Schematic diagram depicting the theorized involvement of corticotrophin-releasing factor (CRF) in the regulation of Aβ. Stress stimulates classic hormonal and neuroregulator-like functions that may directly or indirectly influence Aβ creation and deposition.
CONCLUSION
The results of the studies reviewed in this manuscript suggest that physical and psychosocial stressors acting on the HPA axis may have an impact on the pathogenesis of AD. The evidence to date suggests that stress may increase brain levels of CRF, which in turn increases the levels of neuronal activity, Aβ release, and ultimately, amyloid aggregation into plaques. There is only very limited information, however, on the relationship between amyloid plaque deposition, tangle formation, and the most important element of AD neuropathology, neuronal degeneration.
With improved understanding of the role of various elements of the HPA axis on the pathogenesis of AD and other neurodegenerative diseases, new treatment options may be possible in the future. Because of the wide distribution of CRF and CRFRs within the central nervous system and the function of CRF as a neurotransmitter as well as a neurohormone, the role of CRF systems in neurodegenerative disorders is likely to be complex. Nonetheless, CRFR antagonists may be an attractive target for the development of new drug and non-drug therapies. Additional studies are needed to determine the functional significance of interactions between factors, such as glucocorticoid receptors and adrenocorticotropin, CRF and glutamate, and the influence of such interactions on the pathogenesis of AD.
ACKNOWLEDGMENTS
This work is supported by a grant from the National Institutes of Health (R01 AG025824) awarded to JGC and HXD.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=65).
REFERENCES
- 1.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topological and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Katzman R. Alzheimer’s disease. N Engl J Med. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- 3.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59:1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 4.Stozicka Z, Zilka N, Novak M. Risk and protective factors for sporadic Alzheimer’s disease. Acta Virol. 2007;51:205–222. [PubMed] [Google Scholar]
- 5.Bruandet A, Richard F, Bombois S, Maurage CA, Deramecourt V, Lebert F, Amouyel P, Pasquier F. Alzheimer’s disease with cerebrovascular disease and vascular dementia: clinical features and course compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2009;80:133–139. doi: 10.1136/jnnp.2007.137851. [DOI] [PubMed] [Google Scholar]
- 6.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 7.Sima AA, Li ZG. Diabetes and Alzheimer’s disease – is there a connection? Rev Diabet Stud. 2006;3:161–168. doi: 10.1900/RDS.2006.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs E, Flugge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23:295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 11.Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets. 2006;5:531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- 12.Miller DB, O’Callaghan JP. Effects of aging and stress on hippocampal structure and function. Metabolism. 2003;52:17–21. doi: 10.1016/s0026-0495(03)00296-8. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky RM. Potential behavioral modification of glucocorticoid damage to the hippocampus. Behav Brain Res. 1003;57:175–182. doi: 10.1016/0166-4328(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 14.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs SC, De Souza EB. Corticotropin-releasing factor antagonists, binding-protein and receptors: implications for central nervous system disorders. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:541–554. doi: 10.1053/beem.1999.0042. [DOI] [PubMed] [Google Scholar]
- 17.Pariante CM. Depression, stress and the adrenal axis. J Neuroendocrinol. 2003;15:811–812. doi: 10.1046/j.1365-2826.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- 18.Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- 19.Jack CR, Peterson RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 20.Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and vascular dementia: an MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 21.Murphy DG, DeCarli CD, Daly E, Gillette JA, McIntosh AR, Haxby JV, Teichberg D, Schapiro MB, Rapoport SI, Horwitz B. Volumetric magnetic resonance imaging in men with dementia of the Alzheimer’s type: correlations with disease severity. Biol Psych. 1993;34:612–621. doi: 10.1016/0006-3223(93)90153-5. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan KL. Stress and the evolution of condition-dependent signals. Trends Ecol Evol. 2000;15:156–160. doi: 10.1016/s0169-5347(99)01812-1. [DOI] [PubMed] [Google Scholar]
- 24.Kerr DS, Campbell LW, Applegate MD, Brodish A, Landfield PW. Chronic stress-induced acceleration of electro-physiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991;11:1316–1324. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann NY Acad Sci. 1997;821:271–84. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky RM. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge, Massachusetts: MIT Press; 1992. [Google Scholar]
- 28.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA2 pyramidal neuron. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 30.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learning Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 33.Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 35.Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: A micro-dialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- 36.Baker KB, Kim JJ. Effects of Stress and Hippocampal NMDA Receptor Antagonism on Recognition Memory in Rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son GH, Geum D, Chung S, Kim EJ, Jo JH, Kim CM, Lee KH, Kim H, Choi S, Kim HT, Lee CJ, Kim K. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci. 2006;26:3309–3318. doi: 10.1523/JNEUROSCI.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiland NG, Orchinik M, Tanapat P. Chronic corti-costerone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neuroscience. 1997;78:653–662. doi: 10.1016/s0306-4522(96)00619-7. [DOI] [PubMed] [Google Scholar]
- 39.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 40.De Souza-Talarico JN, Caramelli P, Nitrini R, Chaves EC. Effect of cortisol levels on working memory performance in elderly subjects with Alzheimer’s disease. Arq Neuropsiquiatr. 2008;66:619–624. doi: 10.1590/s0004-282x2008000500003. [DOI] [PubMed] [Google Scholar]
- 41.Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56:1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- 42.Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC. Cortisol secretion and Alzheimer’s disease progression. Biol Psychiatry. 1997;42:1030–1038. doi: 10.1016/s0006-3223(97)00165-0. [DOI] [PubMed] [Google Scholar]
- 43.Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathé AA, Johns CA, Horvath TB. Cortisol and Alzheimer’s disease, I: Basal studies. Am J Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen S, Nasman B, Carlstrom K, Olsson T. Increased levels of adrenocortical and gonadal hormones in mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;13:74–79. doi: 10.1159/000048637. [DOI] [PubMed] [Google Scholar]
- 45.Swanwick GRJ, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s Disease. Am J Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- 46.Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: A cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen WA, Wan R, Mattson MP. Impact of aging on stress-responsive neuroendocrine systems. Mech Ageing Dev. 2001;122:963–983. doi: 10.1016/s0047-6374(01)00250-0. [DOI] [PubMed] [Google Scholar]
- 48.Näsman B, Olsson T, Viitanen M, Carlström K. A subtle disturbance in the feedback regulation of the hypothalamic-pituitary-adrenal axis in the early phase of Alzheimer’s disease. Psychoneuroendocrinology. 1995;20:211–220. doi: 10.1016/0306-4530(94)00054-e. [DOI] [PubMed] [Google Scholar]
- 49.Hoogendijk WJ, Meynen G, Endert E, Hofman MA, Swaab DF. Increased cerebrospinal fluid cortisol level in Alzheimer’s disease is not related to depression. Neurobiol Aging. 2006;27:780.e1–780.e2. doi: 10.1016/j.neurobiolaging.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Bissette G, Reynolds GP, Kilts CD, Widerlöv E, Nemeroff CB. Corticotropin-releasing factor-like immunoreactivity in senile dementia of the Alzheimer type: reduced cortical and striatal concentrations. JAMA. 1985;254:3067–3069. [PubMed] [Google Scholar]
- 51.De Souza EB. CRH defects in Alzheimer’s and other neurologic diseases. Hosp Pract (Off Ed) 1988;23:59–71. doi: 10.1080/21548331.1988.11703535. [DOI] [PubMed] [Google Scholar]
- 52.Pomara N, Singh RR, Deptula D, LeWitt PA, Bissette G, Stanley M, Nemeroff CB. CSF corticotropin-releasing factor (CRF) in Alzheimer’s disease: its relationship to severity of dementia and monoamine metabolites. Biol Psychiatry. 1989;26:500–504. doi: 10.1016/0006-3223(89)90071-1. [DOI] [PubMed] [Google Scholar]
- 53.Whitehouse PJ, Vale WW, Zweig RM, Singer HS, Mayeux R, Kuhar MJ, Price DL, De Souza EB. Reductions in corticotropin releasing factor-like immunoreactivity in cerebral cortex in Alzheimer’s disease, Parkinson’s disease, and progressive supranuclear palsy. Neurology. 1987;37:905–909. doi: 10.1212/wnl.37.6.905. [DOI] [PubMed] [Google Scholar]
- 54.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 55.De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 56.Davis KL, Mohs RC, Marin DB, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Neuropeptide abnormalities in patients with early Alzheimer disease. Arch Gen Psychiatry. 1999;56:981–987. doi: 10.1001/archpsyc.56.11.981. [DOI] [PubMed] [Google Scholar]
- 57.Hibbard C, Yau JLW, Seckl JR. Glucocorticoids and the ageing hippocampus. J Anat. 2000;197:553–562. doi: 10.1046/j.1469-7580.2000.19740553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landfield P, Waymire J, Lynch G. Hippocampal aging and adrenocorticoids: Quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- 59.Issa A, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged cognitively impaired and cognitively unimpaired aged rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–54. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NPV, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 63.Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NPV. Longitudinal study of basal cortisol levels in healthy elderly subjects: Evidence for subgroups. Neurobiol Aging. 1996;17:95–105. doi: 10.1016/0197-4580(95)02005-5. [DOI] [PubMed] [Google Scholar]
- 64.Jellinger K. Maurer K, Maurer K, Riederer P, Beckman H, editors. Morphology of Alzheimer’s disease and related disorders. In Alzheimer’s disease, epidemiology, neurochemistry, and clinics. 1990:61–77. [Google Scholar]
- 65.Price D. New perspectives on Alzheimer’s disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- 66.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 67.Sisodia SS, St George-Hyslop PH. Gamma-Secretase, Notch, Abeta and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki T, Koo EH, Selkoe DJ. Cell surface amyloid β-protein precursor colocalizes with b1 integrins at substrate contact sites in neural cells. J Neurosci. 1997;17:1004–1010. doi: 10.1523/JNEUROSCI.17-03-01004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopatho-logic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 71.Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Litchfield S, Smith A, Barnetson L, Smith AD. Relative roles of plaques and tangles in the dementia of Alzheimer’s disease: correlations using three sets of neuropathological criteria. Dementia. 1995;6:21–31. doi: 10.1159/000106918. [DOI] [PubMed] [Google Scholar]
- 72.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 73.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 74.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 75.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 76.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 77.O’Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic–pituitary–adrenal axis function in depression and Alzheimer’s disease. Br J Psychiatry. 1996;168:679–687. doi: 10.1192/bjp.168.6.679. [DOI] [PubMed] [Google Scholar]
- 78.Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry. 1987;48:9–15. [PubMed] [Google Scholar]
- 79.Belanoff JK, Jurik J, Schatzberg LD, DeBattista C, Schatzberg AF. Slowing the progression of cognitive decline in Alzheimer’s disease using mifepristone. J Mol Neurosci. 2002;19:201–206. doi: 10.1007/s12031-002-0033-3. [DOI] [PubMed] [Google Scholar]
- 80.DeBattista C, Belanoff J. C-1073 (mifepristone) in the adjunctive treatment of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:125–129. doi: 10.2174/1567205053585954. [DOI] [PubMed] [Google Scholar]
- 81.Kulstad JJ, McMillan PJ, Leverenz JB, Cook DG, Green PS, Peskind ER, Wilkinson CW, Farris W, Mehta PD, Craft S. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J Neuropathol Exp Neuro. 2005;64:139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- 82.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 83.Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice mice. J Comp Neurol. 2007;500:311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 85.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPsw transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropath Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576 mice) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 87.Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, SLee SH, Emson PC, Suh YH. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- 89.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rissman RA, Lee KF. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. Neurosci. 2007;27:6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev Neurosci. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- 93.Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice mice via generation of metabolic oxidative stress. J Neurochem. 2009;108:165–75. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- 94.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 mice transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Souza EB, Battaglia G. Corticotropin-releasing hormone (CRH) receptors in brain. Adv Exp Med Biol. 1988;245:123–136. doi: 10.1007/978-1-4899-2064-5_9. [DOI] [PubMed] [Google Scholar]
- 96.Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med. 2005;7:3–36. doi: 10.1385/nmm:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmolesky MT, De Ruiter MM, De Zeeuw CI, Hansel C. The neuropeptide corticotropin-releasing factor regulates excitatory transmission and plasticity at the climbing fibre-Purkinje cell synapse. Eur J Neurosci. 2007;25:1460–1466. doi: 10.1111/j.1460-9568.2007.05409.x. [DOI] [PubMed] [Google Scholar]
- 100.Harkany T, Abraham I, Timmerman W, Laskay G, Tóth B, Sasvári M, Kónya C, Sebens JB, Korf J, Nyakas C, Zarándi M, Soòs K, Penke B, Luiten PG. Beta-amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. Eur J Neurosci. 2000;12:2735–2745. doi: 10.1046/j.1460-9568.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- 101.Nakagami Y, Oda T. Glutamate exacerbates amyloid β1–42-induced impairment of long-term potentiation in rat hippocampal slices. Jpn J Pharmacol. 2002;88:223–226. doi: 10.1254/jjp.88.223. [DOI] [PubMed] [Google Scholar]
- 102.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Green-gard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 103.Tyszkiewicz JP, Yan Z. Beta-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons. J Neurophysiol. 2005;93:3102–3111. doi: 10.1152/jn.00939.2004. [DOI] [PubMed] [Google Scholar]
- 104.Coussens CM, Kerr DS, Abraham WC. Glucocorticoid receptor activation lowers the threshold for NMDA-receptor-dependent homosynaptic long-term depression in the hippocampus through activation of voltage-dependent calcium channels. J Neurophysiol. 1997;78:1–9. doi: 10.1152/jn.1997.78.1.1. [DOI] [PubMed] [Google Scholar]
- 105.Krugers HJ, Goltstein PM, Van der Linden S, Joëls M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J Neurosci. 2006;23:3051–3055. doi: 10.1111/j.1460-9568.2006.04842.x. [DOI] [PubMed] [Google Scholar]
- 106.Harris-White ME, Chu T, Miller SA, Simmons M, Teter B, Nash D, Cole GM, Frautschy SA. Estrogen (E2) and glucocorticoid (Gc) effects on microglia and A beta clearance in vitro and in vivo. Neurochem Int. 2001;39:435–448. doi: 10.1016/s0197-0186(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 107.Abrahám I, Harkany T, Horvath KM, Veenema AH, Penke B, Nyakas C, Luiten PG. Chronic corticosterone administration dose-dependently modulates Abeta (1–42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol. 2000;12:486–494. doi: 10.1046/j.1365-2826.2000.00475.x. [DOI] [PubMed] [Google Scholar]