Abstract
Our research, inspired by the pioneering works of Isaac Witz in the 1980s, established that 40% of human metastatic melanomas express ectopically inhibitory Fc gamma receptors (FcγRIIB), while they are detected on less than 5% of primary cutaneous melanoma and not on melanocytes. We demonstrated that these tumoral FcγRIIB act as decoy receptors that bind the Fc portion of antimelanoma IgG, which may prevent Fc recognition by the effector cells of the immune system and allow the metastatic melanoma to escape the humoral/natural immune response. The FcγRIIB is able to inhibit the ADCC (antibody dependent cell cytotoxicity) in vitro. Interestingly, the percentage of melanoma expressing the FcγRIIB is high (70%) in organs like the liver, which is rich in patrolling NK (natural killer) cells that exercise their antitumoral activity by ADCC. We found that this tumoral FcγRIIB is fully functional and that its inhibitory potential can be triggered depending on the specificity of the anti-tumor antibody with which it interacts. Together these observations elucidate how metastatic melanomas interact with and potentially evade humoral immunity and provide direction for the improvement of anti-melanoma monoclonal antibody therapy.
1. Introduction
The analysis of different types of tumor biopsies by immunohistochemistry for the expression of low affinity receptors for the Fc portion of IgG antibodies (FcγRII) showed that melanomas are one of the rare non-hematopoietic tumors that express FcγRIIB. This ectopic expression of FcγRIIB is restricted to metastatic melanoma and is acquired during the metastasis process. The tumor FcγRIIB found are fully functional. They bind the Fc portion of IgG and retain the ability to initiate an inhibition of cellular phosphorylation cascades.
We will present the context of this expression of Fcγ receptor. We will review the consequences of the FcγRIIB expression by metastatic melanoma assayed in mouse models. Finally, we will discuss its implications for human therapeutic approaches.
2. FcγR in the Immune System
2.1. Extra- and Intracellular Characteristics of FcγR
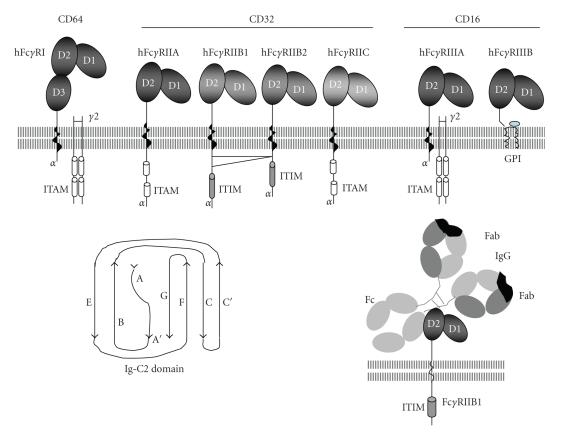
The FcγR are glycoproteins that belong to the Immunoglobulin Superfamily (IgSF) [1]. Their extracellular portions are folded in two or three globular domains called “Ig-C2” domains of approximately 90 amino acids each. A typical “Ig-C2” domain possesses an alternating succession of 8 β sheets (A, A′, B, C, C′, E, F and G) and 7 α loops (AA′, A′B, BC, CC′, C′E, EF and FG) (Figure 1). The β sheets form a bundle stabilized by a disulfide bond between the two opposing B and F β sheets. The BC, CC′, EF and FG loops possess asparagines, whose lateral chain NH function are candidates for glycosylation. The FcγRI possess 3 Ig-C2 domains: the N terminal D1, the D2 and the membrane proximal D3. The FcγRII and FcγRIII possess two of these domains: the N terminal D1 and the D2. The D1 is usually the more glycosylated domain [1–3]. Glycosylation represents between 30 and 45 percent of the molecular weight of the FcγR α chain. The FcγR interact with the immunoglobulin G by their domains D2, which recognize the hinge region of the IgG.
Figure 1.
The human Fc gamma Receptors. Structure of Human Fc gamma Receptors CD64, CD32, and CD16. The FcγR belongs to the Ig superfamily and possess Ig-C2 types of extracellular domains. The domain D2 of the FcγRIIB binds to the hinge region of the IgG.
The variations in size and structure of the IgG's hinge region, the nature and degree of glycosylation in the Fc portion of the IgG, and the number of D domains and differences in glycosylation of the FcγR are parameters that modulate the binding affinity of the different IgG isotypes to each one of the FcγR (Table 1).
Table 1.
Affinity of FcγR for the IgG. Relative affinity of human and mouse Fc gamma receptors for the different isotypes of human and mouse Immunoglobulins G.
| hFcγRIIB | hFcγRIIA | hFcγRIIIA | hFcγRI | |
|---|---|---|---|---|
| Ka | <107 M | <107 M | 1-2 × 107 M | 108-109 M |
| human IgG | 3 > 1 = 4 ⋙ 2 | HR 3 > 1 ⋙ 2,4 | 3 > 1 ⋙ 2,4 | 3 = 1 > 4 ⋙ 2 |
| LR 3 > 1 = 2 ⋙ 4 | ||||
| mouse IgG | 2a = 2b > 1,3 | HR 2a = 2b = 1 | 3 > 2a > 2b ⋙ 1 | 2a = 3 ≫ 1,2b |
| LR 2a = 2b ⋙ 1 | ||||
|
| ||||
| mFcγRIIB | mFcγRIII | mFcγRIV | mFcγRI | |
|
| ||||
| Ka | <107 M | <107 M | 3×107 M | 108 M |
| mouse IgG | 1 = 2b > 2a ⋙ 3 | 2a = 2b = 1 ⋙ 3 | 2a = 2b ⋙ 1,3 | 2a ⋙ 1,2b, 3 |
| human IgG | 3 > 1 > 2 ≫ 4 | 1 = 3 > 2 ≫ 4 | 1,3 | 3 > 1 > 4 ≫ 2 |
The N-terminal extracellular domain of the FcγR is followed by a transmembrane domain (TM) of about 20 amino acids and by a C-terminal intracytoplasmic tail called α chain, except in FcγRIIIB. This latter has an extracellular domain covalently linked to a GPI (Glyco-Phosphatidyl Inositol) moiety, which is anchored in the cytoplasmic membrane [4].
The intracytoplasmic domains are more heterogeneous. The FcγRI, FcγRIIA, FcγRIIB2 and FcγRIIIA are endocytic receptors while the FcγRIIB1 possess a specific sequence of membrane retention. The FcγRII are self-sufficient. The intracytoplasmic domain of FcγRIIA and FcγRIIC contain a signal-transducing unit called ITAM (Immunoreceptor Tyrosine-based Activation Motif) [5, 6] whereas the FcγRIIB contain an ITIM (Immunoreceptor Tyrosine-based Inhibition Motif) [7, 8] The FcγRI and FcγRIIIA do not possess a signal-transducing unit but form a complex with a dimer of a polypeptide chain containing an ITAM called “FcR γ chain” (or FcεR γ chain, as it was first described associated with the FcR for IgE). The association of the α chains FcγRI and FcγRIIIA with the FcR γ chains is required for cell surface expression of these receptors [9] (Figure 1).
2.2. FcγR and Immune Functions
The Fcγ Receptors are of high affinity (FcγRI) when their extracytoplasmic domains recognize monomeric IgG. They are of low affinity (FcγRII, FcγRIII) when they loosely recognize monomeric IgG but bind avidly IgG complexed with their antigens (immune complexes) [10–13] (Table 1). All FcγR belong to the Ig superfamily and share sequence homologies for their extracellular regions, but they differ in their cytoplasmic regions. They are further defined by the way their aggregation initiates or influences the intracellular phosphorylation cascades, which promote cellular activations versus cellular inhibitions. The aggregation of activation receptors (FcγRI, FcγRIIA, FcγRIIC and FcγRIIIA) initiates the phosphorylation of tyrosine of the ITAM by src-family protein tyrosine kinase (PTK) that recruits kinases like Syk. This leads to the phosphorylation by Syk of downstream targets and to cellular activations, which depend upon the cell type and include cell degranulation, cytokine secretion, and phagocytosis of immune complexes. In addition, the activating FcγR on monocytes, macrophages, neutrophils, eosinophils, and dendritic cells can mediate killing of IgG-sensitized target cells by ADCC (Antibody Dependant Cell Cytotoxicity).
Alternative splicing produces two isoforms of the human inhibitory receptors FcγRIIB. The FcγRIIB1 possess a 19 amino acid membrane retention sequence absent from the FcγRIIB2. The FcγRIIB2 are endocytic receptors expressed by monocytes, macrophages and dendritic cells and are involved in regulation of antigen processing and presentation. The FcγRIIB1 are non-endocytic receptors expressed in B cells and mastocytes. The inhibition receptor FcγRIIB1 coaggregation with an activation receptor containing a pITAM, like a triggered BCR (B Cell Receptor), initiates an inhibition message. The pITAM recruits the src-family protein tyrosine kinase Lyn, which phosphorylates the tyrosine of the FcγRIIB1 ITIM. Then the pITIM recruits the hematopoietic specific inositol phosphatases SHIP, (the ubiquitous SHP1 or SHP2 tyrosine phosphatases can potentially be recruited), which shut down the phosphorylation cascade of activation [14]. FcγRIIB homoaggregation alone may or may not induce the ITIM tyrosine phosphorylation. In human B cells, it induces the phosphorylation of the ITIM, which leads to cellular inactivation of human peripheral IgM (+) B cells [15]. In mice, it does not phosphorylate the ITIM but induces a Btk dependent apoptosis on class-switched IgG (+) B cells and plasma cells [16–18].
2.3. The FcγR-Dependent Anti-Tumor Defenses
In some patients suffering from cancer lesions, serum IgG antibodies are present that recognize cancer cells, form immune complexes and consequently activate FcγR. A sustained serological anti-tumor response occurs in patients with melanoma, which includes IgG antibodies against cell surface tumor antigens such as the cancer/testis antigens (NY-ESO-1, MAGE, SSX) and tyrosinase [19–21]. An indication that the serological response is beneficial comes from vaccination studies, which have demonstrated a significant association between the development of an anti-tumor antibody response and survival of melanoma patients [22–24]. The efficacy of therapeutic IgG antibodies against hematopoietic and epithelial tumors argues for an important role of IgG antibodies in anti-tumor defenses [25, 26].
Immune complexes can be involved in the afferent part of the anti-tumor immune response and increase antigen presentation [27, 28]. Moreover, anti-tumor IgG can participate in tumor destruction by activating the cytotoxic activity of FcγR positive cells. Indeed, in vitro studies have demonstrated Fc-dependant mAb mediated ADCC of cell lines derived from solid and lymphatic tumors by FcγR expressing monocytes, macrophages, eosinophils, neutrophils, and NK cells [29, 30]. In early studies, the capability of mAb to elicit tumor regression was found to depend on FcγR-expressing effector cells and the rate of tumor rejection was correlated with the density of FcγR-expressing effector cell infiltration at the tumor site after antibody therapy [31]. The Ab isotype, which induced the strongest depletion of tumor cells, correlates with the most active subclass in ADCC [30–32]. In a clinical study comparing isotype switch variants of alemtuzumab specific for CD52, the strongest depletion of malignant cells was obtained with the antibody isotype that most effectively induced ADCC in vitro [29]. Studies in Fc receptor deficient nude mice indicate that anti-tumor effects of mAbs, such as anti-CD20 mAb (rituximab) and anti-HER2 (trastuzumab), need the presence of the FcR γ chain to be efficient. Macrophages in the lung have been implicated [33]. In the B16 metastatic mouse melanoma model FcγRI [34] and FcγRIV(CD16-2) [35], the equivalent of the human FcγRIIIA, were found to play a major role in the therapeutic effect of the TA99 mAb (a mAb anti-tyrosinase associated protein gp75) [36, 37].
The FcγR-dependent therapeutic effects of IgG anti-tumor antibodies are counterbalanced by the inhibitory FcγRIIB. Indeed, it was shown that the coexpression of host FcγRIIB and activation FcγR on effector cells when engaged together down modulates the anti-tumor efficiency of rituximab and trastuzumab [38].
However, early complement component such as degradation products of C3 might be involved as illustrated by the lower susceptibility of CD11c/CD18 deficient mice to TA99-antibody therapy against mouse metastatic melanoma B16 [39].
3. Expression of Tumor FcγRIIB
3.1. FcγRII and Tumor: First Observations
Since the 1970's, it has been suspected that FcγR could be expressed by non-hematopoietic cell types and tumors.
Neural crest derived cells have been reported to express the FcγR. Examples are the Langehrans cells [40, 41], which express the FcγRIIB2 [42], the Schwann cells, which express the FcγRIII [43] and the Purkinje cells, which express the FcγRIIB [44]. Endothelial and trophoblastic cells of the placenta express FcRn, as well as FcγRII and FcγRIII [45, 46]. In Graves' Disease, the thyroid epithelial cells (thyrocytes) express the FcγRIIB2 [47].
Milgrom et al. first proposed a tumoral expression of FcγR. They used a cryostat hemadsorption technique to demonstrate that tissue sections of different mouse sarcomas adsorbed antibody-coated sheep erythrocytes (SRBC) [48].
In humans, Tonder and Thunold reported that various non-hematopoietic malignant tumors adsorbed SRBC in a manner dependent on the Fc portion of the antibody [49]. At the same time, Isaac Witz showed the presence of IgG within non-lymphoid tumor tissue and observed that these IgG did not exhibit any antibody activity against tumor antigens, suggesting that they bound to tumor cells via their Fc region [50]. This hypothesis was supported by further experiments where primary cultures of tumors and SRBC formed rosettes [51, 52]. In addition, it was observed that tumor cells injected in mice immunized with non-tumoral antigens were able to bind antibody via their Fc region [53]. However, the ectopic expression of FcγR by non-hematopoietic tumors cells was controversial because the presence of inflammatory cells, which can express FcγR, was demonstrated early at the tumor site [54] and because FcγR expression was lost during short-term tumor cell in vitro culture [55]. Nevertheless, it was shown that the experimental tumors regained expression after a single passage in vivo [56] and that some tumor cell lines express FcγR [57]. Moreover Gorini et al. described the presence of FcγR on the cell surface of two human neuroblastoma cell lines using immunohistochemistry and flow cytometry analysis [58]. It is worth noting neuroblastoma are tumors of young children that can arise from any part of the neural crest.
Using experimental models, it was previously suggested that host factors are involved in the induction of FcγR expression by tumor cells. Ran et al. observed that anaplastic carcinoma originally induced by polyomavirus (PyV) and BALB/c 3T3 cells transformed in vitro with PyV acquired FcγR expression only after being passaged in syngeneic animals as solid tumors [56].
3.2. Melanoma Express the FcγRIIb: Immunohistochemical Analysis
The analysis of the expression of FcγR by non-hematopoietic human tumor cells has been realized by immunohistochemistry on a small number of frozen sections of biopsies [59]. Later on, the technique was adapted for 259 primary tumors and 187 metastatic lesions from 12 different cancer types on paraffin-embedded sections with an anti-FcγRIIB rabbit antibody specific for the intracytoplasmic domain of the receptor [60]. The morphology of the cells and double staining with a tumor marker allowed identification of the expressing cells as cancer cells and not infiltrating cells. The analyses show that 24% (49/203 biopsies) of melanoma lesions were positive for the expression of FcγRII. Metastatic melanoma appeared as the tumor with the highest percentage of FcγRIIB expression at 34% (45/121 biopsies), far more than primary melanoma at 5% (4/82) and other non-hematopoietic carcinoma (ovary, brain and colon carcinoma counted, respectively, at 14%, 5% and 4% positive biopsies). By comparing the origin of the lesions, it appears that liver and lymph node metastases with, respectively, 69% and 44% of positive tumors express higher levels of FcγRIIB while lung and skin metastasis express lower levels of 20% and 10%, respectively. This global pattern leads to the proposition that the expression of FcγRIIB correlates with the metastatic progression of the tumor. This hypothesis is reinforced by the finding that patients, for which there are time point biopsy histories, show an absence of FcγRIIB expression in primary lesions and an expression at metastatic stages only in the liver and lymph nodes, and not in the skin [60].
3.3. Gain and Stability of FcγRIIB Expression by Melanoma Cells
It is still unknown how non-hematopoietic tumors acquire the expression of FcγRII. Viral induction has been proposed by Witz for anaplastic carcinoma induced by polyomavirus (PyV). In mouse 3T3 cells transformed by PyV, expression was acquired in vivo and lost in vitro [56]. The loss of FcγRIIB expression following in vitro culture was reported for other non-hematopoietic expressers like Schwann cells or Purkinje cells [61].
When cells derived from frozen biopsies were selectively enriched for the FcγRIIB1 expression and positive clones were established as cell lines in vitro, the phenotype analysis confirmed the melanoma identity of most of these cell lines by the expression of melanoma marker (GD2, Mel/14) and the absence of hematopoietic cells marker (CD45, CD14, CD1a, CD4, CD15, CD20). The analysis of these cell lines karyotypes, which never showed tetraploidy but presented very specific melanoma chromosomal abnormalities, reinforced the identification and excluded fusion with hematopoietic cells. Interestingly, the expression of FcγRIIB was stable in culture in vitro for several years. The biochemical analysis established the expression of only the FcγRIIB1 isoform [59].
Several hypotheses can be proposed to explain the acquisition of FcγRIIB1 expression at the metastasis stage. One possibility might be that the presence of IgG-containing immune complexes within tumors may upregulate FcγRII expression on tumor cells, a phenomenon previously observed in vitro in cell lines of hematopoietic origin [62]. It could be that cytokines in the tumor microenvironment may induce the expression of FcγRIIB1 on tumor cells. One example is IL-4, a cytokine known to upregulate FcγRIIB on human macrophages [63]. Interestingly, melanocytes are of neural crest origins and melanoma express de novo some neural crest cell specific proteins, like bone morphogenic proteins BMP-4 and BMP-7, expression of which is dependent on the master transcription factor Ets-1. BMP proteins are expressed in metastatic melanoma and are prometastatic [64]. It could be that FcγRIIB are derepressed or actively transcribed by a similar dedifferentiation process.
4. Physiological Consequence of Melanoma FcγRIIB1 Expression
4.1. Effect of PyV-Induced FcγRIIB on Carcinoma Metastasic Behavior
Witz's experiments suggested that the induction of FcγRIIB in vivo in PyV induced carcinoma would be the result of a positive “selection” by the gain of metastatic or proliferative capabilities, a process called immunoediting of tumors [65]. Zusman et al. demonstrated that in 3T3 cells transfected to express the mouse FcγRIIB1, the mouse FcγRIIB2 or a deletant for the intracytoplasmic domain of the mouse FcγRIIB, only the full FcγRIIB conferred enhanced tumorigenicity (the IIB1 more than the IIB2) [66] and that the intracellular domain of the receptor was involved in the phenotype [67]. The hypothesis was that the Complement Dependant Cytotoxicity (CDC) and the ADCC defense mechanism would be impaired by the tumor FcγRIIB1. It was not clear to which extent the intracytoplasmic domain of the receptor was needed. It could be necessary for the receptor retention at the cell membrane, for the signaling through the ITIM and the triggering of phosphatase SHP or for both of these properties of the IC domain.
4.2. Xenograft of FcγRIIB1+ Metastatic Melanoma in Immunocompromised Mice
To evaluate the effects of tumor FcγRIIB1 expression on melanoma growth and metastasis, the tumors derived from biopsies were grafted to immunocompromised nude and SCID mice [59]. Nude mice are impaired for the T cell response but able to mount a B cell xenogenic response characterized by the secretion of antimelanoma antibodies, principally of the mouse IgM and IgG3 isotypes. These mouse immunoglobulins have no affinity for mouse FcγR but mIgG3 binds human FcγR. SCID mice are unable to engage in BCR and TCR gene recombination; they show no mature T or B cells, have no serum Ig titer and reject neither allo- nor xenografts. Spontaneous expressers of human tumor FcγRIIB1 as well as transfectant melanoma cell lines were inhibited in their subcutaneous growth in nude mice. This inhibition was dependent of the intracytoplasmic domain of the human FcγRIIB1 as transfected melanoma expressing human FcγRIIB1 lacking the intracytoplasmic domain were not affected in their growth. In SCID mice as well as in vitro, the proliferation of the tumors were unaffected by the FcγRII expression. Antimelanoma mIgG3 bind to the tumor antigen by its Fab region and to the human tumor FcγRIIB1 by its Fc region. This crosslink triggered the phosphorylation of the ITIM domain of the receptor, whose pTyrosine interacts with the SH2 domain of SHP-2 (no co-precipitation of SHP-1 or SHIP was detected in HT144 melanoma cell line). The phosphatase activity inhibits cell proliferation and tumor development. This system does not trigger the immune system of the host, as mouse FcγR have no affinity for mouse IgG3 (Table 1), and ADCC is bypassed. One intriguing question is relative to the role of SHP-2. The effects of upregulation of SHP-2 are controversial; some studies attribute to SHP-2 overexpression an increase in metastatic potential [68, 69], others a decrease [70]. In particular, in a recent study on lung adenocarcinoma, SHP-2 upregulation has been associated with an inhibition of migration (by phosphorylation of Hef1/Cas-L), which suggests that a downregulation of SHP-2 expression could be associated with a gain of migratory potential [70]. One can ask if highly metastatic melanoma, which express FcγRIIB1, would not be low SHP-2 expressers.
4.3. Allogenic and Syngenic Graft of Mouse FcγRIIB1+ Melanoma in Immunocompetant Mice
To evaluate the immunologic host-tumor relationship a murine model was used [60]. The C57BL/6 (H2b) melanoma cell line B16 is indeed slightly immunogenic and as a pigmented cell line, extremely sensible to the effect of anti-tyrosinase antibodies [71]. With this model, a hallmark of success in immunization protocols or passive transfer of anti-tyrosinase antibodies is post-treatment vitiligo [72]. The B16 melanoma transfected to express the mouse FcγRIIB1 receptors either intact or ITIM invalidated by Y→A mutation were grafted in syngeneic host C57BL/6 mice [60]. The tumor uptake and the growth in the syngeneic host were indifferent to the presence of mFcγRIIB1. In the allogeneic host BALB/c (H2d) (albino mice that do not express tyrosinase [73]), the B16 tumor was strongly rejected and purified anti-B16 antibodies from BALB/c mice were sufficient to transfer the rejection of B16 in SCID mice in an ADCC dependent way [60]. The more striking observation was the ability of B16 (FcγRIIB1) or B16 (FcγRIIB1 ITIMY→A) grafted subcutaneously in BALB/c mice to counteract the allogenic rejection and grow (Joel Cohen-Solal, unpublished data). These results propose that FcγRIIB1 possess the ability to protect the melanoma from the humoral antimelanoma immunity and from the allogenic rejection. This later consideration could highlight the mechanism of allogenic tolerization of the fetus proposed by Clark Anderson. The yolk sac membranes, which are of fetal origin, express FcRn and FcγRIIB as well as paternal/maternal MHC molecules and are protected from maternal allogenic antibody mediated cell cytotoxicity.
5. Mechanism of Action of Melanoma FcγRIlB
5.1. Inhibition of ADCC In Vivo and In Vitro
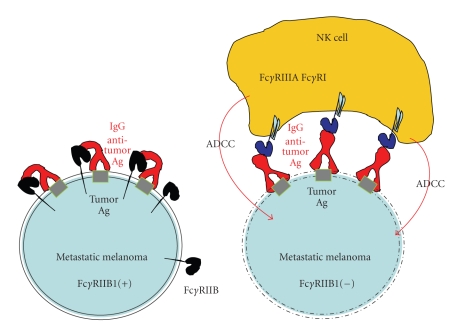
In C57BL/6 mice, the growth of the melanoma B16 is inhibited by treatment of the mice with the mouse IgG2a monoclonal antibody TA99 anti-Tyrosinase Associated Protein TYRP-1/gp75 [72]. In this model, the antibody action is entirely dependent of the FcR γ chain of the effector cells of the host. These receptors are the FcγRI, FcγRIII and FcγRIV (CD16-2) and the cells that express them are NK cells, macrophages, monocytes and neutrophils [39, 72, 74–76]. The expression of the tumor FcγRIIB and FcγRIIB invalidated ITIMY→A antagonize the therapeutic effect of TA99. The ADCC inhibition is also observed in vitro with human melanomas expressing FcγRIIB1 full or deleted of its intracytoplasmic domain [60]. This result suggests that the expression of FcγRIIB1 has the ability to inhibit the ADCC mechanism independently of the ITIM function. The simplest view is a mechanism of decoy receptor: the anti-tumor antibody binds the tumor by its Fab portion while its Fc portion is caught by the tumor FcγRIIB1 and cannot be recognized by the FcγR of the effector cell (Figure 2).
Figure 2.
Tumor FcγRIIB1 expression protects metastatic melanoma from ADCC. Metastatic melanomas that express the FcγRIIB1 are resistant in vitro and in vivo in immunocompetent mice to the ADCC mediated by FcR γ chain dependent effector cells (NK cells, neutrophiles monocytes, or macrophages). The tumor FcγRIIB1 are decoy receptors. The Fc portions of anti-tumor antibodies are captured by the tumor FcγRIIB1 and cannot interact with the Fcγ of the immune effector cells.
5.2. Inhibition of Growth In Vivo and In Vitro
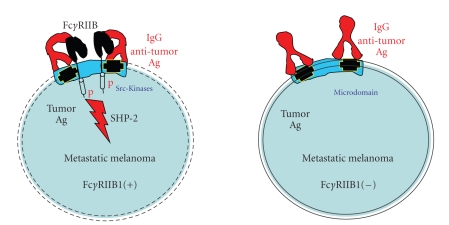
The nevis and primary melanoma express the gangliosides GD3 and GM3. Upon metastasis, they acquire the expression of the enzyme N-acetylgalactosaminyl transferase that converts GD3 into GD2 [77]. Anti-GD2 monoclonal antibodies are used in radiotherapy, coupled to radioelements like 131I or 188Re. There exist two well-documented mouse anti-GD2 antibodies called 7A4 (mouse IgG3) and 3F8 (mouse IgG3) [78, 79]. Mouse IgG3 bind to human FcγR and have a cell cytotoxicity, which depends of activation FcγR and complement receptor of the human host effector cells. They have no effect in vitro on the melanoma proliferation. Surprisingly, the melanomas that express the FcγRIIB1 are inhibited in their in vitro proliferation as well as in vivo when grafted in SCID mice following treatment with 7A4 [59]. The inhibition is dependent on the intracellular domain of the receptor and the ITIM module is phosphorylated and recruits the tyrosine phosphatase SHP-2 [80]. The simplest explanation of the phosphorylation is the recruitment of a scr-kinase linked to the antigen that is recognized by the anti-tumor antibody, which crosslinks it to the FcγRIIB1 by its Fc portion. Then the scr-kinase phosphorylates the ITIM, which in the case of the availability of a phosphatase to bind the pITIM, transduces a dephosphorylation wave that inhibits cell proliferation (Figure 3).
Figure 3.
Tumor FcγRIIB1 expression inhibits metastatic melanoma growth. Metastatic melanomas that express the FcγRIIB1 receptor are sensible to the action of anti-GD2 mAb. In vitro as well as in vivo in immunocompromised mice the tumor FcγRIIB1 that are aggregated with the ganglioside GD2 by the mAb are subjected to phosphorylation of the ITIM that recruit the phosphatase SHP-2. SHP-2 dephosphorylates the Protein Tyrosine kinases and initiates an inhibition of tumor growth.
5.3. The Nature of the Tumor Antigen Matters
These results propose that the FcγRIIB1 retains its functionality and that its expression could be selected as well as counter-selected depending on the type of antigen recognized by the anti-tumor antibody. Gangliosides like GD3 and GD2 are localized in cholesterol rich microdomains where src-kinases are present. If they attract the FcγRIIB1 in the microdomains, they may promote the phosphorylation of their ITIM and give a selective advantage to the melanoma that do not express FcγRIIB1 [59]. Antigens like TYRP-gp75, and Mel14-gp90MEL, which are transmembrane proteins not associated with kinase activities, will not promote the ITIM phosphorylation but rather promote the confinement of the antimelanoma Ab and give a selective advantage to the melanoma that express the FcγRIIB1 [60].
6. Anti-Melanoma Monoclonal Antibodies and Patient Care
6.1. Clinical Trials
Several clinical trials are reported in the USA that use antimelanoma mAb; the main targets are GD2, GD3, Mel14 and melanin (http://clinicaltrials.gov/). One protocol is an immunization with an anti-idiotype antibody 4B5 (Ab2), which forms an “internal image” of the antigen GD2. The goal is to mount an anti-GD2 response. The other therapies are based on mAb coupled to radiolabeled elements (131I and 188Re). With the exception of the anti-idiotype vaccination, the other therapies do not require the immune effector cells of the host, moreover they try to avoid it by the use of F(ab)′2 fragment of the antibodies. These therapies are based on the local irradiation of the cells that are bound to the antimelanoma antibodies. By avoiding the binding to activator FcγR these antibodies do not deplete the host immune effector cells. For these protocols, the tumoral expression of FcγRIIB is without effect. For the anti-idiotype vaccination this could partially explain the poor response of patients. In protocols of active vaccination in contrast to induction of anti-tumor IgM, the increase of circulating anti-melanoma IgG was associated with a poor prognosis [81–83].
6.2. Optimizing the Fc of mAb
The recent studies of the structure of IgG and their interactions with the different activator FcγR have allowed the design of optimized antibody Fc fragments, which is already a reality with two products on the market (eculizumab and catumaxomab) and about ten candidates in clinical trial. The main modifications target the glycosylation and the binding sites for C1q and FcγR. The effects of the modification are evaluated on ADCC, Antibody Dependent Cell Phagocytosis (ADCP) and CDC [84–86]. The treatment of melanoma will benefit from this amazing work realized over ten years, which allows enhanced binding to FcγRIIA and FcγRIIIA and lower binding to the FcγRIIB and which is exactly what is needed to counteract the tumor FcγRIIB1 expression. An increase of 100-fold in ADCC efficiency is commonly reported with these antibodies [84, 87]. This high level of improvement and the safety of the product will certainly give a second chance to the neglected mAb therapy based on the immune effector function in melanoma treatment.
6.3. Modulation of the Immune System with an Anti-Human FcγRIIB Antibody
The immunization of a mouse transgenic for the human FcγRIIA with the extra-cellular moiety of the human FcγRIIB allowed the development of a set of mouse anti-human FcγRIIB monoclonal antibodies. One of them, 2B6, was selected for its ability to interact solely with the FcγRIIB without crossreacting with either the FcγRIIA or the FcγRIIC and to block the Fc-FcR interaction [88]. 2B6 was modified to reduce its immunogenicity in human by chimerism (xi-mAb) and humanization (zu-mAb). It successfully depletes B cells expressing the FcγRIIB by ADCC [89]. It can potentially modulate the immune system. It has been postulated that T cell mediated anti-melanoma immunity could be enhanced by B cell depletion as melanoma development is impaired in B cell deficient μMT mice [90]. The use of multi-monoclonal antibody therapy could be envisioned with anti-melanoma antibodies and anti-FcγRIIB antibodies targeting both melanoma and B cells. However, recent work based on the B16 melanoma model showed the preponderant role of the B cell compartment in the anti-B16 immune response [91]. To address this issue, further studies are needed that will specifically evaluate the extent to which the use of 2B6 (or another anti FcγRIIB mAb) associated to a monoclonal anti-melanoma therapy could be beneficial.
7. Conclusion
The work that we accomplished over the last ten years demonstrated and quantified the ectopic expression of FcγR by melanoma. We established that FcγR are selected during the course of the metastasis and that their expression culminates at the later stages in the liver or the lymph nodes. We observed that the expression of FcγR is restricted to the IIB1 form of receptor, which possess an inhibitor motif ITIM and a membrane retention intracytoplasmic sequence. The expression of the receptor was heterogeneous inside tumor lesions and was dependent on the site of metastasis and most probably to the relation tumor-host. FcγRIIB1 seem immuno-edited by the presence of activity, which they antagonize in vitro and in vivo in mouse models. In contrast, they are counter-selected in absence of the pressure of effector cells, as observed in vitro and in vivo in nude mice.
On one hand, FcγRIIB1 are not detected in melanocytes. They are rarely seen in primary tumors where the Fc dependent effector functions are described as impaired. Effectively, in primary melanoma lesion, suppressor T cells and tolerizing dendritic cells (DC) deactivate the immune response. TGFβ1/2 and IL-10 are the most effective cytokines responsible for the lack of DC maturation [92]. TGFβ have the ability to downregulate the FcR γ chain expression, which reduces the membrane expression of activator FcγR in myeloid effector cells [93] and IL-10 upregulates inhibitor FcγRIIB1/2 [94]. Additionally, TGFβ inhibit IFNγ secretion and ADCC in human NK cells [95]. On the other hand, FcγRIIB1 is highly expressed in metastatic tumors in the spleen or in the lymph nodes [60]. These melanomas have gained a high metastatic power and reside in the liver, which is an environment rich in NK and NKT cells. Tumor FcγRIIB1 expression appears as a decoy mechanism, which is able to counteract the ADCC mediated humoral immunity. The suppressive effect is powerful enough to allow the allogenic uptake and growth of the C57BL/6 derived B16 tumor in BALB/c mice, which suggest that it should be preponderant in the weak efficiency of the passive transfer of anti-tumor antibodies and of antibody-based vaccination.
The optimization of Fc domain of therapeutic antibodies is now possible and allows for a higher ADCC, ADCP and CDC potential. In the case of anti-FcγRIIB1+ metastatic melanoma therapy, the optimization will be reached by lowering the Fc binding to the FcγRIIB binding and by increasing the Fc binding to FcγRIIIA and FcγRI. The reality of these efficiency improvements and the innocuousness of humanized antibodies ensure that mAb anti-melanoma approaches will be revisited in a near future.
Acknowledgments
The authors would like to thank Association pour la Recherche sur le Cancer (ARC), Institut National du Cancer (INCa), Canceropole Ile de France, La Ligue Nationale contre le Cancer, Inserm, University Pierre et Marie Curie, and University Paris-Descartes. Also they thank all our collaborators, including M.F. Avril (Hopital Tarnier), X. Sastre-Garrau (Insitut Curie), and S. Chouaib (Institut Gustave Roussy).
References
- 1.Kaas Q, Ehrenmann F, Lefranc M-P. IG, TR and IgSF, MHC and MhcSF: what do we learn from the IMGT Colliers de Perles? Briefings in Functional Genomics and Proteomics. 2007;6(4):253–264. doi: 10.1093/bfgp/elm032. [DOI] [PubMed] [Google Scholar]
- 2.Brümmendorf T, Rathjen FG. Cell adhesion molecules 1: immunoglobulin superfamily. Protein Profile. 1995;2(9):963–1108. [PubMed] [Google Scholar]
- 3.Halaby DM, Mornon JPE. The immunoglobulin superfamily: an insight on its tissular, species, and functional diversity. Journal of Molecular Evolution. 1998;46(4):389–400. doi: 10.1007/pl00006318. [DOI] [PubMed] [Google Scholar]
- 4.Qiu WQ, de Bruin D, Brownstein BH, Pearse R, Ravetch JV. Organization of the human and mouse low-affinity Fc γ R genes: duplication and recombination. Science. 1990;248(4956):732–735. doi: 10.1126/science.2139735. [DOI] [PubMed] [Google Scholar]
- 5.Reth M. Antigen receptor tail clue. Nature. 1989;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 6.Cambier JC. Antigen and Fc receptor signaling the awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) Journal of Immunology. 1995;155(7):3281–3285. [PubMed] [Google Scholar]
- 7.Amigorena S, Bonnerot C, Drake JR, et al. Cytoplasmic domain heterogeneity and functions of IgG Fc receptor in B lymphocytes. Science. 1992;256(5065):1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 8.Daëron M, Latour S, Malbec O, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc γ RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3(5):635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 9.Kinet J-P, Launay P. Fc α/μR: single member or first born in the family? Nature Immunology. 2000;1(5):371–372. doi: 10.1038/80805. [DOI] [PubMed] [Google Scholar]
- 10.Fridman WH, Bonnerot C, Daeron M, Amigorena S, Teillaud J-L, Sautes C. Structural bases of Fc γ receptor functions. Immunological Reviews. 1992;(125):49–76. doi: 10.1111/j.1600-065x.1992.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 11.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Advances in Immunology. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 12.Ravetch JV, Bolland S. IgG Fc receptors. Annual Review of Immunology. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Solal JF, Cassard L, Fridman W-H, Sautès-Fridman C. Fc γ receptors. Immunology Letters. 2004;92(3):199–205. doi: 10.1016/j.imlet.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Nimmerjahn F, Ravetch JV. Analyzing antibody-Fc-receptor interactions. Methods in Molecular Biology. 2008;415:151–162. doi: 10.1007/978-1-59745-570-1_9. [DOI] [PubMed] [Google Scholar]
- 15.Fournier EM, Sibéril S, Costes A, et al. Activation of human peripheral IgM+ B cells is transiently inhibited by BCR-independent aggregation of Fc gammaRIIB. Journal of Immunology. 2008;181(8):5350–5359. doi: 10.4049/jimmunol.181.8.5350. [DOI] [PubMed] [Google Scholar]
- 16.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc γ RIIB-induced B cell apoptosis. Immunity. 1999;10(6):753–760. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng S-J, Bolland S, Inabe K, Kurosaki T, Pierce SK. The B cell inhibitory Fc receptor triggers apoptosis by a novel c-Abl family kinase-dependent pathway. Journal of Biological Chemistry. 2005;280(42):35247–35254. doi: 10.1074/jbc.M505308200. [DOI] [PubMed] [Google Scholar]
- 18.Xiang Z, Cutler AJ, Brownlie RJ, et al. FcγRllb controls bone marrow plasma cell persistence and apoptosis. Nature Immunology. 2007;8(4):419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 19.Sahin U, Türeci Ö, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(25):11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jäger E, Chen Y-T, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. Journal of Experimental Medicine. 1998;187(2):265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lake DF, Huynh WC, Hersh EM. Natural and induced human antibody response to cancer. Cancer Investigation. 2000;18(5):480–489. doi: 10.3109/07357900009032819. [DOI] [PubMed] [Google Scholar]
- 22.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston PO. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. International Journal of Cancer. 2000;85(5):659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Lutzky J, Gonzalez-Angulo AM, Orzano JA. Antibody-based vaccines for the treatment of melanoma. Seminars in Oncology. 2002;29(5):462–470. doi: 10.1053/sonc.2002.35241. [DOI] [PubMed] [Google Scholar]
- 24.Ehlken H, Schadendorf D, Eichmüller S. Humoral immune response against melanoma antigens induced by vaccination with cytokine gene-modified autologous tumor cells. International Journal of Cancer. 2004;108(2):307–313. doi: 10.1002/ijc.11537. [DOI] [PubMed] [Google Scholar]
- 25.White CA, Berlfein JR, Grillo-López AJ. Antibody-targeted immunotherapy for treatment of non-Hodgkin’s lymphoma. Current Pharmaceutical Biotechnology. 2000;1(4):303–312. doi: 10.2174/1389201003378889. [DOI] [PubMed] [Google Scholar]
- 26.Carter PJ. Potent antibody therapeutics by design. Nature Reviews Immunology. 2006;6(5):343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama K, Ebihara S, Yada A, et al. Targeting apoptotic tumor cells to Fc γ R provides efficient and versatile vaccination against tumors by dendritic cells. Journal of Immunology. 2003;170(4):1641–1648. doi: 10.4049/jimmunol.170.4.1641. [DOI] [PubMed] [Google Scholar]
- 28.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. Journal of Clinical Investigation. 2002;110(1):71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams DO, Hall T, Steplewski Z, Koprowski H. Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype contain increased numbers of macrophages activated for a distinctive form of antibody-dependent cytolysis. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(11 I):3506–3510. doi: 10.1073/pnas.81.11.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denkers EY, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. Journal of Immunology. 1985;135(3):2183–2186. [PubMed] [Google Scholar]
- 31.Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–2466. [PubMed] [Google Scholar]
- 32.Dyer MJS, Hale G, Hayhoe FGJ, Waldmann H. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood. 1989;73(6):1431–1439. [PubMed] [Google Scholar]
- 33.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bevaart L, Jansen MJH, van Vugt MJ, Sjef Verbeek J, van de Winkel JGJ, Leusen JHW. The high-affinity IgG receptor, FcγRI, plays a central role in antibody therapy of experimental melanoma. Cancer Research. 2006;66(3):1261–1264. doi: 10.1158/0008-5472.CAN-05-2856. [DOI] [PubMed] [Google Scholar]
- 35.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23(1):41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Ravetch JV. Fcγ receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Otten MA, van der Bij GJ, Verbeek SJ, et al. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. Journal of Immunology. 2008;181(10):6829–6836. doi: 10.4049/jimmunol.181.10.6829. [DOI] [PubMed] [Google Scholar]
- 38.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Medicine. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 39.Van Spriel AB, van Ojik HH, Bakker A, Jansen MJH, van de Winkel JGJ. Mac-1 (CD11b/CD18) is crucial for effective fc receptor-mediated immunity to melanoma. Blood. 2003;101(1):253–258. doi: 10.1182/blood.V101.1.253. [DOI] [PubMed] [Google Scholar]
- 40.Muretto P. Immunohistochemical study of dendritic cells in foetal skin and lymph-nodes supporting the hypothesis for the neural crest origin of Langerhans cells. Italian Journal of Anatomy and Embryology. 2008;113(4):237–247. [PubMed] [Google Scholar]
- 41.Muretto P. The relationship of langerhans cells to melanocytes and schwann cells in mature cystic teratomas of the ovary. International Journal of Surgical Pathology. 2007;15(3):266–271. doi: 10.1177/1066896907302227. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt DA, Hanau D, Bieber T, et al. Human epidermal Langerhans cells express only the 40-kilodalton Fc γ receptor (FcRII) Journal of Immunology. 1990;144(11):4284–4890. [PubMed] [Google Scholar]
- 43.Vedeler CA, Matre R, Kristoffersen EK, Ulvestad E. IgG Fc receptor heterogeneity in human peripheral nerves. Acta Neurologica Scandinavica. 1991;84(3):177–180. doi: 10.1111/j.1600-0404.1991.tb04933.x. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Hirai H, Torashima T, et al. CD3 and immunoglobulin G Fc receptor regulate cerebellar functions. Molecular and Cellular Biology. 2007;27(14):5128–5134. doi: 10.1128/MCB.01072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedmak DD, Davis DH, Singh U, van de Winkel JGJ, Anderson CL. Expression of IgG Fc receptor antigens in placenta and on endothelial cells in humans. An immunohistochemical study. American Journal of Pathology. 1991;138(1):175–181. [PMC free article] [PubMed] [Google Scholar]
- 46.Lyden TW, Robinson JM, Tridandapani S, et al. The Fc receptor for IgG expressed in the villus endothelium of human placenta is Fc γ RIIb2. Journal of Immunology. 2001;166(6):3882–3889. doi: 10.4049/jimmunol.166.6.3882. [DOI] [PubMed] [Google Scholar]
- 47.Estienne V, Duthoit C, Reichert M, et al. Androgen-dependent expression of FcγRIIB2 by thyrocytes from patients with autoimmune Graves’ disease: a possible molecular clue for sex dependence of autoimmune disease. FASEB Journal. 2002;16(9):1087–1092. doi: 10.1096/fj.01-0998hyp. [DOI] [PubMed] [Google Scholar]
- 48.Milgrom F, Humphrey LJ, Tönder O, Yasuda J, Witebsky E. Antibody-mediated hemadsorption by tumor tissues. International Archives of Allergy and Applied Immunology. 1968;33(5):478–492. doi: 10.1159/000230063. [DOI] [PubMed] [Google Scholar]
- 49.Tonder O, Thunold S. Receptors for immunoglobulin Fc in human malignant tissues. Scandinavian Journal of Immunology. 1973;2(3):207–215. doi: 10.1111/j.1365-3083.1973.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 50.Witz IP. The biological significance of tumor-bound immunoglobulins. Current Topics in Microbiology and Immunology. 1973;61:151–171. doi: 10.1007/978-3-642-65531-9_4. [DOI] [PubMed] [Google Scholar]
- 51.Biran H, Mavligit GM, Moake JL. Receptor sites for complement and for immune complexes on human nonhemopoietic tumor cells. Cancer. 1979;44(1):131–135. doi: 10.1002/1097-0142(197907)44:1<131::aid-cncr2820440123>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 52.Noltenius HW. Fc and complement receptors on malignant tumor cells. Cancer. 1981;48(8):1761–1767. doi: 10.1002/1097-0142(19811015)48:8<1761::aid-cncr2820480812>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 53.Braslawsky GR, Yaackubowicz M, Frensdorff A, Witz IP. Receptors for immune complexes on cells within a non lymphoid murine tumor. Journal of Immunology. 1976;116(6):1571–1578. [PubMed] [Google Scholar]
- 54.Svennevig J-L, Andersson TR. Cells bearing Fc receptors in human malignant solid tumours. British Journal of Cancer. 1982;45(2):201–208. doi: 10.1038/bjc.1982.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ran M, Teillaud J-L, Fridman WH, et al. Increased expression of Fc γ receptor in cancer patients and tumor bearing mice. Molecular Immunology. 1988;25(11):1159–1167. doi: 10.1016/0161-5890(88)90151-4. [DOI] [PubMed] [Google Scholar]
- 56.Ran M, Katz B, Kimchi N, et al. In vivo acquisition of Fc γ RII expression on polyoma virus-transformed cells derived from tumors of long latency. Cancer Research. 1991;51(2):612–618. [PubMed] [Google Scholar]
- 57.Szymaniec S, James K. Studies on the Fc receptor bearing cells in a transplanted methylcholanthrene induced mouse fibrosarcoma. British Journal of Cancer. 1976;33(1):36–50. doi: 10.1038/bjc.1976.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorini G, Ciotti MT, Starace G, Vigneti E, Raschellà G. Fc gamma receptors are expressed on human neuroblastoma cell lines: lack of correlation with N-myc oncogene activity. International Journal of Neuroscience. 1992;62(3-4):287–297. doi: 10.3109/00207459108999781. [DOI] [PubMed] [Google Scholar]
- 59.Cassard L, Cohen-Solal JFG, Galinha A, et al . Modulation of tumor growth by inhibitory Fc(gamma) receptor expressed by human melanoma cells. Journal of Clinical Investigation. 2002;110(10):1549–1557. doi: 10.1172/JCI15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassard L, Cohen-Solal JFG, Fournier EM, et al. Selective expression of inhibitory Fcγ receptor by metastatic melanoma impairs tumor susceptibility to IgG-dependent cellular response. International Journal of Cancer. 2008;123(12):2832–2839. doi: 10.1002/ijc.23870. [DOI] [PubMed] [Google Scholar]
- 61.Vedeler CA, Scarpini E, Beretta S, Doronzo R, Matre R. The ontogenesis of Fc γ receptors and complement receptors CR1 in human peripheral nerve. Acta Neuropathologica. 1990;80(1):35–40. doi: 10.1007/BF00294219. [DOI] [PubMed] [Google Scholar]
- 62.Neauport-Sautes C, Daëron M, Teillaud JL, Blank U, Fridman WH. The occurrence, structural and functional properties of immunoglobulin Fc receptors on murine neoplastic cells. International Reviews of Immunology. 1986;1(3-4):237–271. doi: 10.3109/08830188609056609. [DOI] [PubMed] [Google Scholar]
- 63.Tridandapani S, Wang Y, Marsh CB, Anderson CL. Src homology 2 domain-containing inositol polyphosphate phosphatase regulates NF-κ B-mediated gene transcription by phagocytic Fc γ Rs in human myeloid cells. Journal of Immunology. 2002;169(8):4370–4378. doi: 10.4049/jimmunol.169.8.4370. [DOI] [PubMed] [Google Scholar]
- 64.Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff A-K. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Research. 2005;65(2):448–456. [PubMed] [Google Scholar]
- 65.Ran M, Langer ABB, Eliassi I, et al. Possibilities of interference with the immune system of tumor bearers by non-lymphoid Fc γ RII expressing tumor cells. Immunobiology. 1992;185(2–4):415–425. doi: 10.1016/s0171-2985(11)80657-1. [DOI] [PubMed] [Google Scholar]
- 66.Zusman T, Gohar O, Eliassi I, et al. The murine Fc-γ (Fc γ) receptor type II B1 is a tumorigenicity-enhancing factor in polyoma-virus-transformed 3T3 cells. International Journal of Cancer. 1996;65(2):221–229. doi: 10.1002/(SICI)1097-0215(19960117)65:2<221::AID-IJC16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 67.Zusman T, Lisansky E, Arons E, et al. Contribution of the intracellular domain of murine FC-gamma receptor type IIB1 to its tumor-enhancing potential. International Journal of Cancer. 1996;68(2):219–227. doi: 10.1002/(SICI)1097-0215(19961009)68:2<219::AID-IJC14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 68.Yu D-H, Qu C-K, Henegariu O, Lu X, Feng G-S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. Journal of Biological Chemistry. 1998;273(33):21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 69.Wang F-M, Liu H-Q, Liu S-R, Tang S-P, Yang L, Feng G-S. SHP-2 promoting migration and metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1β in vivo and in vitro. Breast Cancer Research and Treatment. 2005;89(1):5–14. doi: 10.1007/s10549-004-1002-z. [DOI] [PubMed] [Google Scholar]
- 70.Yo K, Iwata S, Hashizume Y, et al. SHP-2 inhibits tyrosine phosphorylation of Cas-L and regulates cell migration. Biochemical and Biophysical Research Communications. 2009;382(1):210–214. doi: 10.1016/j.bbrc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Vijayasaradhi S, Bouchard B, Houghton AN. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. Journal of Experimental Medicine. 1990;171(4):1375–1380. doi: 10.1084/jem.171.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. Journal of Experimental Medicine. 1995;182(5):1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shibahara S, Okinaga S, Tomita Y, et al. A point mutation in the tyrosinase gene of BALB/c albino mouse causing the cysteine-serine substitution at position 85. European Journal of Biochemistry. 1990;189(2):455–461. doi: 10.1111/j.1432-1033.1990.tb15510.x. [DOI] [PubMed] [Google Scholar]
- 74.Takechi Y, Hara I, Naftzger C, Xu Y, Houghton AN. A melanosomal membrane protein is a cell surface target for melanoma therapy. Clinical Cancer Research. 1996;2(11):1837–1842. [PubMed] [Google Scholar]
- 75.Trcka J, Moroi Y, Clynes RA, et al. Redundant and alternative roles for activating Fc receptors and complement in an antibody-dependent model of autoimmune vitiligo. Immunity. 2002;16(6):861–868. doi: 10.1016/s1074-7613(02)00327-8. [DOI] [PubMed] [Google Scholar]
- 76.van Spriel AB, Leusen JHW, van Egmond M, et al. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97(8):2478–2486. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- 77.Thurin J, Thurin M, Kimoto Y. Monoclonal antibody-defined correlations in melanoma between levels of G(D2) and G(D3) antigens and antibody-mediated cytotoxicity. Cancer Research. 1987;47(5):1229–1233. [PubMed] [Google Scholar]
- 78.Gross N, Beck D, Portoukalian J, Favre S, Carrel S. New anti-GD2 monoclonal antibodies produced from gamma-interferon-treated neuroblastoma cells. International Journal of Cancer. 1989;43(4):665–671. doi: 10.1002/ijc.2910430421. [DOI] [PubMed] [Google Scholar]
- 79.Saito N, Yu RK, Cheung NKV. Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochemical and Biophysical Research Communications. 1985;127(1):1–7. doi: 10.1016/s0006-291x(85)80117-0. [DOI] [PubMed] [Google Scholar]
- 80.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc γ RIIB-induced B cell apoptosis. Immunity. 1999;10(6):753–760. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 81.Litvak DA, Gupta RK, Yee R, Wanek LA, Ye W, Morton DL. Endogenous immune response to early- and intermediate-stage melanoma is correlated with outcomes and is independent of locoregional relapse and standard prognostic factors. Journal of the American College of Surgeons. 2004;198(1):27–35. doi: 10.1016/j.jamcollsurg.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 82.Gupta RK, Leitch AM, Morton DL. Nature of antigens and antibodies in immune complexes isolated by staphylococcal protein A from plasma of melanoma patients. Cancer Immunology, Immunotherapy. 1983;16(1):40–47. doi: 10.1007/BF00199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsueh EC, Gupta RK, Qi K, Morton DL. Correlation of specific immune responses with survival in melanoma patients with distant metastases receiving polyvalent melanoma cell vaccine. Journal of Clinical Oncology. 1998;16(9):2913–2920. doi: 10.1200/JCO.1998.16.9.2913. [DOI] [PubMed] [Google Scholar]
- 84.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Current Opinion in Biotechnology. 2009;20(6):685–691. doi: 10.1016/j.copbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 85.Sibéril S, Dutertre C-A, Fridman W-H, Teillaud J-L. FcγR: the key to optimize therapeutic antibodies? Critical Reviews in Oncology/Hematology. 2007;62(1):26–33. doi: 10.1016/j.critrevonc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Sibéril S, Dutertre C-A, Boix C, et al. Molecular aspects of human FcγR interactions with IgG: functional and therapeutic consequences. Immunology Letters. 2006;106(2):111–118. doi: 10.1016/j.imlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 87.De Romeuf C, Dutertre C-A, Le Garff-Tavernier M, et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcγRIIIA/CD16. British Journal of Haematology. 2008;140(6):635–643. doi: 10.1111/j.1365-2141.2007.06974.x. [DOI] [PubMed] [Google Scholar]
- 88.Veri M-C, Gorlatov S, Li H, et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcγ-receptor IIB (CD32B) from the activating Fcγ-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121(3):392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rankin CT, Veri M-C, Gorlatov S, et al. CD32B, the human inhibitory Fc-γ receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood. 2006;108(7):2384–2391. doi: 10.1182/blood-2006-05-020602. [DOI] [PubMed] [Google Scholar]
- 90.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and T1 cytokine responses by B cells. International Journal of Cancer. 2005;117(4):574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 91.Dilillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. Journal of Immunology. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polak ME, Borthwick NJ, Gabriel FG, et al. Mechanisms of local immunosuppression in cutaneous melanoma. British Journal of Cancer. 2007;96(12):1879–1887. doi: 10.1038/sj.bjc.6603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tridandapani S, Wardrop R, Baran CP, et al. TGF-β1 supresses myeloid Fc γ receptor function by regulating the expression and function of the common γ-subunit. Journal of Immunology. 2003;170(9):4572–4577. doi: 10.4049/jimmunol.170.9.4572. [DOI] [PubMed] [Google Scholar]
- 94.Joshi T, Ganesan LP, Cao X, Tridandapani S. Molecular analysis of expression and function of hFcγRIIbl and b2 isoforms in myeloid cells. Molecular Immunology. 2006;43(7):839–850. doi: 10.1016/j.molimm.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 95.Trotta R, Col JD, Yu J, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. Journal of Immunology. 2008;181(6):3784–3792. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]