Abstract
BACKGROUND
Although there are several large-species animal models for saccular aneurysms, there is a need for a simple, reproducible saccular aneurysm model in mice.
OBJECTIVE
To develop a murine saccular aneurysm model, which replicates key characteristics that occur in the formation of human cerebral aneurysms.
METHODS
Elastase is applied extravascularly to the right common carotid artery. We induced saccular aneurysm formation by our method in C57BL/6 mice (n = 30). Aneurysms and control arteries (left common carotid arteries) were harvested at 1 week, 2 weeks, and 3 weeks postinjury (n = 10 for each time point), measured, and stained for elastin content. To demonstrate BMP-derived cell recruitment to the aneurysms, bone marrow from UBC-gfp transgenic mice was transplanted into irradiated C57BL/6 recipients to create C57BL/6.gfp chimeras. Additionally, bone marrow from DsRed transgenic mice was transplanted into irradiated C57BL/6 recipients to create C57BL/6.DsRed chimeras, and bone marrow from B5/EGFP transgenic mice was transplanted into irradiated FVB recipients to create FVB.gfp chimeras. The elastase injury or sham operations were performed in the C57BL/6.gfp, C57BL/6.DsRed, and FVB.gfp chimeras. Aneurysms and sham vessels were harvested at 3 weeks and examined for BMP-derived cell recruitment. Additionally, aneurysms were stained for matrix metalloproteinase-9, which is overexpressed in human cerebral aneurysm tissue.
RESULTS
Aneurysms consistently demonstrated significant loss of elastin in the vessel wall and had significantly larger diameters than control vessels (591 ± 238 µm vs 328 ± 61µm; P = .003 for aneurysms 3 weeks postinjury). Aneurysms from C57BL/6.gfp, FVB.gfp, and C57BL/6.DsRed chimeras consistently revealed significant BMP-derived cell recruitment in the aneurysm wall that was not seen in sham-operated vessels nor in control left common carotid arteries. Aneurysms demonstrated overexpression of matrix metalloproteinase-9.
CONCLUSION
We describe a novel murine elastase saccular aneurysm model that replicates the histopathology and BMP-derived cell–mediated processes that will be a valuable instrument for studying the cell-mediated processes in cerebral aneurysm formation.
Keywords: Aneurysm, Animal model, Bone marrow, Matrix metalloproteinase, Murine, Progenitor cell
The process by which cerebral saccular aneurysms form is currently unknown and controversial. It is thought that aneurysms possibly occur as a result of hemodynamic or other process-induced injury to the endothelium.1,2 Aneurysms are characterized by a degeneration of the medial layer of the vessel wall that affects tensile structural integrity. However, the pathophysiologic process by which the vessel wall medial layer is degraded and aneurysm formation occurs is not known. There is evidence for an intense inflammatory response with macrophages, T cells, B cells, and complement activation found in human cerebral aneurysm tissue.3–6
Bone marrow progenitor (BMP)-derived cells play a significant role in other models of vascular disease, such as intimal hyperplasia.7–10 Bone marrow-derived cells have also been found in human abdominal aortic aneurysms.11 Consequently, an animal aneurysm model that enables the study of BMP-derived cell–mediated processes during the formation of cerebral aneurysms would be valuable.
A number of animal aneurysm models have been described.12–23 These aneurysm models are in larger animal species such as dogs, pigs, or rabbits because the vessel sizes are well suited for catheters and devices for the study of endovascular therapies. Murine models have been less favored because of their small size; however, they could be valuable instruments for studying biological processes because of the availability of transgenic species.
Other murine saccular aneurysm models have not demonstrated significant recruitment of BMP-derived cells to the aneurysm,24 probably because they are microsurgical anastomosis models that do not replicate the damage to the internal elastic lamina that occurs in human cerebral aneurysms.25We describe a novel murine saccular aneurysm model suitable for studying BMP-derived cell–mediated processes in aneurysm formation.
Our murine saccular aneurysm model replicates key features of human cerebral aneurysms, including: disruption of the internal elastic lamina, enlargement of the aneurysm over time, and an inflammatory cell–mediated vascular response. Additionally, a key feature found in human cerebral aneurysms is the overexpression of matrix metalloproteinase-9 (MMP-9),26–28 particularly in human cerebral aneurysms that have ruptured.28 MMP-9 belongs to a family of zinc-dependent endopeptidases that are responsible for the degradation of extracellular matrix proteins, and since it is found in cerebral aneurysms, it is thought to be a key protease responsible for the degradation of the elastic lamina and medial layer of the vessel wall leading to aneurysm formation. Our murine saccular aneurysm model demonstrates overexpression of MMP-9.
MATERIALS AND METHODS
All animal experimentation was performed in accordance with a protocol approved by our institution’s Institutional Animal Care and Use Committee.
Description of Model
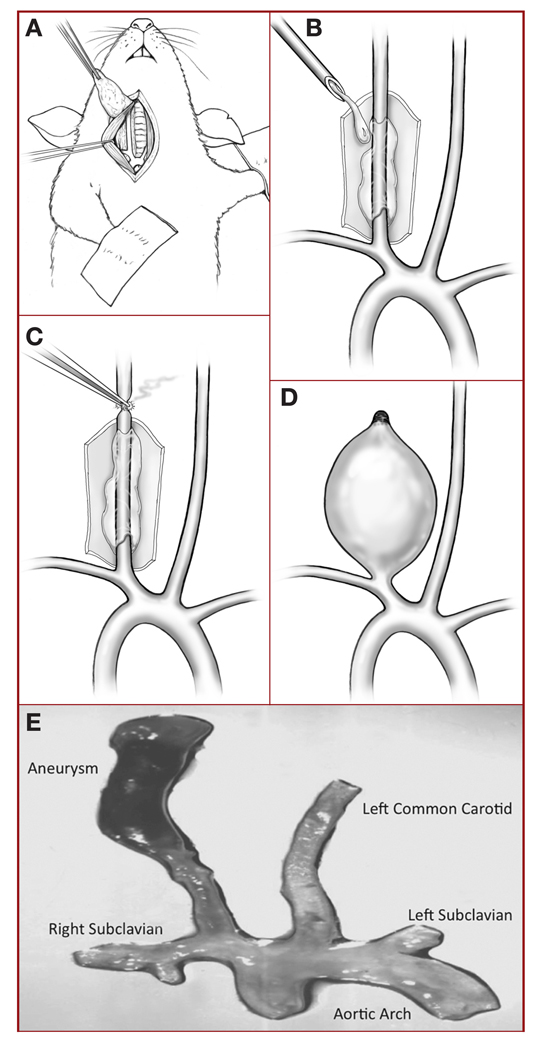
Mice were anesthetized with tribromoethanol working solution (4.5 mg) intraperitoneally. Microsurgical exposure of the right common carotid artery (RCCA) was performed with an operating microscope and adapted from a previously described method29 (Figure 1A). The RCCA was exposed as far proximally and distally as possible (usually about 1–2 cm), and a latex cuff was placed around the vessel. The RCCA was then bathed with porcine pancreatic elastase solution (Worthington Biochemical Corp, Lakewood, NJ), 10 U diluted in 1 mL of 1× phosphate-buffered solution (Invitrogen, Carlsbad, CA) (Figure 1B) for 20 minutes, and the vessel was occluded distally (Figure 1C). A saccular aneurysm formed over the next 3 weeks (Figure 1, D and E). Sham-operated animals underwent microsurgical exposure of the RCCA, which was then bathed with phosphate-buffered saline rather than elastase solution, and no distal occlusion of the artery.
FIGURE 1.
A, drawing illustrating microsurgical exposure of the murine right common carotid artery (RCCA). B, drawing showing latex cuff placed around the RCCA, which is then bathed with porcine pancreatic elastase solution for 20 minutes. C, drawing showing distal occlusion of the RCCA. D, drawing showing a saccular aneurysm that has formed during the 3 weeks postinjury. E, photograph of a typical murine aneurysm (original magnification, ×1).
Creation of Chimeras
Bone marrow was harvested from the femurs and tibias of killed UBC-gfp, B5/EGFP, and DsRed mice. All cell types in the gfp strain express green fluorescent protein, and all cell types in the DsRed strain express red fluorescent protein. The total bone marrow cell populations (5 × 106) were injected into the retro-orbital sinuses of C57BL/6 mice and FVB mice that had been depleted of bone marrow by lethal irradiation with 950 rads to create chimeras designated as C57BL/6.gfp, FVB.gfp, and C57BL/6.DsRed. Whole bone marrow engraftment was confirmed 3 months after transplantation by flow cytometry analysis of peripheral blood mononuclear cells for cell surface markers CD3, B220, and CD11b.
Validation of the Aneurysm Model
The elastase model was performed in C57BL/6 mice (n = 30), and aneurysms and control vessels (left common carotid artery) were harvested at 1, 2, and 3 weeks (n = 10 for each time point) postinjury. Vessels were fixed with 4% paraformaldehyde for 24 hours at 4°C, rinsed with phosphate-buffered saline, and then transferred to 18% sucrose for 24 hours at 4°C. They were then embedded in a cassette with optical cutting temperature compound (OCT; Sakura/Tissue-Tek Co, Torrance, CA) and frozen with dry ice and 2-methylbutane. Specimens were stored at −80°C before they were sectioned by cryostat into 5-µm sections.
The specimens were stained with hematoxylin and eosin and with an elastic stain kit (Richard-Allen Scien tific, Kalamazoo, MI) in which Weigert’s iodine solution stained for elastin, and counterstained with Van Geison stain solution. Diameters of the aneurysms and control vessels were measured under microscopy using Image-Pro Plus software (MediaCybernetics, Silver Spring, MD). Statistical analysis was performed by Student’s t test with P < .05 considered significant.
Demonstration of BMP-Derived Cell Recruitment
Elastase saccular aneurysms were created in C57BL/6.gfp, FVB.gfp, and C57BL/6.DsRed radiation chimeras. Sham operations were performed as controls. Aneurysms and sham-operated vessels were harvested at 3 weeks post-elastase and examined with confocal fluorescent microscopy for evidence of incorporation of bone marrow-derived cells into the wall of the aneurysm(green fluorescent cells in the C57BL/6.gfp, B5/EGFP.gfp, and FVB.gfp chimeras, and red fluorescent cells in the C57BL/6.DsRed chimeras).
Demonstration of MMP-9 Expression
Immunohistochemistry was performed on day 1, day 3, week 1, week 2, and week 3 on 5-µm cross sections from 4% paraformaldehyde- fixed, OCT-embedded aneurysms and control arteries using fluorescent-linked goat anti–MMP-9 (1:150; R&D Systems, Minneapolis, MN). Sections were treated with acetone at −20°C for 5 minutes and air dried before being stained. OCT was removed from the slides with 1× wash solution (Dako, Carpinteria, CA), and then antigen retrieval was performed as needed. Slides were blocked in 3% horse serum for 20 minutes before the application of primary antibody overnight at 4°C. Sections were mounted in VectaShield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Inc, Burlingame, CA) before imaging. Positive control tissues and concentration-matched immunoglobulin controls were included with each immunoassay.
RESULTS
Enlarged saccular aneurysms were consistently produced in the model, with progressive aneurysm growth from week 1 to week 3 post-elastase injury. Diameters of aneurysms versus control vessels were (mean ± standard deviation): 431 ± 120 µm versus 343 ± 55 µm (P = .05); 537 ± 139 µm versus 356 ± 50 µm (P = .001); and 591 ± 238 µm versus 328 ± 61 µm (P = .003) for weeks 1, 2, and 3 postinjury, respectively.
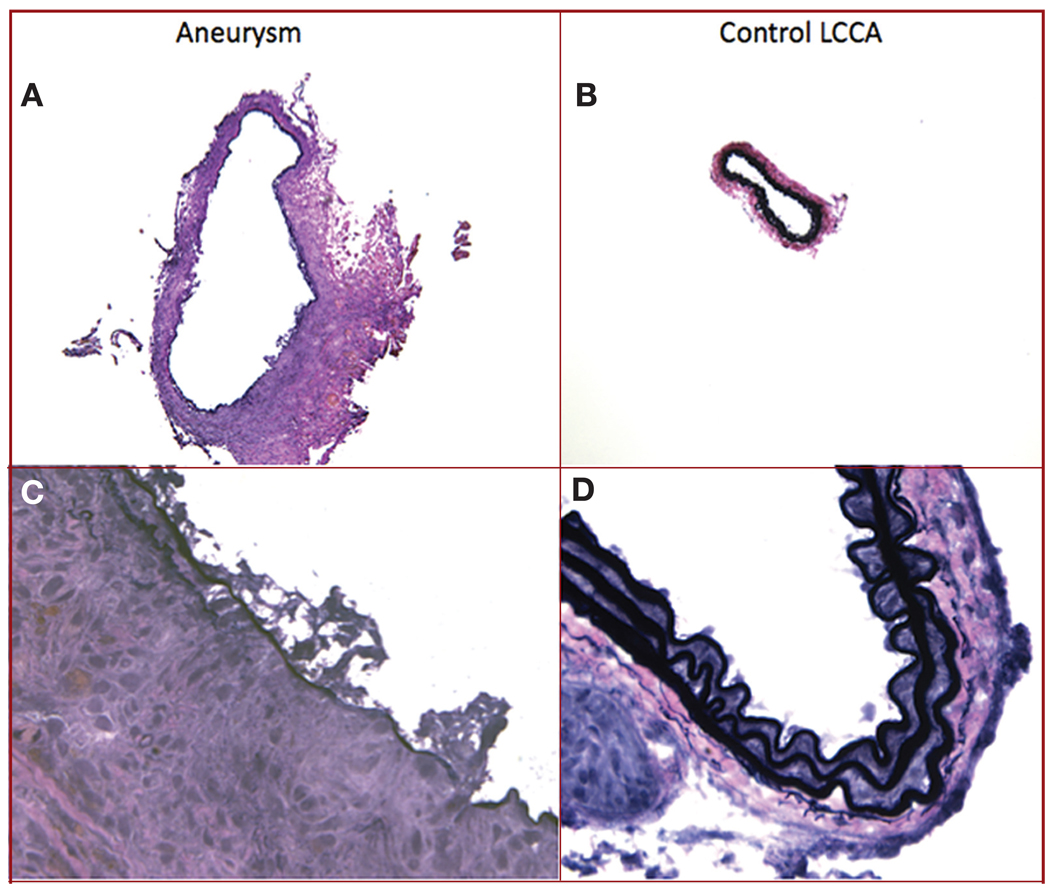
A characteristic feature of human cerebral aneurysms is the disruption of the internal elastic lamina in the aneurysm wall. Aneurysms in this model consistently demonstrated loss of the internal elastic lamina in the aneurysm wall, whereas control vessels demonstrated intact internal elastic lamina (Figure 2).
FIGURE 2.
Elastin staining of cross sections of aneurysm (A and C) and control left common carotid artery (LCCA) (B and D), demonstrating that the aneurysm has a larger cross sectional maximal diameter and has degeneration of the internal elastic lamina in the vessel wall compared with the control LCCA (original magnification, ×10 [A and B] and ×60 [C and D]). An elastic stain kit (Richard-Allen Scientific, Kalamazoo, MI) was used in which the internal elastic lamina stains black and can be seen intact in the LCCA as wavy bands (D).
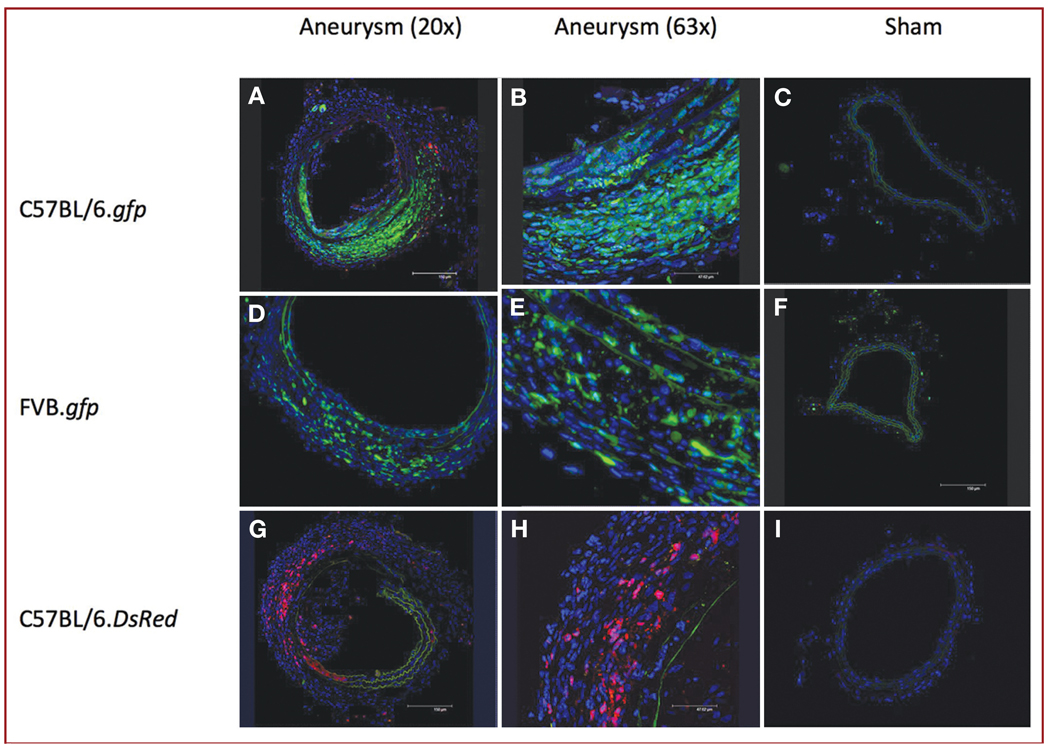
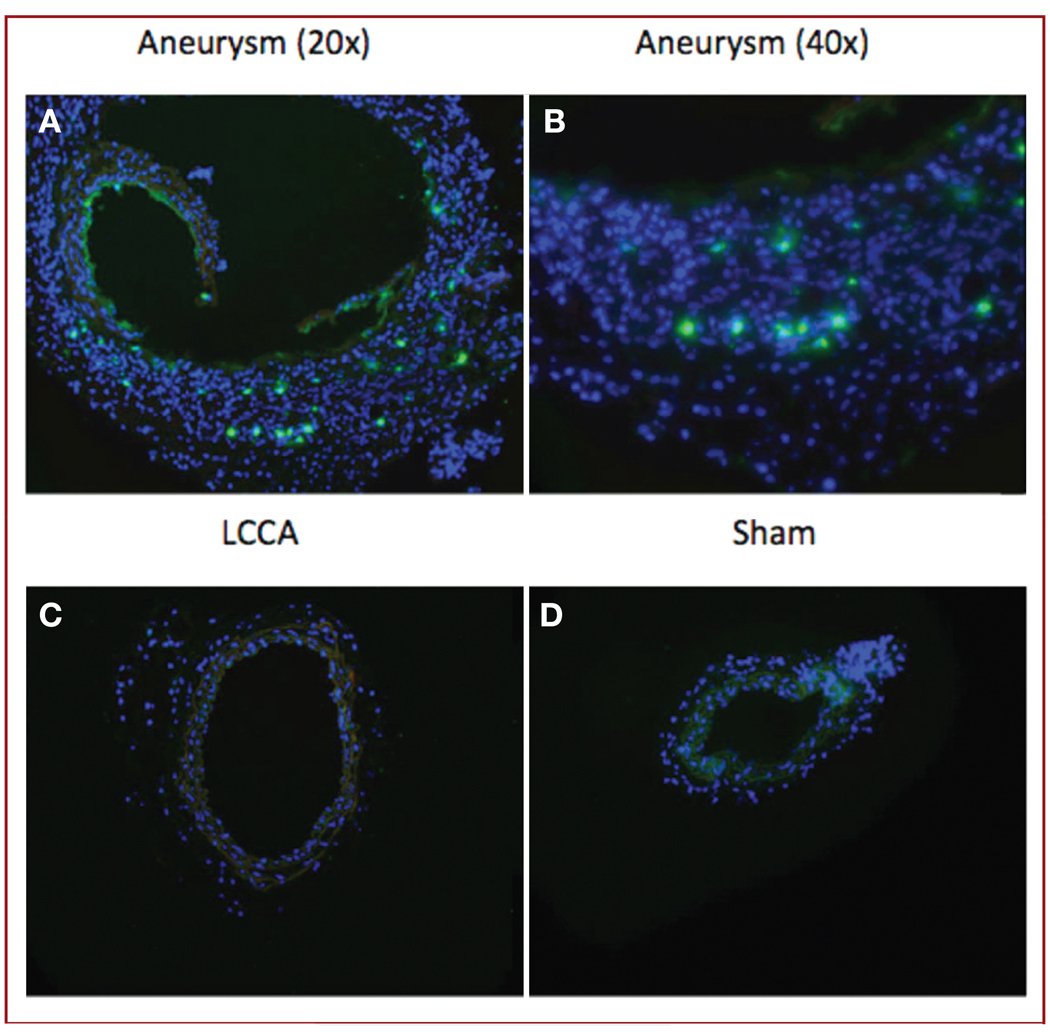
Aneurysms from C57BL/6.gfp, FVB.gfp, and C57BL/6.DsRed chimeras all consistently demonstrated BMP-derived cell recruitment response at the aneurysm site, whereas sham-operated vessels demonstrated no BMP-derived cells (Figure 3). The murine aneurysms replicated the MMP-9 overexpression seen in human cerebral aneurysms. Immunohistochemistry demonstrated MMP-9 expression in our aneurysm model at day 1, day 3, week 1, week 2, and week 3 after injury (Figure 4).
FIGURE 3.
Immunohistochemical staining showing abundant bone marrow–derived cells in the walls of aneurysms (original magnification, ×20 [A, D, and G] and ×63 [B, E, and H]), but not in sham-operated RCCAs (original magnification, ×20 [C, F, and I]) of C57BL/6.gfp (A–C), FVB.gfp (D–F), and C57BL/6.DsRed (G–I) radiation chimeric mice. Blue, 4′,6-diamidino-2-phenylindole (DAPI); green, gfp cells; red, DsRed cells.
FIGURE 4.
Immunohistochemical staining for matrix metalloproteinase-9 (MMP-9) expression, showing detection of MMP-9 at week 2 in cross sections of aneurysms (original magnification, ×20 [A] and ×40 [B]), but not in control LCCAs (original magnification, ×20 [C]) or sham-operated RCCAs (original magnification, ×20 [D]). Fluorescent green, positive staining for MMP-9; blue, DAPI (original magnification, ×20).
DISCUSSION
The pathophysiology of cerebral aneurysm formation is not well understood and is controversial. Cerebral aneurysms are thought to form after an initial endothelial disruption, perhaps induced by hemodynamic or other factors.1,2 Human cerebral aneurysm specimens demonstrate evidence for an intense inflammatory response with macrophages, T cells, B cells, and complement activation.3–6 BMP-derived cells acting as mediators of a repair response to vascular injury have been demonstrated in animal models of intimal hyperplasia.8,9,30 BMP-derived cells (CD34-positive and vascular endothelial growth factor–positive) have also been found in tissue sections of human abdominal aortic aneurysms11 but, to our knowledge, have not been studied in human cerebral aneurysms. We postulate that BMP-derived cell–mediated processes occur in cerebral aneurysm formation. A valid animal aneurysm model is a critical element for studying this problem.
Although a number of animal models have been described, most are in larger species such as dogs, pigs, or rabbits.12–23 These animal models are well suited for the study of surgical and endovascular treatments because their vessel sizes can accommodate catheters and endovascular devices. However, large species are of limited use for studying the mechanisms involved in recruitment of bone marrow–derived cells to the aneurysm site because of the lack of transgenic lines. The lack of transgenic species in large animals is a result of their genetic heterogeneity.
The development of a murine aneurysm model is valuable because of the availability of transgenic murine lines. In addition, new transgenic murine species can be created for future investigations. A murine aneurysm model previously described by Morimoto et al31 consisted of ligation of the left common carotid artery and bilateral renal arteries and placement of the mice on a high-salt diet, which produced aneurysmal changes at the right anterior cerebral artery–olfactory artery bifurcations. However, in their report, the aneurysms were not consistently produced and tended to be microscopic in scale and thus would be difficult to use for demonstration of BMP-derived cell recruitment.
In another murine aneurysm model, described by Frösen et al,24 a pouch graft from syngeneic thoracic aorta was microsurgically anastomosed end-to-side to the abdominal aorta. This model did not demonstrate significant BMP-derived cell recruitment at the aneurysm, possibly because microsurgical anastomosis models induce scarring at the suture line. Additionally, this model did not demonstrate disruption of the internal elastic lamina, a key feature of cerebral aneurysms.25
Our elastase model consistently produced saccular aneurysms and demonstrated progressive aneurysm growth over a 3-week period. Histologically, the aneurysms resembled human cerebral aneurysms with loss of the internal elastic lamina in the aneurysm wall. Significant BMP-derived cell recruitment was consistently demonstrated across several murine strains and with multiple different bone marrow transplant chimeras. MMP-9 expression, a key feature of human cerebral aneurysms, was demonstrated in the murine aneurysms.
Elastase has been used in other species to produce saccular aneurysms.22,23,32–36 However, in those models, the elastase was administered intraluminally in the artery. We show that the extravascular technique still induced aneurysm formation. We found this extravascular technique to be simpler, easier to perform, and more reproducible.
There are several limitations to this model. First, elastase is not known to occur in the process of naturally occurring cerebral aneurysms in humans. In this model, elastase was administered to lead to aneurysm formation. This limitation is a general type of criticism that applies to all animal model research. Previously described animal aneurysm models involved suturing vein or artery patch grafts to vessels, applying elastase, or ligating the carotid artery and renal arteries, none of which occur with naturally occurring human brain aneurysms.12–24,31 The application of elastase is necessary to the aneurysm model because it leads to the degeneration of elastin in the aneurysm wall, a histologically critical characteristic of human cerebral aneurysms, which is not replicated in other animal aneurysm models, such as those that involve suturing an artery or vein graft to create an aneurysmal pouch.
A second limitation of this model is that the aneurysm was created in the extracranial carotid artery and thus was not an intracranial aneurysm. This limitation was attributable to the size constraints of working with murine species. Nearly all previously described animal aneurysm models involved extracranial arteries.12–24 One previously described aneurysm model created aneurysms that were intracranial31; however, these aneurysms were microscopic in scale and not large enough to study pathophysiologic processes at the cellular level in the aneurysm walls. As the purpose of this model was intended to study cell-mediated processes, intracranial aneurysms would be too small.
CONCLUSIONS
We describe a novel murine saccular aneurysm model that produced consistent saccular aneurysms, demonstrated loss of internal elastic lamina, consistently demonstrated BMP-derived cell recruitment across several murine strains and multiple bone marrow transplant chimeras, and demonstrated MMP-9 expression. This will be a valuable instrument for the future study of cerebral aneurysm pathophysiology.
Acknowledgments
This research was funded by the American Association of Neurological Surgeons Neurosurgery Research and Education Foundation Young Clinician Investigator Award. Brian L. Hoh, MD, has previously served as a consultant for Micrus Endovascular, Codman Neurovascular, and Actelion Pharmaceuticals. Edward W. Scott, PhD, is chief scientific officer for RegenMed, Inc.
ABBREVIATIONS
- BMP
bone marrow progenitor
- MMP-9
matrix metalloproteinase-9
- RCCA
right common carotid artery
Footnotes
Disclosures
The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENTS
The investigators describe a new animal model of arterial aneurysms, namely extracranial common carotid aneurysms in mice induced by the application of elastase to the adventitial side of the arterial wall. As shown in the diagrams accompanying the article, the aneurysm is arguably a fusiform aneurysm, since it is a circumferential dilation of the common carotid artery proximal to its ligation. A case is made that this model is analogous enough to human intracranial aneurysms (the histopathology is similar) that useful investigation of cell-mediated processes can be undertaken (such as recruitment of bone marrow progenitor cells to aneurysmal tissue, for example) and the results can be extrapolated to our understanding of the pathogenesis of aneurysms in man, which is currently unknown. This is interesting work, and we will see where it takes this group of investigators in the future.
J. Max Findlay
Edmonton, Canada
The authors continue the quest for a better brain aneurysm model. This is a very elegant and innovative idea to use mice and generate various chimeras. The benefits of this model are that the mouse is used, and quite definitive experiments can be conducted, such as those here, showing that bone marrow progenitor cells populate the aneurysms. The questions are whether systemic artery aneurysms are similar enough to those in the brain that the pathogenetic and treatment-related information can be translated between the two. Also, elastase was used to create the aneurysms, and whether this is involved in human intracranial aneurysms has not been investigated yet.
I suspect that the model is too far removed from the human situation to permit useful conclusions about human brain aneurysms to be derived, but the authors may be able to answer some interesting questions about vascular disease and are encouraged to pursue work on the model.
R. Loch Macdonald
Toronto, Canada
Contributor Information
Brian L. Hoh, Department of Neurological Surgery, University of Florida, Gainesville, Florida.
Gregory J. Velat, Department of Neurological Surgery, University of Florida, Gainesville, Florida.
Erin N. Wilmer, Department of Neurological Surgery, University of Florida, Gainesville, Florida.
Koji Hosaka, Department of Neurological Surgery, University of Florida, Gainesville, Florida.
Robert C. Fisher, Program in Stem Cell Biology, Department of Molecular Genetics and Microbiology, University of Florida, Gainesville, Florida.
Edward W. Scott, Program in Stem Cell Biology, Department of Molecular Genetics and Microbiology, University of Florida, Gainesville, Florida.
REFERENCES
- 1.Kim C, Cervos-Navarro J, Kikuchi H, Hashimoto N, Hazama F. Alterations in cerebral vessels in experimental animals and their possible relationship to the development of aneurysms. Surg Neurol. 1992;38(5):331–337. doi: 10.1016/0090-3019(92)90017-h. [DOI] [PubMed] [Google Scholar]
- 2.Nagata I, Handa H, Hasimoto N, Hazama F. Experimentally induced cerebral aneurysms in rats: VII. Scanning electron microscope study. Surg Neurol. 1981;16(4):291–296. doi: 10.1016/0090-3019(81)90063-x. [DOI] [PubMed] [Google Scholar]
- 3.Tulamo R, Frösen J, Junnikkala S, et al. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery. 2006;59(5):1069–1077. doi: 10.1227/01.NEU.0000245598.84698.26. [DOI] [PubMed] [Google Scholar]
- 4.Frösen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35(10):2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30(7):1396–1401. doi: 10.1161/01.str.30.7.1396. [DOI] [PubMed] [Google Scholar]
- 6.Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45(5):1137–1147. doi: 10.1097/00006123-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93(8):783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 8.Shoji M, Sata M, Fukuda D, et al. Temporal and spatial characterization of cellular constituents during neointimal hyperplasia after vascular injury: potential contribution of bone-marrow-derived progenitors to arterial remodeling. Cardiovasc Pathol. 2004;13(6):306–312. doi: 10.1016/j.carpath.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22(10):1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 10.Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93(10):980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Matsubara J, Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Expression of angiogenesis and angiogenic factors in human aortic vascular disease. J Surg Res. 2002;106(2):239–245. doi: 10.1006/jsre.2002.6468. [DOI] [PubMed] [Google Scholar]
- 12.German WJ, Black PW. Experimental production of carotid aneurysms. N Engl J Med. 1954;250(3):104–106. doi: 10.1056/NEJM195401212500303. [DOI] [PubMed] [Google Scholar]
- 13.Forrest MD, O’Reilly GV. Production of experimental aneurysms at a surgically created arterial bifurcation. AJNR Am J Neuroradiol. 1989;10(2):400–402. [PMC free article] [PubMed] [Google Scholar]
- 14.Graves VB, Ahuja A, Strother CM, Rappe AH. Canine model of terminal arterial aneurysm. AJNR Am J Neuroradiol. 1993;14(4):801–803. [PMC free article] [PubMed] [Google Scholar]
- 15.Guglielmi G, Ji C, Massoud TF, et al. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology. 1994;36(7):547–550. doi: 10.1007/BF00593518. [DOI] [PubMed] [Google Scholar]
- 16.Massoud TF, Ji C, Guglielmi G, Viñuela F, Robert J. Experimental models of bifurcation and terminal aneurysms: construction techniques in swine. AJNR Am J Neuroradiol. 1994;15(5):938–944. [PMC free article] [PubMed] [Google Scholar]
- 17.Cawley CM, Dawson RC, Shengelaia G, Bonner G, Barrow DL, Colohan AR. Arterial saccular aneurysm model in the rabbit. AJNR Am J Neuroradiol. 1996;17(9):1761–1766. [PMC free article] [PubMed] [Google Scholar]
- 18.Spetzger U, Reul J, Weis J, Bertalanffy H, Thron A, Gilsbach JM. Microsurgically produced bifurcation aneurysms in a rabbit model for endovascular coil embolization. J Neurosurg. 1996;85(3):488–495. doi: 10.3171/jns.1996.85.3.0488. [DOI] [PubMed] [Google Scholar]
- 19.Bavinzski G, Al-Shameri A, Killer M, et al. Experimental bifurcation aneurysms: a model for in vivo evaluation of endovascular techniques. Minim Invasive Neurosurg. 1998;41(3):129–132. doi: 10.1055/s-2008-1052027. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald RL, Mojtahedi S, Johns L, Kowalczuk A. Randomized comparison of Guglielmi detachable coils and cellulose acetate polymer for treatment of aneurysms in dogs. Stroke. 1998;29(2):478–486. doi: 10.1161/01.str.29.2.478. [DOI] [PubMed] [Google Scholar]
- 21.Miskolczi L, Guterman LR, Flaherty JD, Hopkins LN. Saccular aneurysm induced by elastase digestion of the arterial wall: a new animal model. Neurosurgery. 1998;43(3):595–601. doi: 10.1097/00006123-199809000-00110. [DOI] [PubMed] [Google Scholar]
- 22.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. AJR Am J Roentgenol. 2000;174(2):349–354. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 23.Hoh BL, Rabinov JD, Pryor JC, Ogilvy CS. A modified technique for using elastase to create saccular aneurysms in animals that histologically and hemodynamically resemble aneurysms in human. Acta Neurochir (Wien) 2004;146(7):705–711. doi: 10.1007/s00701-004-0276-6. [DOI] [PubMed] [Google Scholar]
- 24.Frösen J, Marjamaa J, Myllärniemi M, et al. Contribution of mural and bone marrow-derived neointimal cells to thrombus organization and wall remodeling in a microsurgical murine saccular aneurysm model. Neurosurgery. 2006;58(5):936–944. doi: 10.1227/01.NEU.0000210260.55124.A4. [DOI] [PubMed] [Google Scholar]
- 25.Abruzzo T, Shengelaia GG, Dawson RC, 3rd, Owens DS, Cawley CM, Gravanis MB. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. AJNR Am J Neuroradiol. 1998;19(7):1309–1314. [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SC, Singh M, Huang J, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41(3):642–647. doi: 10.1097/00006123-199709000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Caird J, Napoli C, Taggart C, Farrell M, Bouchier-Hayes D. Matrix metalloproteinases 2 and 9 in human atherosclerotic and non-atherosclerotic cerebral aneurysms. Eur J Neurol. 2006;13(10):1098–1105. doi: 10.1111/j.1468-1331.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 28.Jin D, Sheng J, Yang X, Gao B. Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surg Neurol. 2007;68 Suppl 2:S11–S16. doi: 10.1016/j.surneu.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 29.Connolly ES, Jr, Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery. 1996;38(3):523–532. doi: 10.1097/00006123-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Takamiya M, Okigaki M, Jin D, et al. Granulocyte colony-stimulating factormobilized circulating c-Kit+/Flk-1+ progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2006;26(4):751–757. doi: 10.1161/01.ATV.0000205607.98538.9a. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, Hashimoto N. Mouse model of cerebral aneurysm: experimental induction by renal hypertension and local hemodynamic changes. Stroke. 2002;33(7):1911–1915. doi: 10.1161/01.str.0000021000.19637.3d. [DOI] [PubMed] [Google Scholar]
- 32.Ding YH, Danielson MA, Kadirvel R, et al. Modified technique to create morphologically reproducible elastase-induced aneurysms in rabbits. Neuroradiology. 2006;48(8):528–532. doi: 10.1007/s00234-006-0093-0. [DOI] [PubMed] [Google Scholar]
- 33.Cloft HJ, Altes TA, Marz WF, et al. Endovascular creation of an in vivo bifurcation aneurysm in rabbits. Radiology. 1999;213(1):223–228. doi: 10.1148/radiology.213.1.r99oc15223. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara NH, Cloft HJ, Marx WF, Short JG, Jensen ME, Kallmes DF. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR Am J Neuroradiol. 2001;22(4):698–703. [PMC free article] [PubMed] [Google Scholar]
- 35.Short JG, Fujiwara NH, Marx WF, Helm GA, Cloft HJ, Kallmes DF. Elastase-induced saccular aneurysms in rabbits: comparison of geometric features with those of human aneurysms. AJNR Am J Neuroradiol. 2001;22(10):1833–1837. [PMC free article] [PubMed] [Google Scholar]
- 36.Kallmes DF, Fujiwara NH, Berr SS, Helm GA, Cloft HJ. Elastase-induced saccular aneurysms in rabbits: a dose-escalation study. AJNR Am J Neuroradiol. 2002;23(2):295–298. [PMC free article] [PubMed] [Google Scholar]