Abstract
Gastrointestinal (GI) neuroendocrine tumors (NETs), such as carcinoids, are rare neoplasms characterized by the production of bioactive markers like 5-hydroxytryptamine (5-HT) and chromogranin A (CgA). Besides surgery, there are limited curative and palliative treatments available. Therefore, a great need exists for the development of new pharmacologic strategies to reduce tumor burden and control symptoms in patients with metastatic carcinoid tumors and the carcinoid syndrome. In the present review, we explore several pathways thought to be involved in GI NET carcinogenesis and offer insight into the novel approaches currently in development to target these pathways.
Keywords: Carcinoid, neuroendocrine, ZM336372, histone deacetylase (HDAC) inhibitors, glycogen synthase kinase-3β (GSK3β), phosphatidylinositol-3 kinase (PI3K)-Akt signaling pathway, Lithium
Introduction
Gastrointestinal (GI) neuroendocrine tumors (NETs) comprise a heterogeneous group of carcinomas that may be divided into two main categories: carcinoids and pancreatic endocrine tumors (PETs). GI carcinoid tumors are composed of cells with APUD (amine precursor uptake and decarboxylation) cytochemical characteristics that are thought to arise from the enterochromaffin cells throughout the gut. Conversely, PETs arise from pancreatic islet cells and are classified as either functional or nonfunctional depending upon whether the tumor secretes bioactive hormones. Functional PETs are further stratified into insulinomas, glucagonomas, gastrinomas, vasoactive intestinal peptide (VIP)-secreting tumors (VIPomas), somatostatinomas, and pancreatic polypeptide (PP)-producing tumors (PPomas) based upon the dominant hormone secreted by the tumor. Due to their relatively higher incidence compared to PETs (2.5:100,000 vs. 0.2:100,000), the majority of research on GI NETs has focused on carcinoid tumors [1].
Compared to most GI adenocarcinomas, GI NETs display a relatively slower growth pattern and more indolent disease course. However, they are the second most common cause of isolated hepatic metastases after colorectal carcinoma [2]. Patients with widespread metastases often suffer from incapacitating symptoms, including diarrhea, flushing, wheezing, and skin rashes, that result from the production of excess biogenic amines, neuropeptides, and hormones. Surgical resection is the only potentially curative treatment for GI NETs. However, a large number of patients present with unresectable disease [2]. Therapeutic options for these patients with widespread disease include somatostatin analogs, chemotherapy, ablative procedures, chemoembolization, and liver transplantation. While these treatments may alleviate symptoms or even prolong survival, they do not offer patients a cure [3–6]. Therefore, novel therapies are needed to treat and palliate patents with advanced GI NETs.
The growth, differentiation, phenotype, and hormonal expression of GI NETs depend upon a network of cellular signaling cascades. At this time, several pathways and individual molecules have been implicated in the tumorigenesis of GI NETs. However, a comprehensive review of all of these topics is beyond the scope of this paper. Here we will focus on potential new pathways of interest in GI NETs including alterations in raf-1/MEK/ERK pathway [7–9], Notch1 [10–11], glycogen synthase kinase-3β (GSK-3β) [12], and phosphatidylinositol-3 kinase (PI3K)-Akt [S Pitt, M Kunnimalaiyaan, H Chen, unpublished data] signaling. In this focused review, we will elucidate the areas of these pathways that may provide targets for the development of novel drug therapies in patients with GI NETs.
Molecular Pathogenesis
The molecular genetic mechanism of development and progression of GI NETs, specifically carcinoid tumors, is presently under investigation. Commonly identified genes involved in tumorigenesis include the multiple endocrine neoplasia type 1 (MEN1) gene, chromosomal 18q defects, and p53 alterations. Interestingly, with the application of current molecular techniques, researchers have begun to elucidate novel critical genetic pathways involved in the development of specific NE phenotypes.
Overexpression of the Notch1 intracellular domain in GI NETs
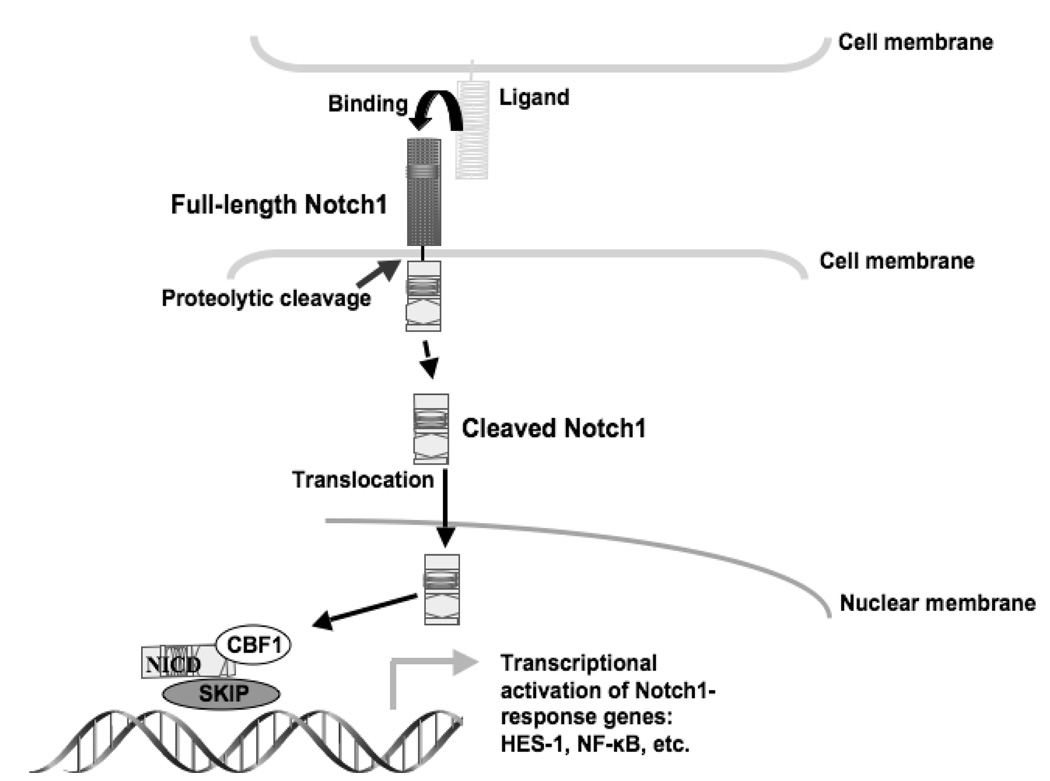
Notch1 is a multifunctional transmembrane receptor that regulates cellular differentiation, proliferation, and survival [13–15]. Binding of any one of the Notch ligands (Delta1 (DLL-1) or Jagged1 (JAG-1), for example) promotes a sequence of proteolytic cleavages resulting in the activated Notch1 intracellular domain (NICD). The active NICD then translocates to the nucleus and binds with the DNA-binding protein complex CSL (CBF1, Su (H), and LAG-1), resulting in the transcriptional activation of target genes such as hairy enhancer of split-1 (HES-1) [17] (figure 1). In human cancer cells, Notch1 has a dual role as either a tumor suppressor or an oncogene. In many types of cancer, including pancreatic, colon, non-small cell lung, cervical, renal cell, and several lymphomas, Notch1 is upregulated. Research suggests that expression of Notch1 signaling prevents cellular differentiation and inhibits apoptosis in these cancers. Conversely, Notch1 signaling is very minimal or absent in prostate cancer and NETs such as small-cell lung cancer (SCLC), pancreatic carcinoid, and medullary thyroid cancer (MTC) [17]. These apparent but paradoxical functions clearly indicate that the role of Notch signaling is dependent on its cellular context.
Figure 1. The Notch1 signaling pathway.
Notch1 is a transmembrane receptor that is activated by proteolytic cleavage following ligand binding. The Notch1 intracellular domain (NICD) translocates into the nucleus where it forms a complex with CBF1 and other proteins to activate gene transcription.
Activation of Notch1 signaling is an attractive target for the development of therapeutic and palliative strategies for carcinoids and other GI NETs. We have previously shown that activation of the Notch1 signaling pathway reduces both neuroendocrine (NE) markers and cellular proliferation in GI carcinoid tumor cells [10, 18]. More recently, we have shown the histone deacetylase (HDAC) inhibitors, valproic acid (VPA) and suberoyl bis-hydroxamic acid (SBHA), to be strong Notch1 activators in NETs [11, 19]. HDAC inhibitors represent a class of diverse molecules that modulate gene transcription by increasing histone acetylation; the resulting alteration in chromatin structure is believed to possess antineoplastic effects in preclinical and clinical studies in neuroblastoma cells and a variety of other cancers [20–23]. In our studies, SBHA and VPA treatment of human GI carcinoid tumor cells resulted in dose-dependent inhibition of cancer cell growth in vitro [11, 19]. Furthermore, treatment with these HDAC inhibitors suppressed expression of the NET markers achaete-scute complex like-1 (ASCL1) and chromogranin A (CgA) in vitro. The critical role of Notch1 activation was confirmed in a mouse tumor xenograft experiment where VPA treatment inhibited carcinoid tumor growth and suppressed ASCL1 in vivo [19]. Currently, we are seeking to develop phase II clinical trials at our institution to determine the efficacy of these HDAC inhibitors as part of a comprehensive therapy in patients with metastatic GI NETs.
Role of the Raf-1/mitogen-regulated extracellular kinase (MEK)/extracellular regulated kinase (ERK) pathway in GI NETs
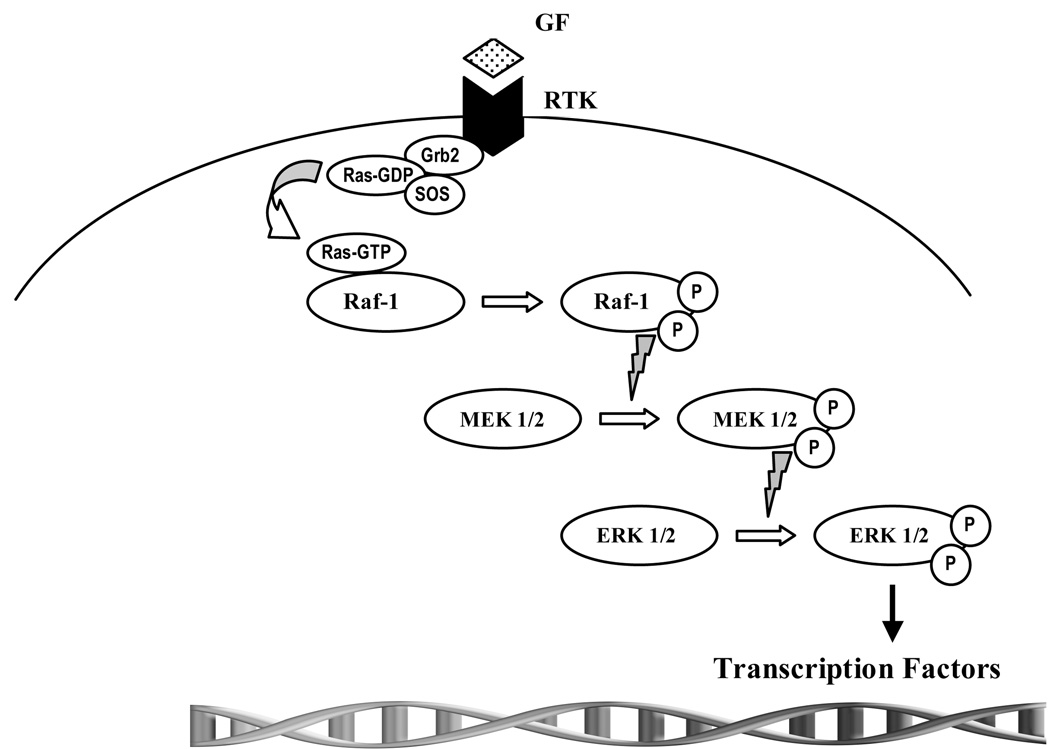
The raf-1/MEK/ERK pathway has long been recognized for its role in cancer biology. In this pathway, ras signaling is thought to involve activation of Raf-1, a cytosolic serine/threonine kinase. Once activated, Raf-1 phosphorylates MEK1/2, which then activates the downstream effector ERK1/2 (figure 2). While activation of this signaling pathway is commonly considered to be growth promoting in several cancers, in certain cell-specific subtypes, Raf-1 activation results in growth suppression [24]; specifically, activation of the Raf-1 signaling pathway in SCLC, MTC, and carcinoid cancer has been shown to result in phenotypic change and reduction in cellular proliferation [22, 25–27]. Indeed, Raf-1 activation may be a novel approach in treating certain cancers such as GI NETs.
Figure 2. The Raf-1/mitogen-regulated extracellular kinase (MEK)/extracellular regulated kinase (ERK) pathway.
Activated ras binds Raf-1, setting off a complex process of phosphorylation of activation sites and dephosphorylation of inhibitory sites. Activated Raf-1 subsequently phosphorylates and activates MEK, which then goes on to phosphorylate and activate the extracellular signal regulated kinase, ERK. ERK has many substrates within the cell but most notable are the transcription factors that get phosphorylated upon ERK translocation to the nucleus. These activated transcription factors then facilitate the transcription of genes that are necessary for cellular growth and differentiation.
Using an estrogen-inducible Raf-1 construct in human GI carcinoid tumor cells, we have previously shown that Raf-1 activation results in carcinoid cell morphological change accompanied by a marked decrease in NE secretory granules by electron microscopy; likewise, Raf-1 induction in carcinoid tumor cells leads to significant reductions in 5-HT, CgA, and synaptophysin (SYP) levels in vitro [7]. Though methods to deliver activated Raf-1 to carcinoid cancer cells are limited, pharmacologic activators of the Raf-1 pathway are currently being studied. Initially identified as a potent and specific inhibitor of Raf isoforms [28], the small molecule ZM336372 (BioMol, Plymouth Meeting, PA) paradoxically showed a >100-fold induction of Raf-1 activity when used in specific culture systems. In our laboratory, we have demonstrated that ZM336372 results in dose-dependent phosphorylation of Raf-1 pathway mediators in human GI carcinoid tumor cells in vitro [9]. Furthermore, exposure to ZM336372 results in a significant reduction of bioactive hormones, such as CgA, as well as the transcription factor ASCL1 in carcinoid cells [9]. Importantly, treatment with ZM336372 also demonstrates marked suppression of carcinoid cancer cell proliferation in vitro. Clearly, this compound warrants further investigation as a novel therapeutic and palliative treatment strategy for patients with refractory GI NETs.
The function of GSK-3β in GI NETs
GSK-3β is a serine/threonine kinase which has been implicated in cell fate, protein synthesis, cell differentiation, proliferation, and apoptosis [24]. Unlike other kinases, GSK-3β is unique in that it is constitutively active in the unphosphorylated state. Phosphorylation of GSK-3β on serine residue 9 (Ser9) inactivates the enzyme by causing a conformational change [24]. Akt, protein kinases A and C, p70s6k, and integrin-linked kinase (ILK) are all proteins capable of phosphorylating GSK-3β at Ser9 and, thus, inhibiting kinase activity [29]. In general, GSK-3β is antiapoptotic; as such, GSK-3β inhibition upregulates apoptosis and causes cell death. Several cellular signaling pathways, such as the raf-1/MEK/ERK, PI3K-Akt, and Wnt/β-catenin cascades, involve GSK-3β [29]. In the cytosol, GSK-3β has been shown to associate with a large protein complex that phosphorylates β-catenin promoting nuclear translocation and eventual degradation [30]. Interestingly, researchers have shown the accumulation of β-catenin in the cytoplasm and nucleus of human GI NET samples [31–32]. Other downstream targets of GSK-3β include p53, c-Myc, Bcl-2, HSF-1, c-Jun, and cyclin D1 [29]. Inactivation of GSK-3β in other non-GI NETs, namely MTC, leads to a decrease in both cell growth and NE markers [24].
Inhibition of GSK-3β has been highlighted as a potential target in GI NETs as well. The aforementioned Raf-1 activating compound ZM336372 also was observed to independently phosphorylate and subsequently inactivate GSK-3β in human GI carcinoid tumor cells, supporting the role of ZM336372 as a GSK-3β inhibitor [12]. Similarly, lithium (Li+) is another well characterized drug capable of inhibiting GSK-3β. Treatment of pheochromocytoma (PC-12) cells with Li+ causes a dose-dependent inactivation of GSK-3β activity that is accompanied by decreased cellular growth and NE hormone suppression [33]. Furthermore, unpublished data from our lab recapitulates these results in GI carcinoid tumor cells. Taken together these data support the identification of GSK-3β as a potential target for novel therapies that may treat or palliate patients with complicated GI NETs. One advantage to the use of Li+ as a GSK-3β inhibitor is that the drug lithium carbonate has been utilized in the management of psychiatric conditions including bipolar disorder, schizophrenia, and depression with minimal adverse side effects for many years. Presently at our institution, we are conducting phase II clinical trials to determine the efficacy of lithium carbonate in the treatment of GI NETs. In addition to Li+, numerous additional GSK-3β inhibitors exist including SB216763 [24], SB415286, CHIR99021, and azakenpaullone [34].
Downregulation of PI3K-Akt Signaling in GI NETs
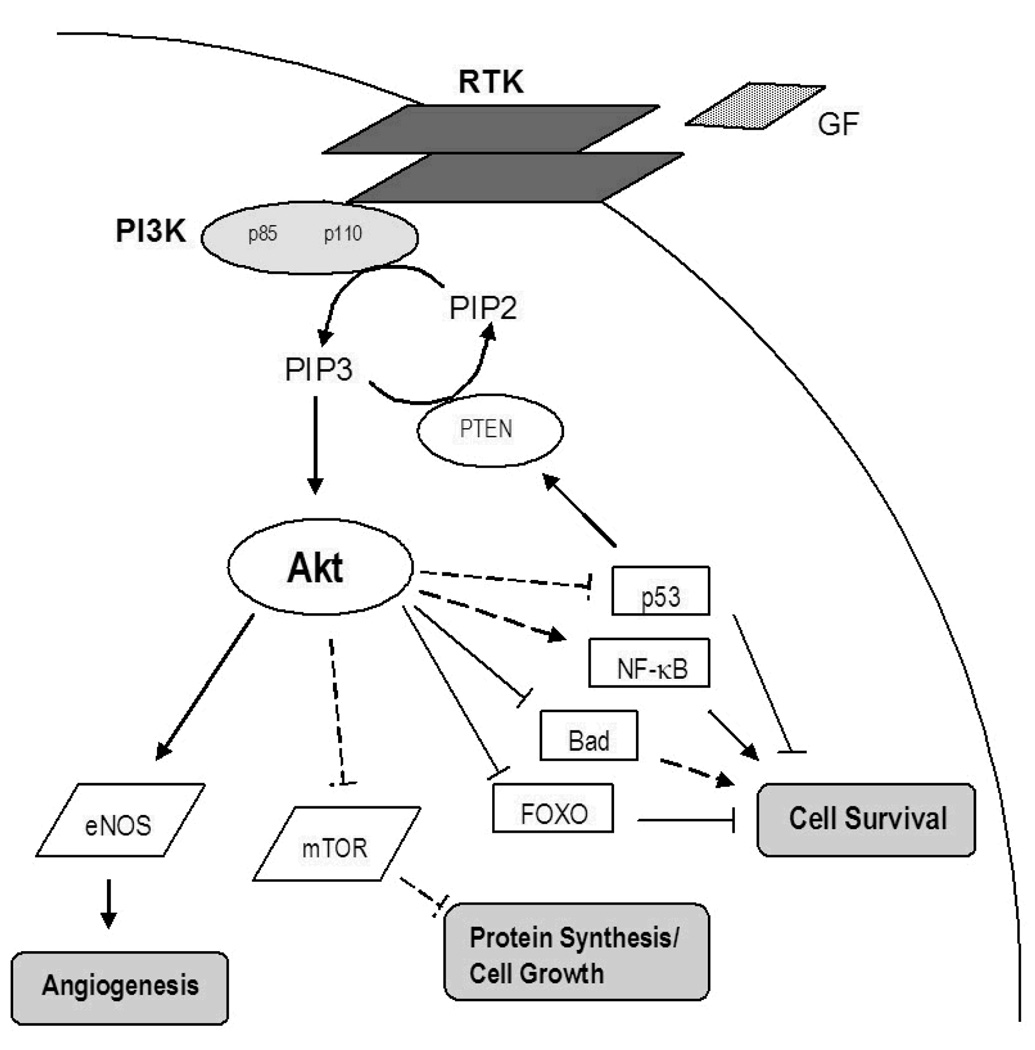
The PI3K-Akt pathway is over-expressed and genetically selected for during tumorigenesis in several cancers [35]. Normal cellular functions, including growth, survival, proliferation, and motility, are regulated by PI3K-Akt signaling and exploited by developing malignancies. Akt activation requires upstream binding of growth factor receptor tyrosine kinases (RTKs) or G-protein coupled receptors that activate PI3K, a heterodimer with two subunits, p85 (regulatory) and p110 (catalytic) (figure 3). PI3K subsequently phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) generating phosphatidylinositol-3,4,5-triphosphate (PIP3). The PIP3 molecule then recruits both Akt (a serine/threonine kinase alternately named protein kinase B) and inositol phosphate-dependent pyruvate dehydrogenase kinase-1 (PDK1), and phosphorylates Akt either directly or through PDK1. Full activation of Akt occurs when both the threonine 308 and serine 473 residues are phosphorylated [35]. Activated Akt then interacts with a number of downstream substrates including mammalian target of rapamycin (mTOR), various caspases, and Forkhead transcription factors among others. The actions of phosphatase and tensin homolog (PTEN), a well-known tumor suppressor, turn off PI3K signaling. Furthermore, loss-of-function mutations in PTEN are known to cause Akt activation [35]. Upregulation of the PI3K-Akt pathway has been shown to play a role in the tumorigenesis of MTC, a non-GI NET [36].
Figure 3. The Phosphatidylinsoitol-3 kinase (PI3K)-Akt Signaling Pathway.
PI3K is activated by binding of growth factors (GF) to a receptor tyrosine kinase (RTK) through direct interaction or adapter molecules. PIP2 is then phosphorylated by PI3K to form PIP3, which then engages Akt and PDK1 resulting in phosphorylation of Akt (either directly or by PDK1). Activated Akt then stimulates pathways involved in cell growth and protein synthesis (mTOR), activates pro-survival molecules (NF-κB), and inhibits pro-apoptotic molecules (FOXO, Bad, p53) thereby promoting cell survival and proliferation. PTEN negatively regulates PI3K-Akt signaling.
In concert with this evidence in MTC, downregulation of PI3K-Akt signaling is another strategy that may be employed in the development of novel drugs to treat or palliate patients with GI NETs. However, at this time only preliminary, unpublished data support this approach. The PI3K, the Akt molecule, and various other pathway mediators are all potential targets for shutting off aberrant Akt activation. By targeting the PI3K and blocking the p110 catalytic domain, LY294002 and wortmannin are drugs that have been used both in vivo and in vitro in animal models [35]. On the other hand, targeting the Akt molecule itself would be a different approach for inhibiting PI3K-Akt dependent cell proliferation. Triciribine phosphate monohydrate (also known as API-2, TCN-PM, or VD-0002) is a synthetic tricylic nucleoside that has antiproliferative effects that may in part be due to inhibition of Akt phosphorylation and activation of Akt-1, -2, and -3 without effecting PI3K or PDK1. This drug is currently in a multicenter Phase I clinical trial in adult patients with hematologic malignancies, but has not to our knowledge been investigated in GI NETs. Similar drugs that targeted either PDK1 or PIP3 may also be effective. BAG956 is dual inhibitor of both PI3K and PDK1, but also has not been used in GI NETs [37].
Conclusion
The molecular pathways that regulate tumorigenesis in GI NETs are numerous. Further investigation is needed to clarify the roles and relative contributions of each individual signaling cascade. At this time, the Notch1, Raf1/MEK/ERK 1/2, GSK3-β, and PI3K-Akt signal transduction pathways have all emerged as potential targets for the treatment and palliation of GI NETs. Up and/or downstream molecules involved in the regulation of these pathways, such as mTOR, may prove to more selectively inhibit tumor cell growth. Phase II clinical trials in GI NETs are currently ongoing or have been conducted for the Notch1 activator valproate (VPA, depakote), the GSK3-β inhibitor lithium carbonate, and the mTOR inhibitors RAD001 (everolimus) and CCI-779 (temsirolimus). Unfortunately, the scope of this review precludes a discussion of the countless prospective methods for treating GI NETs, as additional approaches including antiangiogenic drugs (i.e. bevacizumab, sunitinib malate, sorafenib, vatalanib) and EGFR inhibitors (i.e. gefitinib) show potential. Clealry, however, manipulation of the various signal transduction pathways or downstream mediators involved in GI NET carcinogenesis provide a promising approach for future studies.
References
- 1. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105.. This compilation of several large United States-based databases comprising patients from 1950 to 1999 examines 13,715 carcinoid tumors and provides epidemiologic information regarding the natural history and evolution of the detection and diagnosis of carcinoid disease.
- 2.Chen H, Hardcare JM, Uzar A, Cameron JL, Choti M. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–92. doi: 10.1016/s1072-7515(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 3.Touzous JG, Keily JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, Pitt HA. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–783. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musunuru S, Chen H, Rajapal S, Stephani N, McDermott JC, Holen K, Rikkers LF, Weber SM. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg. 2006;141:1000–1004. doi: 10.1001/archsurg.141.10.1000. [DOI] [PubMed] [Google Scholar]
- 5.Van Gompel JJ, Sippel RS, Warner TF, Chen H. Gastrointestinal carcinoid tumors: factors that predict outcome. World J Surgery. 2004;28:387–392. doi: 10.1007/s00268-003-7019-3. [DOI] [PubMed] [Google Scholar]
- 6.Miller CA, Ellison EC. Therapeutic alternatives in metastatic neuroendocrine tumors. Surg Oncol Clin N Am. 1998;7:863–879. [PubMed] [Google Scholar]
- 7. Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–G254. doi: 10.1152/ajpgi.00420.2002.. The intracellular signaling pathways that regulate production of bioactive molecules had not been completely understood prior to the publication of these data. These results show that raf-1 induction suppresses neuroendocrine marker and hormone production in human gastrointestinal carcinoid cells via a pathway dependent on MEK activation.
- 8. Kunninmalaiyaan M, Chen H. The raf-1 pathway: a molecular target for treatment of select neuroendocrine tumors? Anticancer Drugs. 2006;17:139–142. doi: 10.1097/00001813-200602000-00004.. In this review, the authors discuss the possibility that activation of the Ras/Raf signaling pathway may be a therapeutic target for patients with select NE tumors. They suggest that in-vitro activation of Raf-1 in NE tumors either by expression of the ectopic catalytic domain of Raf-1 or by a pharmacologic drug, ZM336372, resulted in growth inhibition and significant reduction in NE markers such as serotonin, chromogranin A and calcitonin. These data support development of Raf-1-activating compounds for treatment of patients with NE tumors of selective subtypes.
- 9.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4:910–917. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 10.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G636–G642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 11.Greenblatt DY, Cayo M, Ning L, Jaskula-Sztul R, Haymart M, Kunnimalaiyaan M, Chen H. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg. 2007;11:1515–1520. doi: 10.1007/s11605-007-0249-1. [DOI] [PubMed] [Google Scholar]
- 12. Kunnimalaiyaan M, Ndiaye M, Chen H. Neuroendocrine tumor cell growth inhibition by ZM336372 through alterations in multiple signaling pathways. Surgery. 2007;142:959–964. doi: 10.1016/j.surg.2007.09.020.. This study highlights the role of GSK3-β in NE tumors.
- 13.Kadesch T. Notch signaling: The demise of elegant simplicity. Curr Opin Genet Dev. 2004;14:506–512. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Maillard I, Pear WS. Notch and cancer: Best to avoid the ups and downs. Cancer Cell. 2003;3:203–205. doi: 10.1016/s1535-6108(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 15.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: Insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 16. Kunnimalaiyaan M, Haymart M, Chen H. Tumor suppressor role of Notch1 and Raf-1 signaling in medullary thyroid cancer cells. Translational Oncogenomics. 2007;2:43–47.. This article provides a nice review of the results of Notch1 and Raf-1 pathway activation in a medullary thyroid cancer cell line in vitro. These data clearly support the cell-context dependent role of Notch1 and Raf-1 as either a tumor suppressor or proto-oncogene.
- 17. Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535.. This is a very thorough review of what is currently understoof regarding Notch1 signaling in G NETs.
- 18.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW, Jin N, Collins BJ, Nelkin BD, Chen H, Ball DW. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 19.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 20.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkühler C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007;3:195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 21.Stockhausen MT, Sjölund J, Manetopoulos C, Axelson H. Effects of the histone deacetylase inhibitor valproic acid on notch signaling in human neuroblastoma cells. Br J Cancer. 2005;4:751–759. doi: 10.1038/sj.bjc.6602309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platta CS, Greenblatt DY, Kunnimalaiyaan M, Chen H. The HDAC inhibitor trichostatin A inhibits growth of small cell lung cancer cells. J Surg Res. 2007;142:219–226. doi: 10.1016/j.jss.2006.12.555. [DOI] [PubMed] [Google Scholar]
- 23.Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13:98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 24.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3β, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 25.Younes N, Fulton N, Tanaka R, Wayne J, Straus FH, 2nd, Kaplan EL. The presence of K-12 ras mutations in duodenal adneocarcinomas and the absence of ras mutations in other small bowel adenocarcinomas and carcinoid tumors. Cancer. 1997;79:1804–1808. [PubMed] [Google Scholar]
- 26.Paraskevakou H, Saetta A, Skandalis K, Tseleni S, Athanassiadis A, Davaris PS. Morphological-histochemical study of intestinal carcinoids and K-ras mutation analysis in appendiceal carcinoids. Pathol Oncol Res. 1999;B5:205–210. doi: 10.1053/paor.1999.0193. [DOI] [PubMed] [Google Scholar]
- 27.Yashiro T, Fulton N, Hara H, Yasuda K, Montag A, Yashiro N, Straus F, 2nd, Ito K, Aiyoshi Y, Kaplan EL. Comparison of mutations of ras oncogene in human pancreatic exocrine and endocrine tumors. Surgery. 1993;114:758–763. [PubMed] [Google Scholar]
- 28.Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Boyle FT, Hewitt N, Plant H, Hedge P. Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol. 1999;8:559–568. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 29.Rowe MK, Weist C, Chuang D. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meares GP, Jope RS. Resolution of the nuclear localization mechanism of glycogen synthase kinase-3. J Biol Chem. 2007;282:16989–17001. doi: 10.1074/jbc.M700610200.. This reference describes the known molecular mechanisms of glycogen synthase kinase-3.
- 31. Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin in gastrointestinal carcinoid tumor. Cancer Res. 2001;61:6656–6659.. This study provides insight into the development of GI carcinoid tumors with respect to Wnt/beta-catenin signaling.
- 32.Su MC, Wang CC, Chen CC, Hu RH, Wang TH, Kao HL, Jeng YM, Yuan RH. Nuclear translocation of beta-catenin protein but absence of beta-catenin and APC mutation in gastrointestinal carcinoid tumor. Ann Surg Oncol. 2006;13:1604–1609. doi: 10.1245/s10434-006-9072-2. [DOI] [PubMed] [Google Scholar]
- 33.Kappes A, Vaccaro A, Kunnimalaiyaan M, Chen H. Lithium ions: a novel treatment for pheochromocytomas and paragangliomas. Surgery. 2007;141:161–165. doi: 10.1016/j.surg.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tighe A, Ray-Sinha A, Staples OD, Taylor SS. GSK-3 inhibitors induce chromosome instability. BMC Cell Biol. 2007;8:34. doi: 10.1186/1471-2121-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4.. The article is an extensive review of early PI3K-Akt data describing multiple methods for interfering with pathway upregulation.
- 36. Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–1015. doi: 10.1016/j.surg.2006.06.040.. The potential role for PI3K-Akt pathway inhibition in GI NETs is strongly supported by the results of this study performed in MTC, a non-GI NET.
- 37.Weisburg E, Banerji L, Wright RD, Barrett R, Ray A, Moreno D, Catley L, Jiang J, Hall-Meyers E, Sauveur-Michel M, Stone R, Galinsky I, Fox E, Kung AL, Griffin JD. Potentiation of anti-leukemic therapies by the dual PI3K/PDK-1 inhibition, BAG956: effects on BCR-ABL- and mutant FLT3-expressing cells. Blood. 2008;111:3723–3734. doi: 10.1182/blood-2007-09-114454. [DOI] [PMC free article] [PubMed] [Google Scholar]