Abstract
Whereas most epithelial tissues turn-over and regenerate after a traumatic lesion, this restorative ability is diminished in the sensory epithelia of the inner ear; it is absent in the cochlea and exists only in a limited capacity in the vestibular epithelium. The extent of regeneration in vestibular hair cells has been characterized for several mammalian species including guinea pig, rat, and chinchilla, but not yet in mouse. As the fundamental model species for investigating hereditary disease, the mouse can be studied using a wide variety of genetic and molecular tools. To design a mouse model for vestibular hair cell regeneration research, an aminoglycoside-induced method of complete hair cell elimination was developed in our lab and applied to the murine utricle. Loss of utricular hair cells was observed using scanning electron microscopy, and corroborated by a loss of fluorescent signal in utricles from transgenic mice with GFP-positive hair cells. Regenerative capability was characterized at several time points up to six months following insult. Using scanning electron microscopy, we observed that as early as two weeks after insult, a few immature hair cells, demonstrating the characteristic immature morphology indicative of regeneration, could be seen in the utricle. As time progressed, larger numbers of immature hair cells could be seen along with some mature cells resembling surface morphology of type II hair cells. By six months post-lesion, numerous regenerated hair cells were present in the utricle, however, neither their number nor their appearance was normal. A BrdU assay suggested that at least some of the regeneration of mouse vestibular hair cells involved mitosis. Our results demonstrate that the vestibular sensory epithelium in mice can spontaneously regenerate, elucidate the time course of this process, and identify involvement of mitosis in some cases. These data establish a road map of the murine vestibular regenerative process, which can be used for elucidating the molecular events that govern this process.
Keywords: Balance, Cochlea, Mouse, Hair cell, Regeneration, Vestibular, Gentamicin
1. Introduction
One of the hallmarks of epithelial sheets is cell turnover and renewal, but the efficiency of these processes varies among species and tissue types. In mammals, the hair cells in the epithelium of the inner ear are among the least efficient in their ability to turn over and regenerate. The hair cells in the organ of Corti are perhaps the only epithelial cells that completely lack the ability to be replaced, whereas in the vestibular organs replacement of hair cells is limited (Stone et al., 1998). Auditory and balance impairments caused by loss of hair cells will likely be alleviated if missing hair cells can be replaced by a regenerative treatment. One approach to designing therapies to enhance hair cell regeneration in the mammalian auditory epithelium is to mimic the spontaneous regeneration that occurs in the vestibular epithelium (Forge et al., 1993; Lopez et al., 1997).
The extent of in vivo spontaneous hair cell regeneration in the mammalian vestibular epithelium has been characterized in several species. In the guinea pig model, embryonic-like hair cells appeared several weeks after a moderate lesion caused by aminoglycosides (Forge et al., 1993). The spontaneous regeneration of hair cells could be enhanced by infusion of growth factors and found to be accompanied by functional improvement (Kopke et al., 2001). Spontaneous hair cell regeneration was also found in the chinchilla following hair cell loss induced by ototoxicity (Lopez et al., 1997). The severity of the lesion appeared to influence the ability of the tissue to regenerate. Following a severe vestibular lesion caused by unilateral application of streptomycin to perilymph in guinea pigs, new hair cells could not be detected (Meiteles and Raphael, 1994a). The extent of vestibular hair cell regeneration in mice is not well documented.
Mice serve as the model of choice for hereditary disease and other pathologies. Better understanding of parameters related to the spontaneous hair cell regeneration in the vestibular system in mice should promote novel designs for inducing regeneration in the cochlea. To prepare for studying the molecular mechanism that controls regeneration, it is first necessary to determine the extent of spontaneous hair cell regeneration, the timing for appearance and maturation of the new cells and the involvement of mitosis in this process.
The relative resistance of the peripheral vestibular organs to aminoglycosides complicates the production of experimental lesions. For instance, a recent model for ototoxic elimination of cochlear hair cells failed to induce vestibular hair cell loss (Oesterle et al., 2008). The difficulty in producing significant loss of vestibular hair cells with a systemic regimen of aminoglycosides necessitates use of a local application of the drugs at high concentration. Vestibular hair cell elimination could be accomplished by inoculating neomycin into the perilymph (Staecker et al., 2007).
We aimed to induce a complete loss of vestibular hair cells in the mouse utricle, in order to facilitate quantification as part of documenting the extent of hair cell regeneration. We injected gentamicin into the mouse posterior semicircular canal and observed a complete elimination of vestibular hair cells. We then determined that spontaneous hair cell regeneration in the utricle starts several weeks later, leading to substantial replacement of lost hair cells. Nevertheless, hair cell regeneration in the utricle remained incomplete.
2. Materials and methods
2.1. Animal groups
All animal experiments were approved by the University Committee on the Use and Care of Animals at the University of Michigan and were performed using accepted veterinary standards. We used two strains of mice, CBA/J and FVB/NJ. CBA/J male mice (n = 100) at 3–4 weeks of age were purchased from Jackson Laboratory. On arrival, mice were housed in the University of Michigan Unit for Lab Animal Medicine. The animals were 3–6 weeks of age when the ototoxic aminoglycoside gentamicin (or control solutions) were administered. We also used 11 FVB/NJ transgenic mice with a BAC GFP inserted into the α9 nicotinic acetylcholine receptor gene (Zuo et al., 1999). These mice, kindly provided by Jian Zuo (St. Jude, Memphis, TN), express GFP in all developing hair cells. The expression is sustained in cochlear outer hair cells in mature animals, and appears to also be present in vestibular hair cells. As such, these mice are valuable for establishing presence of hair cells based on GFP detection.
Animals were divided into four groups for histological assessment. The utricles of the first group were prepared as rhodamine phalloidin-stained whole-mounts (total number: n = 23). These animals were sacrificed at 4 (n = 2), 7 (n = 5), 14 (n = 5), 21 (n = 8) or 28 (n = 3) days after the gentamicin treatment. The second group consisted of the GFP mice. These animals were sacrificed at 1 (n = 3), 2 (n = 2), 3 (n = 3), or 4 weeks (n = 3) after the gentamicin treatment and their utricles prepared as whole-mounts for fluorescence analysis. The utricles of the third group (n = 42 mice) were processed for analysis using scanning electron microscopy (SEM). Animals were sacrificed at 1 (n = 7), 2 (n = 10), 3 (n = 9), 4 (n = 6), 8 (n = 4), 16 (n = 3) or 24 weeks (n = 3) after the gentamicin treatment. The fourth group was used for BrdU incorporation studies (n = 31 mice). These animals were sacrificed at 1 (n = 12), 2 (n = 11) or 3 weeks (n = 8) after the gentamicin treatment. These animals received BrdU from the time of the ototoxic insult until they were sacrificed. Four additional animals were injected with artificial perilymph (instead of gentamicin) and served as control for the SEM group. In all animals, gentamicin treatment was given to one ear, and the contralateral ear was used as a normal control.
2.2. Surgery
Following anesthesia, the left post-auricular region was shaved and cleaned. Under the operating microscope (Zeiss OM1), an incision was made behind the left pinna, the muscles were separated and the posterior semicircular canal (PSC) was exposed. A polyethylene tube (inner diameter 0.11″, outer diameter 0.61″, Becton Dickinson, Sparks, MD) was connected to a fine polyimide tube (inner diameter 0.0039″, outer diameter 0.0049″, Microlumen, Tampa, FL) and filled with gentamicin (40 mg/ml). The end of the polyethylene tube was connected by a 30 G needle to a 10 μl Hamilton syringe (Hamilton, Reno, NV) filled with sterile normal saline. Infusion speed was controlled by a syringe pump (Harvard Apparatus Inc., Holliston, MA) at the rate of 0.1 μl/min. Once the device was confirmed to function (normal flow with no leaks), a small hole was made on the PSC with a micro dissecting needle, and the tip of the polyimide tube was inserted into the PSC toward the crus commune. Before the gentamicin injection, the polyimide tube was sealed in the PSC with carboxylate cement (ESPE, Norristown, PA). The tightness of the seal was visually confirmed by lack of fluid leakage.
The animals were injected with gentamicin at the rate of 0.1 μl/min for 10 min., for a total of 1.0 μl (40 μg). To allow the gentamicin to spread throughout the inner ear and stabilize, the tube was left in place for three minutes after the injection. The tube was then removed and the hole was sealed immediately with carboxylate cement. The skin was closed with 4-0 Ethilon suture (Ethicon Inc., Somerville, NJ). The total surgery duration was approximately 30 min.
With this approach to the PSC, it is impossible to be certain whether the drug is administered into vestibular endolymph or perilymph. Since all animals produced a severe utricular lesion with complete (or nearly complete) elimination of hair cells, it appears that the lesion model is applicable regardless of which fluid space is directly infiltrated.
2.3. Phalloidin-stained whole-mounts
Animals were anesthetized and the temporal bones removed and fixed in 4% paraformaldehyde. The utricles were dissected out under the stereoscope, permeabilized in 0.3% Triton X-100 for 10 min, and then incubated with rhodamine phalloidin (Molecular Probes, Eugene, OR) 1:100 in PBS for 30 min. After thorough rinsing, the utricles were mounted on glass slides with Crystal Mount (Biomedia, Foster City, CA), examined with a Leica DMRB epi-fluorescence microscope (Leica, Eaton, PA) using 100X oil objectives and photographed using a SPOT-RT digital camera (Diagnostic Instruments Inc., Sterling Heights, MI) with SPOT-RT Software c3.3.
2.4. Whole-mount GFP analysis
Transgenic mice co-expressing a GFP transgene with endogenous α9 acetylcholine receptor (AchR) (Zuo et al., 1999) were used to validate the model for hair cell elimination and observe the long term outcome using an additional experimental approach. We anesthetized heterozygous or homozygous mice (four weeks old) and injected gentamicin through the PSC as described above. Mice were sacrificed 1, 2, 3 or 4 weeks after the gentamicin treatment, and temporal bones harvested and fixed in 4% paraformaldehyde for 30 min. After rinsing in PBS, the utricles and cochleae were dissected out and mounted on glass slides. Samples were analyzed and photographed under epi-fluorescence microscopy, visualizing the transgenic GFP, with no antibody augmentation of the signal.
2.5. SEM
Animals were anesthetized and perfused systemically with 2% glutaraldehyde (EMS, Ft. Washington, PA) in 0.15% cacodylate buffer, inner ears removed and utricles dissected out and stored in same fixative in 4 °C for 2 h. Utricles were then processed for SEM using the OTOTO method (Davies and Forge, 1987; Osborne and Comis, 1991). The samples were dehydrated in a graded series of ethanols, critical point dried and mounted on stubs using silver paste. Samples were analyzed and digitally recorded using a Philips XL30 field emission gun scanning electron microscope (FEI, Hillsboro, OR).
2.6. Hair cell counts
To estimate the number of the hair cells, hair bundles from photomicrographs of SEM were counted using a method based on that earlier described (Denman-Johnson and Forge, 1999) with a slight modification. Initially, each utricle was observed in low magnification and centered. The magnification was then raised to 3000X and the tissue photographed (center area). Four additional areas of the utricle, adjacent to the center area, were also photographed at the same magnification, and the number of the hair bundles was counted in these five distinct areas and summed (Fig. 4). The size of each picture was 43.45 μm × 33.10 μm and the total area of the five selected areas was 7191 μm2.
Fig. 4.
Mean numbers of two kinds of hair bundles (immature appearance and short bundle) in 1434.6 μm2 fields from the SEM at each post treatment time. Error bar indicates standard deviation.
2.7. Analysis of hair bundles
The hair bundles were scored based on their appearance in SEM. The scoring system included two categories: “immature cells” and “short bundle cells.” Immature cells displayed stereocilia that were of approximately equal height within a bundle (no gradation) and the kinocilium was of a length closely similar to that of the stereocilia and could only be discerned due to its thicker diameter. Short bundle cells displayed a kinocilium that was elongated and stereocilia with some degree of height gradation, but thinner than normal stereocilia. The number of cells in each of these categories was counted and plotted.
2.8. BrdU
We administered BrdU (Sigma, St. Louis, MO) continuously for 1–3 weeks by adding BrdU to the drinking water at a concentration of 2 mg/ml. BrdU administration was initiated immediately after the gentamicin treatment. The water bottle containing BrdU was shielded from light by aluminum foil wrapping. The BrdU–water solution was changed weekly. One, two or three weeks after the gentamicin treatment, animals were anesthetized; temporal bones removed and immersed in 4% paraformaldehyde for 2 h. Utricles were dissected out and processed for immunohistochemistry. Small intestine was obtained as a control and prepared as frozen sections. Tissues were blocked against non-specific binding of the secondary antibody by incubation in 5% normal goat serum for 30 min, and permeabilized with 0.3% Triton X-100 for 10 min followed by 0.3% H2O2 in water for 30 min to quench the endogenous peroxidase activity. After rinsing with PBS, the samples were reated with 2N HCl in PBS for 30 min at room temperature to denature the DNA. Then the specimens were immuno-reacted with a mouse monoclonal anti-BrdU antibody (Sigma, St. Louise, MO) diluted 1:50 in PBS for 60 min and then washed three times with PBS for 15 min. Tissues were then processed with a mouse ABC-peroxidase kit (Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine (DAB) as a chromogen. The specimens were washed with PBS, coverslipped with Gel/Mount (Biomeda, Foster City, CA, USA), and observed and photographed using a Leica DMRB microscope (Leica, Eaton, PA, USA) using ×10, ×40 and ×100 objective lenses. All of these procedures were performed at room temperature.
3. Results
After the surgical procedure, animals recovered with free access to water and food. There were no mortalities and all animals recovered. Gentamicin-treated animals showed severe vestibular dysfunction manifested by severe head tilt to the left (operated) side, circling counter-clockwise or severe twisting and longitudinal body-axis rotating motion. The rotating motion resolved within approximately two weeks after the treatment. Head tilt and circling persisted longer, but resolved by 16 weeks after the insult. These results suggest that the deficit in vestibular function induced by gentamicin partially resolved with time. However, the present data do not allow us to distinguish between central compensation versus functional recovery due to hair cell regeneration.
To assess hair cell morphology and density in utricles, we processed the tissues as whole-mounts which we stained with phalloidin to detect F-actin (Fig. 1). High concentration of F-actin in stereocilia, cuticular plate and adherens junctions provide helpful landmarks for assessing the region of the luminal surface, allowing for visualization of the presence of hair cells versus scars (Meiteles and Raphael, 1994b). Four days after the gentamicin administration, numerous sites of missing hair cells were seen throughout the utricle (Fig. 1a). Hair cells were replaced by scarring supporting cells, determined by the pattern of actin distribution in junctional areas, as previously shown (Meiteles and Raphael, 1994a). Due to the massive loss of hair cells, the exact location of the striola could not be determined.
Fig. 1.

Epi-fluorescence images of central utricular areas in whole-mounts stained with phalloidin. (a) Four days after the gentamicin treatment, numerous scars (arrow head) are seen in the entire area. A few remaining hair cells can be detected based on presence of cuticular plates and/or stereocilia (arrow). (b) Seven days after the gentamicin treatment, numerous scars (arrow head) were seen throughout the utricle. (c) Fourteen days after the treatment, scars are still abundant but their density is decreased. (d) Twenty one days after the treatment, hair cells are present based on stereocilia (arrow). (e) Twenty eight days after the treatment, a large number of hair bundles can be detected (arrow) and the height of those stereocilia is shorter than that of normal utricles (f). Scale bars (e) 25 μm (a–e), (f) 20 μm.
Seven days after treatment (Fig. 1b), the extent of hair cell loss was greater than that seen at the four days time point. Very few hair cells were observed in the sensory epithelium. The luminal surface of the epithelium consisted of scars generated by expanded supporting cells. Fourteen days after the treatment (Fig. 1c), cells with stereocilia or cuticular plate were mostly absent, but the tissue appeared reorganized such that the typical scarring pattern was less prominent. Twenty one days after the treatment (Fig. 1d), the number of scars decreased compared to that of at 14 days and some hair cells were present, based on presence of hair bundles. The bundles were invariably short, appearing no taller than the diameter of the cell. At 28 days (Fig. 1e), numerous hair bundles were detected, and their height was increased compared to that seen in the 14 day utricles. The stereocilia of the hair cells in post-lesion ears (Figs. 1 c–e) were shorter than those found in normal utricles (Fig. 1f). These data demonstrate that gentamicin induced a severe insult leading to loss of most or all utricular hair cells, and that spontaneous hair cell regeneration could lead to partial repopulation of the epithelium.
Fluorescence-conjugated phalloidin staining defines the cellular organization at the luminal surface. The presence of scars, along with the absence of stereocilia bundles is a strong indication for absence of hair cells, as seen in Fig. 1. To further assay for the absence of hair cells in the gentamicin-treated utricles, we used α9 AchR knock-in mice with GFP-positive vestibular and auditory hair cells (Fig. 2). In the auditory epithelium, the specificity of GFP was evident by positive outer hair cells and negative inner hair cells (Fig. 2a), as previously described (Zuo et al., 1999). In the normal mature vestibular epithelium, GFP was detected in hair cells throughout the utricle (Fig. 2b). One week after administration of gentamicin, the utricle was nearly devoid of GFP (Fig. 2c). Similarly, no GFP was detected two weeks after the ototoxic insult (Fig. 2d). At the 3 week time point, numerous GFP-positive cells were seen in the sensory epithelium (Fig. 2e). The density of GFP appeared to increase at the 4 week time point (Fig. 2f), but did not reach the density of GFP positive cells in normal utricles (compare Fig. 2f to 2b). These data confirm that hair cells are indeed absent following the gentamicin insult at all focal places of the utricle. The data also show that new hair cells appear several weeks after the insult.
Fig. 2.
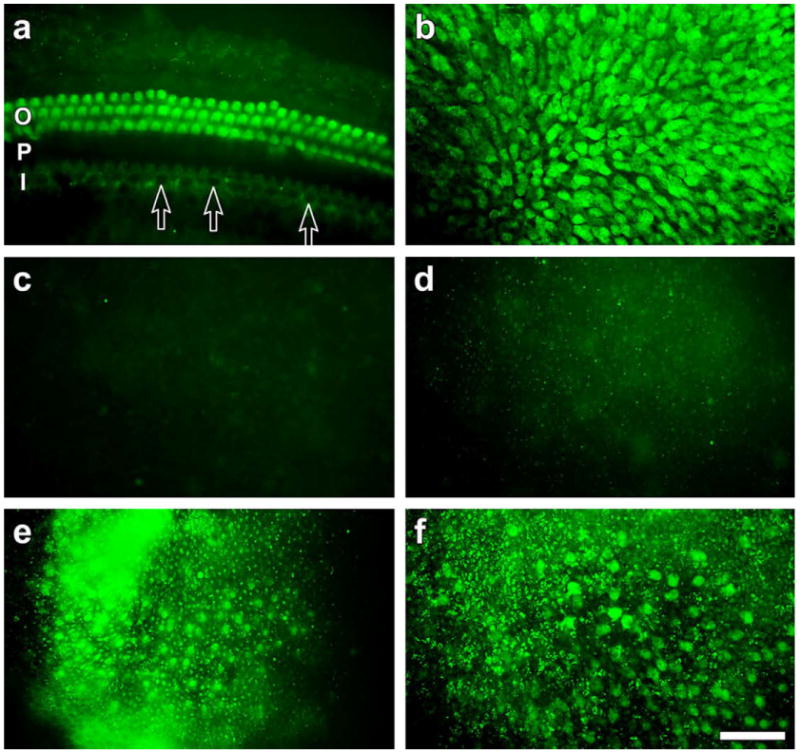
Whole-mounts of the cochlea (a) and utricles (b–f) viewed with epi-fluorescence. (a) GFP is seen in outer hair cells (O) but not in inner hair cells (arrows) of a normal cochlea (P, pillar cells; I, inner hair cells). (b) GFP is densely and evenly distributed in the utricular epithelium. (c and d) One and two weeks after the gentamicin treatment, no GFP signal is observed in the entire utricle (Fig. 2c and d, respectively). (e) Three weeks after the treatment, some GFP–positive cells are seen in the epithelium. The signal appears more diffuse than in the normal ear. (f) GFP observed four weeks after the treatment is better defined than at three weeks, but not as dense and well organized as in normal utricles. Scale bar in f: 25 μm (a–f).
SEM was used to observe the surface of the utricle at higher resolution. In animals injected with artificial perilymph as a vehicle control, no loss of hair cells could be observed, and the bundles appeared normally organized (Fig. 3a), similar to control utricles that received no treatment at all (Fig. 3b). One week after the gentamicin treatment, hair bundles could not be detected at the luminal surface of the utricle (Fig. 3c). At the 2 week time point, most animals did not have any bundles, but 2 out of 9 utricles contained a very small number of hair cell bundles (Fig. 3d). Three weeks after the treatment, a larger number of stereocilia bundles were identified (Fig. 3e). The stereocilia on most of these cells were short and their appearance was embryonic-like, as also seen at the 2 weeks time point. However, some cells had slightly taller stereocilia. These cells also contained a kinocilium. Four weeks after treatment (Fig. 3f) the density of hair cells was higher than that seen at 2 and 3 week time points. At longer time points, 8 and 16 weeks after gentamicin (Fig. 3 g–h, respectively), the morphology of hair cells was more mature, with many cells exhibiting a very tall kinocilium. However, the density of hair cells did not reach that seen in the normal utricle (compare Fig. 3g to 3b). These SEM images corroborate the finding using phalloidin staining and GFP detection, showing that a thorough depletion of vestibular hair cells can be found after gentamicin and that several weeks later the population of hair cells is replenished substantially but not completely.
Fig. 3.
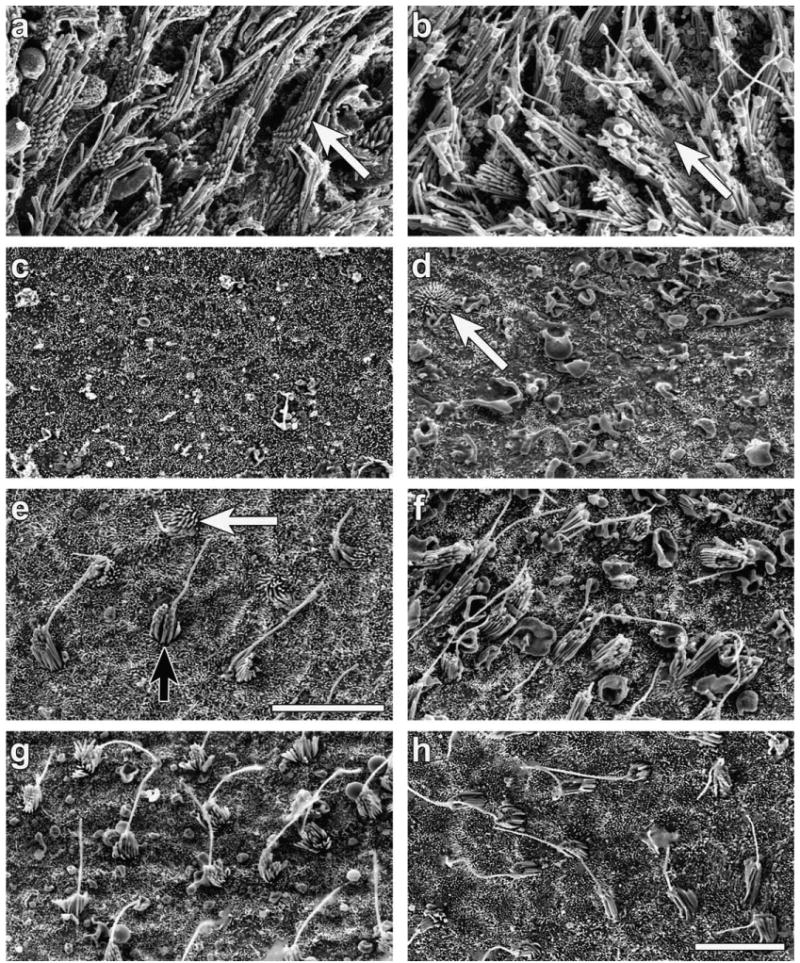
SEM images of the central area of mouse utricles showing control tissue treated with artificial perilymph (a), normal untreated utricle (b) and tissues obtained at several time points after the gentamicin treatment: one week (c), two weeks (d), three weeks (e) four weeks (f), eight weeks (g) or sixteen weeks (h). (a–b) A normal density and appearance of the utricular surface. (c) No stereocilia bundles are seen and the surface is covered with cells exhibiting short microvilli. (d) Most of the area is devoid of stereocilia bundles but one hair cell is seen, with round stereocilia bundles and embryonic-like appearance. (e) A few hair cells are seen with a tall kinocilium (black arrow), but other cells remain less differentiated (white arrow). (f) Hair cell bundles consist of taller stereocilia and a tall kinocilium. (g–h) Bundles density appear similar to those seen at earlier stages. Scale bars represent 10 μm in e (for a–e) and in h (for f–h).
Using SEM images as seen in Fig. 3, we counted the cells that had embryonic-like appearance and either no kinocilium or a kinocilium with height similar to stereocilia, and the cells that displayed a short bundle with a clear kinocilium. Cells were counted in an area at the center of the utricle in all 42 ears processed for SEM (Fig. 4). Results show that up to two weeks after the gentamicin treatment, immature cells were mostly devoid of an elongated kinocilium, whereas starting at three weeks after gentamicin, the number of cells with more mature bundles and a kinocilium was higher than that of immature cells. The number of cells with mature-like bundles increased gradually until the sixteen weeks time point. At twenty four weeks, the number of short bundle cells decreased, but a small number of immature cells were observed at all time points. Cells with very tall stereocilia, resembling surface morphology of type I hair cells, were not observed in the sensory epithelium of the regenerating utricle.
High magnification SEM analysis was used to characterize the surface morphology stereocilia bundles (Fig. 5). At three weeks, most of the hair bundles observed had a short kinocilium positioned at the center of the apical surface, and the stereocilia occupied the entire surface, with the tallest stereocilia being close to the center (Fig. 5a). In some cells, the kinocilium was longer and its position was closer to the periphery of the bundle (Fig. 5b). In these cells, the bundles did not occupy the entire apical surface of the cell. Links that connect stereocilia to each other were present as early as three weeks after the insult (Fig. 5c). Immature cells sometimes appeared in pairs, where adjacent cells were of similar size and morphological features (Fig. 5d). In other cases, adjacent cells had a different degree of maturation (Fig. 5e). At later time points, kinocilia tended to be positioned at the periphery and were much taller than the stereocilia (Fig. 5f–h). The stereocilia bundles were centrally located, surrounded by a peripheral apical surface that was devoid of projections (microvilli or stereocilia) (Fig. 5h). In some of the cells, stereocilia exhibited a height gradient (Fig. 5h) and were connected to their neighbors with tip links (Fig. 5i).
Fig. 5.

SEM images of stereocilia bundles at different time points after gentamicin. a–c, e, h and i are three weeks after gentamicin treatment, whereas (d) and (f) are four weeks after treatment. (a) A bundle with short stereocilia covering the entire apical surface, and a centrally positioned kinocilium. (b) Longer kinocilium positioned closer to the pole of the apical surface, with the latter being devoid of microvilli. (c) High magnification of (b), showing inter-stereocilia links. (d) Pairs of adjacent hair cells with similar surface morphology. (e) Two separated hair bundles observed on the surface of one cell. (f) A hair cell with a tall kinocilium positioned at the pole and an immature stereocilia bundle that appears loosely organized. (g) A cell with closely packed stereocilia lacking gradation in height, and a tall kinocilium. (h) A cell with normally packed stereocilia showing a height gradient and a tall kinocilium positioned at the pole of the bundle. (i) High magnification of the cell in (h), showing stereocilia connecting to each other with links. Scale bars, (a) 1 μm, (b and e–h) 2 μm, (c and i) 200 nm, (d) 5 μm.
BrdU was used to trace new DNA in cells of utricles at one (n = 12), two (n = 11) or three (n = 8) weeks after the gentamicin treatment. BrdU-treated animals did not exhibit weight loss (data now shown). Contralateral utricles and small intestine tissues were used as negative and positive controls, respectively. In gentamicin-treated utricles, only 3 out of 31 utricles showed BrdU positive nuclei around the central region of the utricle (Fig. 6a). Those three samples were part of the one week recovery group (n = 1) and the two week group (n = 2). Some of the BrdU positive nuclei appeared in pairs. Contralateral utricles (no ototoxic lesion) and the remaining 28 of the 31 utricles treated with gentamicin were BrdU negative (Fig. 6b). The small intestines samples from all BrdU-treated animals contained BrdU-positive nuclei (Fig. 6d).
Fig. 6.

Whole-mount of utricles (a–c) and frozen section of the small intestine (d) labeled with BrdU-specific antibody and viewed by light microscopy. (a) Two weeks after the gentamicin, BrdU-positive nuclei are seen in the sensory epithelium of the utricle. Most positive cells are not paired, but some are (arrow). (b) One week after the gentamicin, BrdU positive nuclei appear as a pair at a focal plane beneath the luminal surface. (c) Two weeks after the gentamicin treatment, no BrdU positive nuclei are detected in the hair cell layer. (d) BrdU-positive cells are present in the mucous cells of the gut. Scale bars, (c) 20 μm (for a–c), (d) 50 μm.
4. Discussion
4.1. Summary
Our data demonstrate that a single application of gentamicin into the semicircular canal leads to a severe to complete depletion of hair cells from mouse utricles. Within two weeks hair cells start to reappear in the epithelium and their density and degree of differentiation are enhanced during the following weeks. The morphology of the new hair cells does not return to the pre-lesion condition even after 16 weeks.
4.2. Model for hair cell loss
Models for hair cell elimination are useful for studies on protection, repair and regeneration in inner ear epithelia. While not necessarily mimicking the majority of clinical situations, these models facilitate a baseline in which no hair cells are present, thereby sharpening the evaluation of spontaneous regeneration and any methods whereby it may be enhanced. The mouse model is particularly valuable because of the genetic tools available for research in these animals. However, mice appear more resistant than many other mammals to aminoglycoside ototoxicity, and previous attempts to eliminate vestibular hair cells with systemic drug application have failed. As such, the method we present relies on gentamicin applied to the semicircular canal. Although the effort involved in surgical administration of the drug precludes use of this method for high throughput studies, the method here presented reliably yields complete or nearly complete hair cell loss. This provides a baseline in which no hair cells are present, facilitating evaluation of methods that enhance the regeneration. An alternative approach for elimination of vestibular hair cells using neomycin injection into perilymph was also successful (Staecker et al., 2007).
The scars generated by supporting cells in sites of hair cell loss appear similar to those described in guinea pig ears (Meiteles and Raphael, 1994a). The correlation between presence of scars at the luminal surface and absence of hair cells is supported by the absence of GFP signal in mice with endogenous GFP-positive vestibular hair cells, and further corroborated by SEM analysis. Supporting cells are therefore the most likely source for generating the new hair cells observed as early as two weeks post-insult. The repair of damaged hair cells, shown to occur in developing hair cell systems (Sobkowicz et al., 1996; Sobkowicz et al., 1997), appears not to be involved in hair cell regeneration in the mature murine vestibular epithelium following a complete elimination of hair cells. However, the extent to which damaged hair cells could be repaired in cases where injured hair cells remain in the vestibular epithelium merits investigation.
The functional correlates of the lesion and regeneration in these studies are not easy to interpret, because the lesion was unilateral and as such, central compensation and adaptation have occurred. Future studies with bilateral lesion as well as the use of electrophysiological measurements such as VOR may be able to evaluate the relationship between the observed structural repair and vestibular function. It will also be necessary to study the fate of primary vestibular neurons and the innervation of the new hair cells. Studies of the neurons are especially important considering that in an aging gerbil model the neurons and/or synapses have been shown to degenerate even without the loss of vestibular hair cells (Kevetter et al., 2005; Leonard and Kevetter, 2007).
4.3. Differentiation of the new hair cells
New hair cells differentiate in a morphological sequence that resembles the development of normal hair cells in several specific features. Similar to developing hair cells (Denman-Johnson and Forge, 1999; Mbiene et al., 1984), the kinocilium in regenerating hair cells is more likely to be in the center of the apical surface early on and move to one of the poles later. Also, as cells proceed through maturation, stereocilia become graded in height and the perimeter of the bundles becomes devoid of microvilli.
However, there are some conspicuous differences between development and regeneration. First, it is not clear why some of the regenerated cells do not mature and why the density of hair cells remains lower than in mature normal utricles. Variations in the severity of the lesion may affect a supporting cell's generative capacity, even up to the point of diminishing it outright. The severity of the lesion may have diminished the ability of supporting cells to generate new hair cells. Interestingly, an extremely severe lesion in the avian basilar papilla also impairs the potential for full regeneration (Cotanche et al., 1995). Another difference is the duration of each process; regeneration spanning weeks, compared to the few days needed for development (Sher, 1971). The relative slowness of regeneration has also been noted with vestibular hair cells in guinea pigs (Forge et al., 1993) and during induced hair cell regeneration using forced expression of Atoh1 in the vestibular epithelium (Staecker et al., 2007) and the organ of Corti (Izumikawa et al., 2005). In birds, the duration of hair cell regeneration in the basilar papilla after an aminoglycoside lesion is also relatively long (Stone and Rubel, 2000), but following a partial lesion due to noise exposure, hair cells regenerate much faster and hearing returns quickly (Niemiec et al., 1994; Saunders et al., 1992). It is possible therefore that the severity of the damage influences the speed at which supporting cells can recover and produce new hair cells. Therefore, it would be important to analyze the extent of injury sustained by supporting cells in these trauma paradigms and correlate them with the pace and quality of regeneration.
Incomplete regeneration appears to be common to the mouse and guinea pig vestibular models. It is not clear why the density of regenerated hair cells does not reach normal conditions. Reduction in the number of supporting cells could be one reason for the incomplete repopulation of the epithelium, although it is unclear why remaining supporting cells do not continue to divide, as do their counterparts in the basilar papilla. In attempts to enhance regeneration in clinical settings, it may be necessary to combine mitogenic signals with signals for hair cell differentiation.
4.4. Mechanism of regeneration
In epithelia that renew and regenerate, basal cells or stem cells usually exist, and therefore the contribution of such cells to regeneration should be considered. In the avian basilar papilla, no stem cells or basal cells have been found, and therefore the regenerative process in the bird ear depends entirely on transdifferentiation of supporting cells. While some stem cells have been shown to reside in mature mouse utricles (Li et al., 2003), their number is small and probably insufficient for participating in hair cell regeneration in a substantial way. The paucity of BrdU positive cells observed in our study further contributes to the notion that stem cells are not involved in hair cell regeneration. As such, it is unclear what role these stem cells play and whether they can contribute to slow turnover that may occur in the vestibular epithelium, similar to that seen in birds (Oesterle et al., 1997), yet unable to yield large scale regeneration after a massive trauma.
The process of supporting cell transdifferentiation is rather uncommon yet appears to be conserved during the evolution of inner ear epithelia. Transdifferentiation has been shown in the inner ears of fish (Wilkins et al., 1999), amphibians (Steyger et al., 1997), birds (Stone et al., 1998) and mammals (Forge et al., 1993; Lopez et al., 1997), and probably also accounts for the regeneration that occurs following forced expression of Atoh1 (Izumikawa et al., 2005; Kawamoto et al., 2003; Staecker et al., 2007). The molecular basis of the phenotypic change from supporting cell to hair cell is not well understood, but changes in notch signaling are likely to account at least in part to this process. It is also unclear why mitosis occurs in some cases of regeneration in the mammalian vestibular epithelium (Lopez et al., 1998) and not others. Studies in vitro suggest that overcoming supporting cell quiescence would initiate proliferative hair cell regeneration in mature mammalian vestibular epithelium (Meyers and Corwin, 2007). While our in vivo work shows substantial regeneration without proliferation, it is likely that enhancing mitosis would lead to a more complete regenerative process. Studies in birds, where some supporting cells divide and then undergo transdifferentiation whereas others transdifferentiate without division (Adler and Raphael, 1996; Roberson et al., 1996) may help elucidate the molecular signals that control the choice between the two options.
Only a small portion of the animals in our study showed BrdU positive cells in their utricles, and the number of positive cells in each of those animals was small. While the cause for this variability within the animals in the study is not clear, one possibility is that the severity of the pathology induced in the supporting cells also varied, and influenced the extent of transdifferentiation. The severity of changes in remaining supporting cells could have been related to the concentration of gentamicin they were exposed to, which in turn could be a function of whether the injection resulted in perilymphatic versus endolymphatic inoculation.
4.5. Correlating surface morphology with cytoarchitecture
The ability to understand and quantify tissue via surface examination is a powerful and efficient research tool, but the relationship between surface morphology and overall structure needs to first be worked out. This relationship is not necessarily straightforward and in the traumatized inner ear epithelium it may be complex and dynamic. Supporting cells in the normal vestibular epithelium are coupled to each other with an extensive array of gap junctions. In areas of hair cell loss, scars generated by supporting cells appear neatly organized on the apical surface, but sections fail to depict clear boundaries between supporting cells and the actual shape of each cell is not presently clear (Meiteles and Raphael, 1994a). There is a possibility that supporting cells are organized in a syncytium, or a dynamic state of cell–cell interaction with changing borders. This could explain our finding that some cells appear to contain more than one stereocilia bundle, with differences in the morphology between the bundles. It is unclear whether these cells are fused supporting cells or cells that received mixed/confused positional signals, leading to mis-guided differentiation.
Fixation of vestibular epithelia for SEM analysis with traditional fixatives is not optimal as diffusion of the fixative into the tissue is inefficient. The sub-optimal procedure leads to the appearance of swollen “blobs” in the surface, in both controls tissues and experimental specimens. The presence of intact bundles and tip-links in both control and regenerated hair cells validates the data, but better fixation methods will need to be explored.
4.6. BrdU data
BrdU was administered to mice in our study over long periods of time. The advantage of this long duration is that data are more likely to include a larger proportion of new DNA in the tissue. However, BrdU is toxic to the animals and long duration of exposure may influence their general health and may indirectly impair their ability to regenerate the vestibular epithelium. Once it is determined when the critical period of s-phase takes place, it will be possible to use shorter BrdU exposure times to evaluate the extent of mitosis in healthier animals.
The presence of BrdU in cells of the gut, used here as positive control, demonstrates that sufficient levels of BrdU were circulating in the plasma of these animals. While there is a possibility that brush border cells lining the lumen of the gut take up BrdU directly, the presence of BrdU in deeper cells of the crypts, where cells receive nutrients from the blood supply, indicates that BrdU was available to cells throughout the body. The presence of BrdU in the ear supports this notion.
4.7. Cell types and innervation
Many weeks after the vestibular insult, a large number of regenerated hair cells remain morphologically immature, and therefore it is difficult to determine if these cells are type I or type II vestibular hair cells. Based on surface morphology, cells appear to be type II vestibular hair cells, but further studies using ultrastructural analysis of sections and electrophysiology are necessary for characterizing these cells. It is also likely that the new cells are not completely differentiated into any of the distinctive mature morphologies. Detailed physiology at the single cell level may be of help in determining the functional status of these immature cells, as shown in developing vestibular hair cells (Rusch et al., 1998). While hair cell regeneration in the avian vestibular periphery yields both type I and type II hair cells, type II cells tend to appear first, followed later by the appearance of type I cells (Stone et al., 1998). Observations made in chinchillas (Tanyeri et al., 1995) were similar to data presented here, namely, that new cells are either immature or type II. Further physiological studies may be able to address the functional impact of absence of type I cells.
5. Conclusions
We have shown that a severe or complete depletion of utricular hair cells can be induced by gentamicin in the mature mouse. Transgenic animals expressing GFP in their vestibular hair cells were helpful in assessing hair cell loss. New hair cells are generated over the time course of 24 weeks following the insult. BrdU-positive cells were detected among these new cells suggesting that at least some arise from divisions within the epithelium. Several months after the lesion, the density of hair cells appears lower than in normal utricles and the surface morphology of the new hair cells remains immature. The availability of powerful analytical tools for studies in mice, combined with the model we present for hair cell elimination, can help elucidate the mechanism of hair cell regeneration, which can be used to augment regeneration in the vestibular epithelium and induce regeneration in the auditory epithelium.
Acknowledgments
We thank Jian Zuo for the GFP knock-in mice, Anna Lysakowski for helpful comments and Donald Swiderski for assistance. Work supported by the A. Alfred Taubman Medical Research Institute, the Berte and Alan Hirschfield Foundation, the R. Jamison and Betty Williams Professorship, a Research Grant from Kansai Medical University, and NIH/NIDCD Grants R01-DC01634, R01-DC05401, R01-DC03685 and P30-DC05188.
References
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Messana EP, Ofsie MS. Migration of hyaline cells into the chick basilar papilla during severe noise damage. Hear Res. 1995;91:148–159. doi: 10.1016/0378-5955(95)00185-9. [DOI] [PubMed] [Google Scholar]
- Davies S, Forge A. Preparation of the mammalian organ of Corti for scanning electron microscopy. J Microsc. 1987;147:89–101. doi: 10.1111/j.1365-2818.1987.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Denman-Johnson K, Forge A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol. 1999;28:821–835. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Zimmerman CL, Leonard RB. Hair cell numbers do not decrease in the crista ampullaris of geriatric gerbils. J Neurosci Res. 2005;80:279–285. doi: 10.1002/jnr.20451. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Jackson RL, Li G, Rasmussen MD, Hoffer ME, Frenz DA, Costello M, Schultheiss P, Van De Water TR. Growth factor treatment enhances vestibular hair cell renewal and results in improved vestibular function. Proc Natl Acad Sci USA. 2001;98:5886–5891. doi: 10.1073/pnas.101120898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard RB, Kevetter GA. Structural and functional changes in the cristae ampullares of aged gerbils. Neuroscience. 2007;147:794–802. doi: 10.1016/j.neuroscience.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Lee SC, Schoeman G, Beykirch K. Quantification of the process of hair cell loss and recovery in the chinchilla crista ampullaris after gentamicin treatment. Int J Dev Neurosci. 1997;15:447–461. doi: 10.1016/s0736-5748(96)00103-7. [DOI] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Lee SC, Li G, Beykirch K. Hair cell recovery in the chinchilla crista ampullaris after gentamicin treatment: a quantitative approach. Otolaryngol Head Neck. 1998;119:255–262. doi: 10.1016/S0194-5998(98)70060-9. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Favre D, Sans A. The pattern of ciliary development in fetal mouse vestibular receptors. A qualitative and quantitative SEM study. Anat Embryol. 1984;170:229–238. doi: 10.1007/BF00318726. [DOI] [PubMed] [Google Scholar]
- Meiteles LZ, Raphael Y. Scar formation in the vestibular sensory epithelium after aminoglycoside toxicity. Hear Res. 1994a;79:26–38. doi: 10.1016/0378-5955(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Meiteles LZ, Raphael Y. Distribution of cytokeratins in the vestibular epithelium of the guinea pig. Ann Oto Rhinol Laryn. 1994b;103:149–155. doi: 10.1177/000348949410300212. [DOI] [PubMed] [Google Scholar]
- Meyers JR, Corwin JT. Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci. 2007;27:4313–4325. doi: 10.1523/JNEUROSCI.5023-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemiec AJ, Raphael Y, Moody DB. Return of auditory function following structural regeneration after acoustic trauma: behavioral measures from quail. Hear Res. 1994;79:1–16. doi: 10.1016/0378-5955(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Rubel EW. Induction of cell proliferation in avian inner ear sensory epithelia by insulin-like growth factor-I and insulin. J Comp Neurol. 1997;380:262–274. doi: 10.1002/(sici)1096-9861(19970407)380:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MP, Comis SD. Preparation of inner ear sensory hair bundles for high resolution scanning electron microscopy. Scanning Microsc. 1991;5:555–564. [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct trans differentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit Neurosci. 1996;2:195–205. [Google Scholar]
- Rusch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci. 1998;18:7487–7501. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Adler HJ, Pugliano FA. The structural and functional aspects of hair cell regeneration in the chick as a result of exposure to intense sound. Exp Neurol. 1992;115:13–17. doi: 10.1016/0014-4886(92)90213-a. [DOI] [PubMed] [Google Scholar]
- Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Oto-Laryngol. 1971;285(Suppl):1–77. [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Post-traumatic survival and recovery of the auditory sensory cells in culture. Acta Oto-Laryngol (Stockh) 1996;116:257–262. doi: 10.3109/00016489609137836. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Cellular interactions as a response to injury in the organ of corti in culture. Int J Dev Neurosci. 1997;15:463–485. doi: 10.1016/s0736-5748(96)00104-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Burton M, Hawkins JR, Schuff NR, Baird RA. Calbindin and parvalbumin are early markers of non-mitotically regenerating hair cells in the bullfrog vestibular otolith organs. Int J Dev Neurosci. 1997;15:417–432. doi: 10.1016/s0736-5748(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Temporal, spatial, and morphologic features of hair cell regeneration in the avian basilar papilla. J Comp Neurol. 2000;417:1–16. doi: 10.1002/(sici)1096-9861(20000131)417:1<1::aid-cne1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Stone JS, Oesterle EC, Rubel EW. Recent insights into regeneration of auditory and vestibular hair cells. Curr Opin Neurol. 1998;11:17–24. doi: 10.1097/00019052-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Tanyeri H, Lopez I, Honrubia V. Histological evidence for hair cell regeneration after ototoxic cell destruction with local application of gentamicin in the chinchilla crista ampullaris. Hear Res. 1995;89:194–202. doi: 10.1016/0378-5955(95)00137-7. [DOI] [PubMed] [Google Scholar]
- Wilkins HR, Presson JC, Popper AN. Proliferation of vertebrate inner ear supporting cells. J Neurobiol. 1999;39:527–535. doi: 10.1002/(sici)1097-4695(19990615)39:4<527::aid-neu6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Zuo J, Treadaway J, Buckner TW, Fritzsch B. Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci USA. 1999;96:14100–14105. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]



