Abstract
NCBE (SLC4A10) is a member of the SLC4 family of bicarbonate transporters, several of which play important roles in intracellular-pH regulation and transepithelial transport. Here we characterize a new antibody that was generated in rabbit against a fusion protein consisting of maltose-binding protein and the first 135 amino acids (aa) of the N-terminus of human NCBE. Western blotting—both of purified peptides representing the initial ~120aa of the transporters and of full-length transporters expressed in Xenopus oocytes—demonstrated that the antibody is specific for NCBE versus the two most closely related proteins, NDCBE (SLC4A8) and NBCn1 (SLC4A7). Western blotting of tissue in four regions of adult mouse brain indicates that NCBE is expressed most abundantly in cerebral cortex (CX), cerebellum (CB) and hippocampus (HC), and less so in subcortex (SCX). NCBE protein was present in CX, CB, and HC microdissected to avoid choroid plexus. Immunocytochemistry shows that NCBE is present at the basolateral membrane of E18 fetal and adult choroid plexus. NCBE protein is present by western blot and immunocytochemistry in cultured and freshly dissociated HC neurons but not astrocytes. By western blot, nearly all NCBE in mouse and rat brain is highly N-glycosylated (~150 kDa). PNGase F reduces the MW of natural NCBE in mouse brain or human NCBE expressed in oocytes to approximately the predicted MW of the unglycosylated protein. In oocytes, mutating any one of the three consensus N-glycosylation sites reduces glycosylation of the other two, and the triple mutant exhibits negligible functional expression.
Keywords: slc4a10, central nervous system, brain, oocytes, glycosylation
INTRODUCTION
NCBE belongs to the SLC4 family of bicarbonate transporters, which in humans includes the products of at least ten genes. NCBE is SLC4A10. The SLC4 transporters play essential roles in intracellular-pH regulation, transepithelial transport, and (in the case of the erythrocyte) CO2 transport. Within the SLC4 family are two major groups of transporters: (1) Na-independent anion exchangers (AEs), including AE1, AE2 and AE3; and (2) Na-coupled transporters. The latter can be further subdivided into two subtypes: (2a) electrogenic Na-coupled cotransporters, including NBCe1 and NBCe2; and (2b) electroneutral Na-coupled cotransporters, including NCBE, NBCn1 and NDCBE. The functional grouping of these eight transporters corresponds rather well with groupings based on amino-acid sequence analyses (for reviews, see refs. Romero et al., 2004; Romero, 2005).
NCBE was first cloned by Wang et al. (Wang et al., 2000) from a cDNA library from the mouse insulinoma cell line MIN6. Choi et al. (Choi et al., 2002) isolated two splice variants from human brain and kidney, NCBE-A (the ortholog of the mouse clone) and NCBE-B (which contains a 30aa insert near the middle of the presumed cytoplasmic amino terminal domain). Functional analysis of NCBE in Xenopus oocytes and HEK239 cells suggested that NCBE exchanges extracellular Na+ and for intracellular Cl−. Thus, the clone was initially designated as a Na+-driven chloride/bicarbonate exchanger. When expressed in Xenopus oocytes, hNCBE-B has absolute requirements for Na+ and (Choi et al., 2002) and is also blocked by DIDS (Choi et al, unpublished). However, its Cl− dependence is unclear (Romero et al., 2004; Romero, 2005).
Giffard et al. (Giffard et al., 2003) isolated two splice variants, rb1NCBE and rb2NCBE, from rat (r) brain. The first, rb1NCBE, is analogous to human (h) NCBE-B. The second, rb2NCBE, contains an extra 21aa at the presumed cytoplasmic carboxyl terminus, ending in the consensus PDZ motif ETCL. Recent work (Lee et al., 2006) shows that this PDZ motif of rb2NCBE interacts with ezrin binding protein 50 (EBP50), which in turn binds to the cytoskeleton-anchoring protein ezrin (Bretscher et al., 2000), which binds protein kinase A (PKA). Inhibition of protein kinase A appears to increase the activity of rb2NCBE (Lee et al., 2006).
By RNA blot analysis, NCBE is expressed at high levels in brain and at low levels in pituitary, testis, kidney and ileum (Wang et al., 2000). By in situ hybridization, NCBE is expressed in the CNS very early in development, in a pattern consistent with neuronal expression (Hübner CA et al., 2004). The same study showed that NCBE is expressed in the peripheral nervous systems and in non-neuronal tissues, such as the choroid plexus, the dura and some epithelia. Praetorius et al. developed an antibody generated against an 18-amino acid peptide corresponding to a portion of the C terminus of mouse NCBE (Praetorius et al., 2004). An immunocytochemistry study has demonstrated that NCBE as well as NBCn1 are localized to the basolateral membrane of choroid-plexus epithelial cells, whereas NBCe2 is localized to the apical membrane (Bouzinova et al., 2005; Praetorius et al., 2004). Thus NCBE and NBCn1 may mediate net base influx into choroid-plexus epithelial cells, whereas NBCe2 may facilitate and Na+ transport from the cells into the cerebrospinal fluid, and thereby play an important role in cerebrospinal-fluid production (Bouzinova et al., 2005). However, so far there is no evidence at the protein level for the expression of NCBE in neurons.
In the present study, we characterized a new antibody directed against the amino terminus of hNCBE. By western blotting, the antibody is specific for NCBE versus NDCBE or NBCn1 heterologously expressed in Xenopus oocytes. In mice and rats, the antibody detects a ~150-kDa band in cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC)—including microdissected mouse CX, CB, and HC preparations that were free of choroid plexus. By immunocytochemistry, NCBE is present at the basolateral membrane of choroid plexus in both fetus and adult. In rat HC neuronal cultures, western blotting demonstrates NCBE as a ~150-kDa band. Moreover, immunocytochemistry demonstrates the presence of the protein on the plasma membrane of both cultured and freshly dissociated neurons. Treatment with PNGase reduces the molecular mass to ~127 kDa in mouse brain tissue as well as oocytes heterologously expressing NCBE. Mutating the three consensus glycosylation sites eliminates glycosylation in oocytes.
METHODS
Antibodies
A recombinant fusion protein consisting of maltose-binding protein (MBP) and the first 135 amino acids of human NCBE (GenBank Accession: AY376402) in the cytoplasmic N terminus, was expressed in E. coli on a pMAL™-2 vector (New England Biolabs, MA, USA), and the fusion protein was purified by affinity chromatography. A rabbit was injected with the purified MBP-fusion protein and the resulting antiserum was used for the present study.
For affinity purification (AP) of the NCBE antiserum, we used a CarboxylLink™ Kit (Pierce, Rockford, IL) following the instructions of the manufacturer. Briefly, a His-tagged peptide consisting of the first 123aa of NCBE (see below) was covalently linked to a column of CarboxylLink™ Coupling Gel. The column was equilibrated with 100 mM PBS, pH 7.4. The crude antiserum diluted in PBS was applied to the column for incubation at room temperature (RT) for 2 hours, and then the column was washed with PBS to remove the unbound proteins. The bound antibody was eluted with 100 mM glycine, pH 2.8, and the eluted antibody was neutralized by adding 1/10 volume of 1 M Tris-HCl.
A monoclonal antibody against His-tag was purchased from Novagen (Novagen, San Diego, CA). A monoclonal antibody against glutathione transferase (GST) was purchased from Chemicon (Chemicon International, Temecula, CA). A polyclonal antibody against aquaporin 1 (AQP1) was purchased from Alpha Diagnostics International (Alpha Diagnostic International, San Antonio, TX).
Construction of an Enhanced Green Fluorescent Protein (EGFP)-tagged full-length transporters in a Xenopus expression vector
Creating EGFP ‘donor’ cDNA
Our starting material was NBCe1-A–EGFP.pGH19)—that is, hNBCe1-A cDNA (GenBank Accession: NM_003759) cloned into a pGEM-based vector between the 5′ and 3′ UTRs of the Xenopus β-globin gene. The C terminus (Ct) of NBCe1-A is connected via the 5aa linker ‘SPVAT’ to the N terminus of EGFP (Toye et al., 2006). In this construct the EGFP is flanked at both the 5′ end (i.e., within SPVAT) and 3′ end by AgeI restriction sites. We eliminated the AgeI site at the 3′ flank of EGFP (ACCGGT to ACCCGT) using the Quickchange® Site-Directed Mutagenesis Kit (Stratagene, Cedar Creek, TX). This enabled the isolation of the EGFP cDNA cassette from the construct by digestion of the AgeI site at the 5′ flank of EGFP and the XhoI site at the 3′ flank of EGFP located within the pGH19 vector sequence. The isolated cassette was AgeI-EGFP-XhoI.
Creating NBC-like ‘acceptor’ cDNA
Our starting materials were (1) NCBE-B.pGH19—that is, hNCBE-B cDNA (SLC4A10; GenBank Accession: AY376402; this construct lacks the 30aa cassette A and has a short C terminus) cloned into pGH19 (Trudeau et al., 1995). (2) NDCBE1.pGH19—that is, hNDCBE1 cDNA (SLC4A8; GenBank Accession: NM_004858) cloned into pGH19 and (3) NBCn1-B.pGH19—that is, rNBCn1-B cDNA (SLC4A7; GenBank Accession: NM_058211) cloned into pGH19 (Choi et al., 2003). We introduced the sequence TGCTCACCGGTA (i.e, Cys and Ser codons and an AgeI restriction site) at a position immediately prior to the termination codon for each construct, using the Quickchange mutagenesis kit according to the manufacturer’s protocol. The Ser represents the first residue of the SPVAT linker. The resulting constructs were NCBE-B/AgeI.pGH19, NDCBE1/AgeI.pGH19 and NBCn1-B/AgeI.pGH19.
Creating NBC-like–EGFP.pGH19
The vector sequence between the AgeI site (i.e., 3′ end of the NBC-like sequence) and XhoI site of NCBE-B/AgeI.pGH19, NDCBE1/AgeI.pGH19 and NBCn1-B/AgeI.pGH19 was excised by enzymatic restriction and replaced with AgeI-EGFP-XhoI to create NCBE-B–EGFP.pGH19, NDCBE1–EGFP.pGH19 and NBCn1-B–EGFP.pGH19 that is, NBC-like cDNA cloned into pGH19 between the 5′ and 3′ UTRs of the Xenopus β-globin gene and linked to a Ct EGFP via the 6aa linker ‘CSPVAT’ between the NBC-like sequence and EGFP.
cRNA injection of oocytes
Xenopus oocytes were prepared as previously described (Goldin, 1992). Briefly, female Xenopus laevis were anesthetized in a solution of 0.2% tricaine (ethyl 3-aminobenzoate methanesulfonate or MS-222, Catalog# A5040, Sigma-Aldrich, St. Louis, MO). Ovarian lobes were removed, placed in 0-Ca solution (98 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5), cut into small pieces, and washed five times with 0-Ca solution. Oocytes were dissociated by enzymatic digestion with 2 mg/ml of type IA collagenase (Sigma-Aldrich) in 0-Ca solution. Stage V-VI oocytes were selected and kept at 18 °C in OR3 medium.
We made cRNA encoding the three transporters described in the previous section: (1) hNCBE-B with and without a Ct-EGFP tag, (2) rNBCn1-B with a Ct-EGFP tag, and (3) hNDCBE with a Ct-EGFP tag. Capped cRNAs encoding the above transporters were injected into Xenopus oocytes, which we incubated for 4 to 5 days at 18 °C before collecting them for membrane protein preparation.
Site-directed mutagenesis of NCBE
The putative extracellular loop between the TM5 and TM6 of hNCBE has three potential N-glycosylation motifs (677NGTL, 687NISA and 697NLTV). The three Asn residues were mutated to Gln using a QuikChange® Site-Directed Mutagenesis Kit (Stratagene, Cedar Creek, TX). We created three single mutants (with one of the three Asn replaced for each), three double mutants (with two of the three Asn replaced) and one triple mutant (with all of the three Asn replaced).
Preparation of membrane proteins from mouse or rat brain or Xenopus oocytes
Historically our lab has worked with rat, but is shifting to mouse to take advantage of genetic manipulated animals. In the present study, we used both species.
The mouse or rat brain or oocytes were put in Na-Phosphate buffer containing 7.5 mM NaH2PO4, 250 mM sucrose, 5 mM EDTA, 5 mM EGTA, pH 7.0 and 1% protease inhibitor cocktail for mammalian tissues (Catalog #P8340, Sigma-Aldrich) and were homogenized with a Teflon Pestle probe and tube on Glas-Col® Homogenizer (Glas-Col, Terre Haute, IN), rotating at 5000 rpm. The homogenate was then centrifuged at 3,000 × g at 4 °C for 15 min to remove the cellular debris. The supernatant was ultracentrifuged at 100,000 × g at 4 °C for 1 hr. The pellet was resuspended in protein-suspension buffer containing 5% SDS, 20 mM Tris-HCl and 5 mM EDTA at pH 8.0. The total protein concentration was measured using the BCA™ Protein Assay Reagent (Catalog #23228 and #23224, Pierce, Rockford, IL) according to the manufacturer’s protocol, and the membrane protein preparations were stored in aliquots at −80 °C until use.
Western blotting
Membrane proteins were mixed with 2 × SDS-sample loading buffer containing 6M of urea and were denatured for 8 min at 95 °C. The proteins were then separated on SDS-polyacrylmide gel and transferred onto Immuno-Blot™ PVDF membrane (Bio-Rad, Hercules, CA). The blots were blocked in 5% powdered milk in TBST (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20, pH 7.5) at 4 °C overnight. The blots then were incubated with primary antibody in 1% milk in TBST at RT for 2 hours, followed by five 6-min washes with TBST. The blots were then incubated with secondary antibody in TBST containing 1% milk at RT for 1 hour followed by five 6-min washes with TBST. Chemiluminescent detection was performed with ECL plus™ Western Blotting Detection Reagents (Amersham Biosciences, Piscataway, NJ) prior to X-ray film exposure.
Immunodepletion assay
Primary antibodies at a dilution of 1:2000 in 1% milk in TBST were preabsorbed at RT for 3 hr by incubation with 20 μg/ml (for western blotting) of either His-tagged NCBE N-terminus (see below) or His-tagged NBCn1 N-terminus (see below).
His-tagged NCBE N-terminus
This peptide consisted of a leader sequence (GSSHHHHHH), followed by a thrombin-protease linker sequence (SSGLVPRGS), fused to the first 123 residues of NCBE (MEIK … ELDE). The leader was not removed for affinity-purification and immunodepletion studies.
His-tagged NBCn1 N-terminus
This peptide consisted of a leader sequence (GHHHHHH), directly fused to the first 123 residues after (and not including) the start Met of rNBCn1 (EADG … EMDEL). The leader was not removed for immunodepletion studies.
GST-tagged NDCBE N-terminus
This peptide consisted of the GST followed by a PreScission™ Protease linker (LEVLFQGP) on a vector of pGEX-6p-1 (Amersham Biosciences), fused to the first 116aa of human NDCBE (MPAA … PHEL).
For immunodepletion, the affinity-purified antibody was diluted in PBS and incubated with the column covalently-coupled with the peptide consisting of His-tagged NCBE Nt for 2 hours with shaking. The flow-through was concentrated to the starting volume and was used for negative control to stain rat brain sections.
Deglycosylation reaction
We used PNGase F (500U/μl, New England BioLabs, Ipswich, MA) following the instructions of the manufacturer. Briefly, 80 μg of membrane proteins were mixed with 10× denaturing buffer supplied with the enzyme, and were denatured at 98 °C for 10 min. PNGase F (2 μl) was then added and incubated at 37 °C for 1 hour. Then the sample was mixed with 2× SDS-loading buffer and separated on SDS-gel and transferred onto PVDF membrane for western blotting.
Immunocytochemistry experiments performed on brain sections
The immunocytochemistry protocol for adult brains was performed according to previously described methods (Praetorius and Nielsen, 2006), with some modifications. Briefly, an adult female rat was transcardially perfusion fixed with 4% paraformaldehyde in phosphate buffer (pH 7.4). The brain was removed, immersion-fixed in 4% paraformaldehyde and kept at 4 °C until sectioned. In the case of embryonic day 18 (E18) animals, we dissected the brain as described in the next paragraph, immersion-fixed the brain in 4% paraformaldehyde, and kept the tissue at 4 °C until sectioning. Brain tissue was dehydrated, embedded in paraffin, and sectioned at 5 μm using a rotary microtome. Slides were heated in a 60 °C oven overnight. Sections were then depariffinized, rehydrated and boiled in a 10 mM citrate buffer solution (pH 6.0). Endogenous peroxidase activity was blocked by 0.3% H2O2 in distilled H2O. Sections were then incubated with 0.5 M NH4Cl in TBS (10 mM Tris-HCl, 150 mM NaCl, pH 7.4) and blocked in a solution containing TBS supplemented with 0.1% BSA, 0.05% saponin and 0.2% gelatin. Sections were incubated overnight at 4 °C with the affinity purified NCBE antibody (a 1:200 dilution in TBS supplemented with 0.1% BSA and 0.3% Triton X-100). The immunodepleted antibody (see above) was used as negative control. The next morning, sections were incubated with ImmPRESS™ anti-rabbit IgG peroxidase reagent (Vector Labs, Burlingame, CA), and staining was visualized with the peroxidase substrate Nova Red (Vector labs). Sections were then counterstained with Methyl Green, dehydrated in a graded alcohol series, cleared in xylene, and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ). Images were acquired on a Nikon Eclipse 800 Research microscope equipped with a CCD camera (Optronics Engineering, Goleta, CA).
Immunocytochemistry of cultured and freshly dissociated hippocampal neuron
Rat hippocampal (HC) neurons were cultured as previously described by Wu et al. (Wu et al., 2001), with slight modifications. Briefly, pregnant rats were deeply anesthetized with halothane and euthanized by cervical dislocation. E18 pups were excised from the uterus and placed in a HEPES-buffered solution (HBS). Fetuses were then decapitated and the brain was removed. Hippocampi were microdissected, digested for 12–15 min with 0.03% trypsin, and triturated. Neuron viability was assessed by trypan-blue exclusion. HC neurons were suspended in neurobasal media supplemented with B27, penicillin, streptomycin, glutamate, and fetal bovine serum. Cells were plated at a density of ~35,000 cells/ml on 12-mm poly-L-lysine–treated coverslips and stored at 37 °C in a 5% CO2 incubator. After 3–4 hours, the serum-rich neurobasal media was aspirated and replaced by serum-free media to inhibit astrocyte growth. Fresh media was added weekly until cells were collected to prepare whole lysate for western blotting.
After two to four weeks in culture the neurons on cover slips were photobleached by exposure to the microscopy laser with Rodhamine settings for 5 min to reduce potential background autofluorescence (Neumann and Gabel, 2002). In experiments with freshly dissociated neurons, we found that this photobleaching protocol had little effect on the magnitude or distribution of the NCBE signal. After photobleaching, the cells were immediately fixed in 4% PFA for 20 min, and washed with TBST. The cells were then treated with 0.3% H2O2 in TBS for 15 min, washed with TBST, then blocked in TBST containing 5% normal goat serum (NGS) for 30 min. The cover-slips were incubated with anti-NCBE antibody in TBS containing 2.5% NGS, 0.025% Triton X-100 overnight at 4 °C. The cells were then washed with TBST and incubated with HRP-conjugated goat-anti-rabbit secondary antibody (at 1:400 dilution; Vector Laboratories, Inc. Burlingame, CA) in TBS containing 2.5% NGS, 0.025% Triton X-100 at room temperature for 30 min, then rinsed with TBST. The cells were then treated with the signal amplification reagent TSA™ plus Tetramethylrhodamine System (at 1:100 dilution; PerkinElmer Life Sciences) according the manufacturer’s instruction.
For freshly dissociated mouse neurons, we plated the triturated neurons onto coverslips, and allowed them to adhere for 1 h before using them for immunocytochemistry as described above. In order to identify the cell types, we double-stained the cells with NCBE antibody together with the neuronal marker MAP2 or astrocytic marker GFAP. The coverslips were incubated with NCBE antibody as well as monoclonal mouse anti-MAP2 antibody (1:100 dilution; Cat# MAB3418, Chemicon International, Temecula, CA) or monoclonal mouse anti-GFAP antibody (1:200 dilution; Cat# MAB360, Chemicon International, Temecula, CA) in TBS containing 2.5% NGS, 0.025% Triton X-100 at 4°C overnight. HRP-conjugated goat anti-rabbit or Cy2-goat anti-mouse secondary antibody (1:200 dilution; Jackson ImmunoReseach Laboratories, Inc., West Grove, PA) was incubated with coverslips at room temperature for 30 min, followed by application of the signal amplification reagent TSA™ plus Tetramethylrhodamine System.
Intracellular pH measurements
We monitored intracellular pH in cultured HC neurons using the same approach described previously (Bouyer et al., 2004). Briefly, we loaded neurons with the esterified form of 2′,7′-bis-2-carboxyethyl)-5(and-6) carboxyfluorescein (BCECF; Molecular Probes Inc., Eugene, OR), and used an Olympus IX70 inverted microscope equipped for epi-fluorescence (oil immersion 40× objective, NA 1.35, with a 1.5 × magnification knob). The light source was a 75 W xenon arc lamp, and we used a filter wheel (Ludl Electronic Products Ltd., Hawthorne, NY) to alternate between two excitations filters (440 ± 5 nm and 495 ± 5 nm, Omega Optical Inc., Brattleboro, VT). We collected emitted light, via a band-pass filter (530 ± 35 nm, Omega Optical Inc.), with an intensified CCD camera (Model 350F, Video Scope International LTD, Dulles, VA), averaging 4 video frames for each excitation wavelength acquisition point, and acquiring one data pair every 8 to 10 seconds. Using Optimas (Media Cybernetics, Inc., Silver Spring, MD), we defined an area of interest corresponding mainly to the neuron soma, computed the ratio of pixel intensities at 490 nm (I490) vs 440 nm (I440), and converted the I490/I440 ratio to pHi using the high-K+/nigericin technique (Thomas et al., 1979) with a one-point calibration at pHi 7.00 (Boyarsky et al., 1988).
The composition of the HEPES solution was (in mM): 146 NaCl, 1.3 NaH2PO4, 3 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, 30 HEPES, titrated to pH 7.40 with NaOH, and osmolality adjusted to 300 mOsm. The composition of the solution was (in mM): 124 NaCl, 1.3 NaH2PO4, 22 NaHCO3, 3 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, 5% CO2 (balance O2), pH 7.40, and osmolality adjusted to 300 mOsm. The solutions were delivered to the chamber at 7 ml min−1 at 37°C (Bouyer et al., 2003; Zhao et al., 2003).
Statistics analysis
To compare the distribution of NCBE in difference regions of mouse brain, one-way ANOVA and a Student-Newman-Keuls (SNK) Multiple Comparison were performed by using KaleidaGraph (Version 4, Synergy Software). P < 0.05 was considered significant.
RESULTS
Validation of the polyclonal antibody
Fig. 1 shows the alignment of part of the NCBE N terminus (Nt) with comparable parts of NDCBE and NBCn1. We raised the antiserum against an MBP fusion protein that included the first 135 residues of human NCBE, and examined the specificity of the antibody by using it to probe a western blot of (1) a His-tagged peptide consisting of the first 123aa of hNCBE (His-NCBE-Nt; Fig. 2A, lane 1), (2) a His-tagged peptide consisting of the first 123aa after (and not including) the start Met of rNBCn1 (His-NBCn1-Nt, Fig. 2A, lane 2), or (3) a GST-tagged peptide consisting of the first 116aa of hNDCBE (GST-NDCBE-Nt, Fig. 2B). The results indicate that the antibody specifically recognizes the NCBE-Nt but neither NBCn1-Nt nor NDCBE-Nt.
Fig. 1.
Sequence alignment of the initial portions of the N termini of human NCBE, rat NBCn1 and human NDCBE. The first 135aa of NCBE Nt (beneath the solid + dashed lines) were used to immunize rabbit to generate the antibody. Beneath the solid lines are the portions of NCBE (123aa), NBCn1 (123aa but lacking the initial M), and NDCBE (116aa) used to generate the peptides for affinity purification or immunodepletion. “*” indicates amino acid residues identical to all the three N termini, “:” indicates conserved substitutions. “.” indicates semi-conserved substitutions.
Fig. 2.
Specificity of anti-NCBE antibody in western blots of purified peptides corresponding to the N termini of Na+-coupled transporters. (A) Western blotting of His-NCBE-Nt and His-NBCn1-Nt. Two parallel blots were prepared and probed with crude anti-NCBE antibody (upper panel) and anti-His antibody (middle panel), respectively. A parallel blot was stained with coomassie blue (lower panel). (B) Western blotting of GST-NDCBE-Nt. The blot was probed with anti-NCBE antibody (upper panel), stripped and probed with anti-GST antibody (middle panel). A parallel blot was stained with coomassie blue (lower panel). The antibodies were used at dilution of 1:10,000.
We further assessed the specificity of the antibody by using it to probe western blots of membrane proteins extracted from oocytes injected with H2O or oocytes expressing full-length hNCBE-B, hNCBE-B-EGFP, rNBCn1-EGFP, or hNDCBE-EGFP. Fig. 3 indicates that our NCBE antibody specifically recognizes full-length NCBE (lane 2) and does not cross-react with either full-length NBCn1-EGFP (lane 3) or full-length NDCBE-EGFP (lane 4). Note that the antibody reveals two bands for untagged NCBE (lane 2 in Fig. 3A, and lane 1 in Fig. 3B) expressed in oocytes, one at a molecular weight (MW) of ~127 kDa (predicted MW of hNCBEB, which contains the 30aa-cassette A, is 125.9 kDa), and the other at a MW of ~150 kDa. Similarly, for EGFP-tagged NCBE (Fig. 3B), the antibody also recognizes two bands (predicted MW of EGFP with linker is 28 kDa).
Fig. 3.
Specificity of anti-NCBE antibody in western blots of membrane extracts from oocytes expressing EGFP-tagged NCBE, NBCn1, or NDCBE. The expression of EGFP-tagged transporters was confirmed by confocal microscopy (not shown). Membrane proteins (20 μg per lane) were separated on 4–15% SDS gel. Crude antibody was used at a dilution of 1:2000.
By confocal microscopy of oocytes, we confirmed the expression of EGFP-tagged recombinant proteins at or near the plasma membrane (not shown). We also confirmed the presence of NBCn1-EGFP and NDCBE-EGFP on the western-blot membrane using polyclonal antibodies targeted against the Ct of NBCn1 and the Nt of NDCBE (Michelle Kelly et al, unpublished), both developed by our group (not shown).
NCBE expression in mouse brain
As shown in Fig. 4, when we use crude NCBE antiserum to probe a western blot of a membrane preparation from the mouse cerebral cortex, we detect a major band at a MW of ~150 kDa (lane 1). Minor bands also appear at ~75 kDa, ~32 kDa, and ~27 kDa, and much weaker bands appear at ~48.9 kDa and ~22.2 kDa. Immunodepleting the antiserum with a His-NCBE-Nt greatly reduces the intensities of the 150-kDa and 75-kDa bands (lane 2). On the other hand, immunodepleting with His-NBCn1-Nt does not reduce any of the bands (lane 3). Finally, when we use antiserum that we affinity-purified with His-NCBE-Nt, we see the major band at ~150 kDa as well as very weak bands at the previously noted MWs. In summary, the crude antiserum recognizes a 150-kDa band that presumably represents glycosylated NCBE, as well as minor bands, at least some of which represent cross-reactivity with other proteins. The affinity-purified antiserum would potentially be useful for immunocytochemistry.
Fig. 4.
Western blotting of membrane preparations from mouse cerebral cortex. (Lane 1) The blot was probed with crude antiserum. (Lane 2) The antiserum was preabsorbed with His-NCBE-Nt before probing the blot. (Lane 3) The antiserum was preabsorbed with His-NBCn1-Nt before probing the blot. (Lane 4) The blot was probed with the affinity-purified (AP) antiserum.
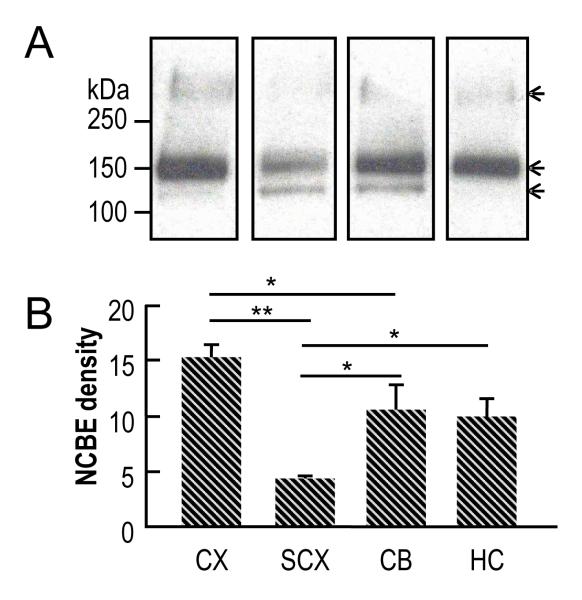
We next used the crude NCBE antiserum to probe western blots of sub-areas of brain from adult mice, 104 days old. As we saw in lane 1 of Fig. 4, the antibody reveals a major band at an MW of ~150 kDa in the cerebral cortex (Fig. 5A, lane 1). In subcortex (lane 2) and cerebellum (lane 3), the antiserum reveals a band not only at ~150 kDa, but also at ~127 kDa, which is in the range of molecular weights predicted for the unglycosylated protein (i.e., 122.5 for the splice variant without cassette A and the short C terminus to 128.3 for the variant with cassette A and the long C terminus). Finally, in hippocampus (lane 4), the antiserum reveals a major band at ~150 kDa and a faint band at ~127 kDa. Thus, most of the NCBE expressed in mouse brain, especially in CX and HC, is at ~150 kDa, which presumably represents glycosylated protein.
Fig. 5.
The relative distribution of NCBE in different regions of mouse cerebral cortex (CX), subcortex (SCX), cerebellum (CB) and hippocampus (HC), as assessed by western blotting. (A) Western blots of membrane preparations. In each lane, 25 μg of total membrane protein was separated on 4–20% SDS gel. Crude antiserum was used at dilution of 1:1000. Depicted here are pooled data from five adult mice at an age of 114 days. The arrows indicate bands at ~300 kDa (presumably the highly glycosylated dimer), ~150 kDa (presumably the highly glycosylated monomer), and ~127 kDa (presumably the slightly glycosylated monomer). (B) Summary of densitometry data from experiments like those in panel A. Values are means ± SE for N = 5 pools of material (each pool representing 5 mice). We made statistical comparisons among the groups using a one-way ANOVA and Student-Newman-Keuls (SNK) Multiple Comparison (* P<0.05, ** P<0.001). The absence of a symbol over a horizontal line indicates a lack of statistical significance between a set of bars.
Fig. 5B summarizes densitometry data (including both the 150-kDa and 127-kDa bands) from five groups of mice. It shows that NCBE is relatively abundant in CX, CB and HC, and less so in SCX. The expression level of NCBE in CX is about two-fold higher than in SCX, and about 50% higher than in CB or HC.
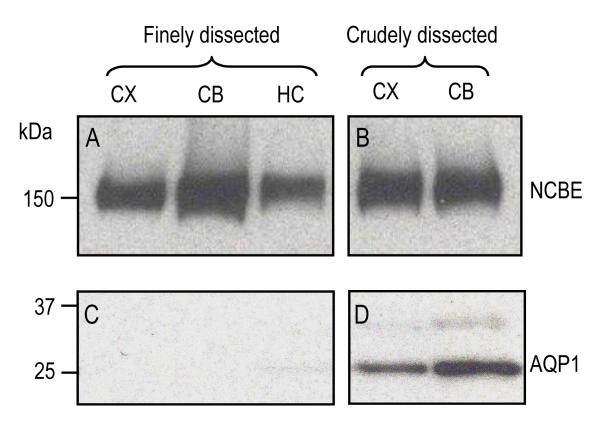
For the data in Fig. 5, we did not take special precautions to avoid completely the choroid plexus, which expresses high levels of NCBE (Praetorius et al., 2004; Praetorius and Nielsen, 2006)and AQP1. Therefore, we finely dissected the brain tissue to avoid the choroid plexus in CX, CB, and HC. The left side of Fig. 6 shows one western blot of this preparation probed for NCBE (top), and a second probed for AQP1 (bottom). The right side of Fig. 6 shows the result for tissues “roughly dissected” as in Fig. 5A. Note that AQP1 levels were substantially lower in the finely dissected tissue, indicating a low level of contamination from choroid plexus. On the other hand, we observed no significant difference in NCBE expression levels between the two methods of dissection. Thus, these three brain regions express substantial amounts of NCBE in tissues other than choroid plexus.
Fig. 6.
Western blotting of membrane preparations of finely dissected vs crudely dissected mouse-brain tissues. (A and C) The tissue was finely dissected to eliminate contamination from choroid plexus. (B and D) The tissue included the whole cerebral cortex (with ventricles) or cerebellum (with contamination from fourth ventricle), as in Fig. 4, Fig. 5, and Fig. 7. The blots were probed with crude NCBE antiserum (panels A and B) or anti-AQP1 antibody (panels C and D).
NCBE expression in rat brain
We also used the NCBE antibody to examine the expression of NCBE in rat brain. As shown in Fig. 7A, the antibody reveals bands with a pattern similar to that in mouse brain. The NCBE antibody recognizes a band with MW of ~150 kDa and additional bands at ~75 kDa, ~32 kDa, and ~27 kDa. Preabsorption of the antiserum with His-NCBE-Nt results in the disappearance of the 150-kDa and 75-kDa bands, and the near-disappearance of the 32-kDa band (Fig. 7B). Thus, these bands presumably represent breakdown products of NCBE. The affinity-purified antibody reveals only one major band at ~150 kDa and a very weak band at MW of 75 kDa (Fig. 7C). Thus, the affinity purification scheme presumably enriched a population of antibodies that tends not to recognize the proteolytic fragments recognized by the crude antiserum.
Fig. 7.
Western blotting of membrane preparations from rat brain. (A) Western blot of cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC). In each lane, ~25 μg of total membrane protein was separated on 4–20% SDS gel. The blot was probed with crude antiserum at dilution of 1:1000. (B) Western blot of cerebral cortex probed with crude antiserum that had been preabsorbed with His-NCBE-Nt. (C) Western blot of cerebral cortex probed with affinity-purified (AP) antiserum.
Fig. 8 shows the results of an immunocytochemistry study of NBCE on the choroid plexus from the rat E18 fetus (panel A) and adult (panel B). The affinity-purified antibody reveals staining in basolateral membrane of choroid plexus (panel A and B), but no major reactivity in other regions of rat brain (not shown). The staining of the choroid plexus was absent (insets) when we immunodepleted the antibody—prior to staining the sections—with His-NCBENt (Fig. 1). Our results with the adult tissue are consistent with those reported previously (Bouzinova et al., 2005; Praetorius et al., 2004). Recall that in Fig. 6, we showed the expression of NCBE in finely dissected mouse-brain tissues that were devoid of choroid-plexus contamination. Taken together, these results indicate that NCBE is expressed in regions of rodent brain other than choroid plexus, but it is not visible by immunocytochemistry.
Fig. 8.
Immunocytochemistry of NCBE in rat choroid plexus. (A) Choroid plexus from lateral ventricle at embryonic day 18 (E18), probed with affinity-purified NCBE antibody or affinity-purified antibody that had previously been immunodepleted (Inset of A). For negative control, the affinity-purified antibody was immunodepleted with a His-NCBE-Nt. (B) Choroid plexus from fourth ventricle from adult (age P75), probed with affinity-purified NCBE antibody or affinity-purified antibody that had previously been immunodepleted (Inset of B). Arrows indicate immunoreactivity at basolateral membranes. Scale bar: 25 μm.
NCBE expression in cultured and freshly dissociated hippocampal neurons
To explore further the question of whether NCBE might be present in neurons, we examined the expression of NCBE in cultured hippocampal neurons. In western blotting of whole-cell lysates of cultured HC neurons, the affinity-purified antibody reveals two major bands, one at ~300 kDa and the other at ~150 kDa (Fig. 9A). The ~300-kDa band, which is also present at a lower intensity in mouse brain (Fig. 4, Fig. 5A) and rat brain (Fig. 7), probably represents a dimer of glycosylated NCBE. As in Fig. 4 and Fig. 7, the lower-MW bands in Fig. 9A presumably represent degradation products, though in Fig. 9A the lower-MW bands have distinctly different MWs than in Fig. 7. When we preincubated the antibody with His-NCBE-Nt, all bands were absent (Fig. 9B). Fig. 9C shows a cultured rat pyramidal neuron, the soma and processes of which stained with affinity-purified antiserum. We found that the antibody similarly stained virtually all pyramidal neurons in culture, as well as many smaller neurons. The antibody did not stain astrocytes (data not shown). Fig. 9D shows another pyramidal neuron at higher magnification, indicating NCBE expression at the plasma membrane of the neuron soma. The staining pattern has a punctate appearance, especially in the processes, as reported by Cooper et al for the closely related NBCn1 protein (Cooper et al., 2005). As shown in Fig. 9E, immunodepleted antiserum did not stain neurons. Fig. 9F shows a DIC image of the neurons shown in panel E.
Fig. 9.
Expression of NCBE in cultured rat hippocampal neurons. (A) Western blot of whole lysate of cultured neurons, in duplicate, probed with affinity-purified antiserum. The arrows indicate bands at ~300 kDa (presumably the highly glycosylated dimer) and at ~150 kDa (presumably the highly glycosylated monomer). (B) Western blot re-probed with affinity-purified antiserum that had previously been immunodepleted with His-NCBE-Nt. This is the same blot as in panel A, but stripped and re-probed. In parallel, we re-probed a third lane from the same blot in panel A (not shown) with the NCBE antiserum, and found that the ~150-kDa and ~300-kDa bands were still present (data not shown). (C) Immunocytochemistry of cultured rat hippocampal neuron with affinity-purified antiserum. Scale bar: 25 μm. (D) A high magnification view of another example of showing the staining of NCBE on the plasma membrane of neuron soma. Scale bar: 20 μm. (E) Parallel immunocytochemical staining of neurons with immunodepleted affinity-purified antiserum. (F) DIC image showing the neurons in panel (E). Arrows indicate neuronal cell bodies. Scale bars in E and F: 25 μm. (G) Representative intracellular pH (pHi) recoveries of cultured hippocampal neurons from acid loads imposed by an prepulse. The two records are from different pyramidal neurons, one exposed to a HEPES buffer (in culture for 21days), and the other exposed to a buffer (in culture for 28 days), and are typical of experiments of 9 neurons in HEPES and of 9 neurons in . The solutions containing were present during the periods indicated by the horizontal black bar.
To verify the health of the cultured pyramidal neurons, we studied their recovery from an acid load. Fig. 9G shows a representative pair of pHi recordings from pyramidal HC neurons, acid loaded by the prepulse technique (Boron and De Weer, 1976). Even though the neuron exposed to has a higher total buffering power (Roos and Boron, 1981), its pHi recovers more rapidly from the acid load, indicating substantial -dependent acid extrusion. Thus, the pHi physiology of these cultured HC neurons is similar to that of freshly dissociated HC neurons (Bevensee et al., 1996; Schwiening and Boron, 1994).
The data in Fig. 9 indicate that the staining of our affinity-purified antibody in culture is specific for neurons and for NCBE, and that the neurons have the expected pHi phenotype.
Because the phenotype of the neurons may have changed during culture, we explored the expression of NCBE in freshly dissociated mouse hippocampal cells. The dissociation process can truncate processes. Therefore, we performed double-staining for neuronal marker MAP2 or astrocytic marker GFAP. Fig. 10A–C show a neuron double-stained with MAP2 (green) and our affinity-purified NCBE antibody (red). Fig. 10D–F show an astrocyte double-stained with GFAP and affinity-purified NCBE antibody. The data indicate that NCBE is expressed in freshly dissociated neurons, but not in astrocytes.
Fig. 10.
Expression of NCBE in freshly dissociated mouse hippocampal neurons. (A-C) Immunocytochemistry of a freshly dissociated HC neurons doubled stained with MAP2 (green) and NCBE antibody (red). (D-F) Immunohistochemistry of a freshly dissociated HC astrocyte double-stained with GFAP (green) and NCBE antibody (red). The results are representative of three experiments. Scale bar: 10 μm.
The above results represent the first evidence—on the protein level—that NCBE is expressed in neurons, not only in culture, but in neurons directly removed from the brain.
Glycosylation of NCBE
Recall that for the oocytes injected with NCBE cRNA, the NCBE antibody recognizes bands at both ~150 kDa and ~127 kDa (Fig. 3). In order to determine if these bands represent differences in the glycosylation of NCBE, we treated a membrane preparation made from 15 oocytes with PNGase F. This enzyme specifically cleaves the N-linked sugar chains on glycoproteins. As shown in Fig. 11A, this treatment collapses the bands into a single band that appears to be at a MW of ~122 kDa (lane 2), consistent with the predicted unglycosylated MW of 125.9 kDa. These results suggest that the two NCBE bands in lane 1 represent highly vs slightly glycosylated proteins.
Fig. 11.
Deglycosylation of the hNCBE heterologously expressed in oocytes. (A) Full-length NCBE without EGFP tag. (B) Full-length NCBE with C-terminal EGFP tag. Membrane-protein extracts from Xenopus oocytes expressing NCBE were treated in the presence or absence of PNGase F at 37 °C for 1 hour. Afterward, 25 μg protein per lane was separated on 4–15% SDS gels, western blotted, and then probed with crude NCBE antibody.
Fig. 11B shows that PNGase F treatment of the fusion protein NCBE-EGFP similarly results in a single band, which runs at an apparent MW of ~150 kDa (see Fig. 3), which is approximately the sum of the predicted MWs of unglycosylated NCBE-B (125.9 kDa) and EGFP plus linker (28 kDa). In other experiments (not shown), we find that NCBE-EGFP is functionally active in oocytes.
Sequence analysis reveals three potential glycosylation motifs (677NGTL, 687NISA and 697NLTV) on the presumed extracellular loop between TM5 and TM6 of NCBE. We generated series of hNCBE mutants to replace the Asn with Gln at these three motifs. When we replace any one of the three Asn residues, leaving the other two intact, the MW of the faint bands (enclosed by the rectangles in Fig. 12A, Lanes 2, 3 and 4) were substantially lower than of the ~150-kDa WT bands (enclosed by the rectangles in Fig. 12A, Lane 1; Fig. 12B, Lane 1, and Fig. 12C). The major bands in Lanes 2, 3 and 4 of Fig. 12A correspond to MWs that are similar to that of the ~127-kDa WT band, which is probably slightly glycosylated (see above comments on Fig. 11A).
Fig. 12.
Western blots of hNCBE N-glycosylation–site mutants, as expressed in oocytes. (A) Wild-type NCBE and the three single mutants (N677Q, N687Q, N697Q). (B) Wild-type NCBE, the three double mutants (N677Q/N687Q, N677Q/N697Q, N687Q/N697Q), and the triple mutant (N677Q/N687Q/N697Q) with a long exposure (4 minutes). (C) Wild-type with a short exposure (10 seconds), from the same blot shown in panel B. 25 μg of total membrane protein was loaded per lane.
For the double mutants, with only one potential glycosylation site intact (Fig. 12B, Lane 2, 3 and 4), western blotting reveals only single bands with MWs similar to that of the ~127-kDa major band of WT NCBE.
For the triple mutant (Fig. 12B, Lane 5), with no potential glycosylation sites intact, we again observe only a single band. Note that the MW of this band is slightly lower than the major band in Lanes 3 and 4. Because the lower expression level of the mutants required a relatively long film exposure (4 minutes) in Fig. 12B, in Fig. 12C we present a short exposure (10 seconds) of Fig. 12B/Lane 1. Consistent with these western-blot data, we were unable to detect pHi recovery from a CO2-induced acid load in oocytes expressing the triple NCBE mutant (not shown).
In summary, the results from Fig. 11 and Fig. 12 suggest that (1) the lower bands for the WT and the three single mutants, as well as the bands for three double mutants represent slightly glycosylated NCBE and (2) the band for the triple mutant represents unglycosylated NCBE.
Fig. 13 shows the deglycosylation of NCBE expressed in the four regions of mouse brain. In light of these results, we can more fully evaluate data presented earlier in the paper. For example, NCBE expressed in mouse brain is mostly N-glycosylated (Fig. 4, Fig. 5, and Fig. 6), especially in CX (Fig. 5A, Lane 1) and HC (Fig. 5A, Lane 4). In SCX and CB, weak bands corresponding to MWs of ~127 kDa may represent slight glycosylation of NCBE (Fig. 5A, Lanes 2 and 3).
Fig. 13.
Deglycosylation of NCBE expressed in mouse brain. Membrane-protein extracts from four different regions of mouse brain—cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC)—were treated in the presence or absence of PNGase F. Afterward, 25 μg protein per lane was separated on 4–15% SDS gel, western blotted, and then probed with crude NCBE antibody. The labels indicate the positions of highly glycosylated NCBE (~150 kDa) and deglycosylated NCBE (~122 kDa).
DISCUSSION
Specificity of the antibody
The sequence alignment (Fig. 1) shows that the first ~120aa of NCBE, NBCn1 and NDCBE are rather similar except for the first 18 residues. However, the new antibody that we describe here—raised against the initial 135 residues of NCBE—still proved to be specific for NCBE (vs NDCBE or NBCn1), both at the level of purified peptides (Fig. 2) and full-length transporters expressed in Xenopus oocytes (Fig. 3). Thus, the real epitope for the anti-NCBE antibody is likely to reside in the first 18 amino acids and/or perhaps some short stretches further downstream (e.g., NCBE residues 28–37, 78–83). To our knowledge, our test of antibody specificity was the first of its kind for an antibody raised against a Na+-coupled transporter.
Tissue distribution of NCBE in brain
By western blotting, NCBE is expressed in all four major brain regions that we tested: cortex, subcortex, cerebellum, and hippocampus (Fig. 5 and Fig. 7). Like Praetorius et al. (Praetorius et al., 2004), we find NCBE by western blotting in samples of finely dissected cerebral cortex and cerebellum macroscopically devoid of choroid plexus (Fig. 6). Moreover, by demonstrating the absence of AQP1 in these microdissected tissues, we verified that these tissues are indeed devoid of choroid plexus. Finally, by comparing finely dissected tissue (i.e., lacking choroid plexus) vs crudely dissected tissue (i.e., containing choroid plexus) in Fig. 6, we can reach an important new conclusion: at the protein level, NCBE expression in the choroid plexus makes only a modest contribution to the total-brain expression of NCBE. In other words, the brain mainly expresses NCBE protein in structures other than the choroid plexus.
In 2003, Giffard et al. (Giffard et al., 2003) published an in-situ hybridization study in which they examined the expression of two NCBE variants in rat brain and spinal cord from embryonic day 19 to postnatal day (P) 15. An NCBE variant with a 90bp insert (cassette A)—in portion of the cDNA corresponding to middle of the cytosolic N terminus (i.e., not part of the immunogen for our antibody)—is apparent at E19 and persists at least to P15. The transcript is present in Purkinje cells of the cerebellum as well as the dentate gyrus and CA regions 1–3 in the hippocampus. Another variant lacking a 39bp insert (cassette B)—corresponding to the extreme C terminus of the protein, and resulting in the creation of a PDZ-binding domain—is more heavily expressed in astrocytes but is also present in neurons.
In 2004, Hübner et al. (Hübner CA et al., 2004) published another in-situ hybridization study, in which they demonstrated the presence of NCBE transcripts in several populations of CNS neurons—as well as in several non-neural tissues such as the choroid plexus—between E12.5 and E18.5.
Previous immunocytochemistry studies of the brain showed NCBE expression only in the basolateral membrane of the choroid-plexus epithelium of adults (Bouzinova et al., 2005; Praetorius et al., 2004; Praetorius and Nielsen, 2006). Our immunocytochemistry data (Fig. 8B) confirm the abundant presence of NCBE in the basolateral membrane of the adult choroid-plexus epithelium, and demonstrate that the protein is also present in the basolateral membrane of choroid-plexus epithelium of the E18 fetus (Fig. 8A).
In cultures of rat hippocampal neurons, we detected abundant NCBE by western blotting (Fig. 9A), and also detected NCBE at the plasma membranes of virtually all cultured pyramidal neurons (Fig. 9C–D). In addition, we detected NCBE protein at the plasma membrane of freshly dissociated neurons (Fig. 10A–C), but not in astrocytes (Fig. 10D–F). To our knowledge, these are the first data to show the expression of NCBE—at the protein level—in a neuron.
It is curious that the relatively small total amount of NCBE protein present in the choroid plexus is easily visible by immunocytochemistry, whereas the relatively large amount elsewhere—readily detected by western blotting—is invisible by immunocytochemistry in fixed sections of brain. It is possible that NCBE is present at especially high density in choroid plexus, or that neuronal NCBE exists in a state that renders the antibody less sensitive than in choroid plexus. Alternatively, the antibody may be more sensitive in a preparation in which individual cells are fully exposed to the fixative and other solutions.
Giffard et al. found by in situ hybridization (Giffard et al., 2003) that NCBE is expressed in cortical astrocytes. On the other hand, our data indicate that NCBE is not expressed in either cultured or freshly dissociated hippocampal astrocytes. Phenotypic differences among astrocytes in different brain regions are well known (Nedergaard et al., 2003).
Glycosylation of NCBE
More than half of all eukaryotic proteins are glycoproteins, and about 90% of these are likely to carry N-linked glycans (Helenius and Aebi, 2004). When we heterologously express wild-type NCBE in Xenopus oocytes, only ~40% is highly N-glycosylated, as evidenced by the 150-kDa band in Fig. 3 and Fig. 11A. The remaining ~60% of NCBE has an apparent MW of ~127 kDa, which may represent core glycosylation (Helenius and Aebi, 2004; Ruddock and Molinari, 2006). On the other hand, in mouse brain (Fig. 4, Fig. 5, Fig. 6) and in rat brain (Fig. 7), almost all NCBE is highly N-glycosylated. Previously, Praetorius et al. found that treating NCBE from rat choroid plexus with PNGase F reduced the apparent MW from ~180 to ~140 kDa (Praetorius et al., 2004), consistent with N-glycosylation of NCBE. In Fig. 13, we find that treating with PNGase F reduces the apparent MW of NCBE from ~150 to ~122 kDa (close to the predicted MW of unglycosylated NCBE) in all four regions of mouse brain.
All of the established Na+-coupled transporters in the SLC4 family have multiple consensus N-glycosylation sites in the putative third extracellular loop between TM5 and TM6. NCBE and NBCe1-A (an electrogenic member of SLC4 family) have three homologous consensus N-glycosylation sites. In NBCe1, disrupting the first of these (N592) has no effect on glycosylation of the transporter heterologously expressed in oocytes (Choi et al., 2003). Disrupting either N597 or N617 has no effect on the fraction of protein that is glycosylated (which remains at ~100%), but each mutation reduces the sugar mass of the remaining glycosylated NBCe1-A by about half. We were surprised to find that the effects of disrupting the consensus N-glycosylation sites of NCBE, heterologously expressed in oocytes, were quite different (Fig. 12). Disrupting any one of the three consensus N-glycosylation sites greatly reduces the fraction of protein that is highly glycosylated, and also markedly reduces the sugar mass of the remaining glycosylated NCBE.
As shown by Fig. 12A and B, even though we loaded equal amounts of total membrane protein on each lane, we detected much less protein for NCBE mutants compared to the WT. Moreover, the larger number of asparagines that we mutated, the greater was the tendency for expression to decrease. In fact, the expression of the triple mutant was so low that we were unable to detect transport activity in oocytes (not shown). Because the expression levels of the single as well as the double mutants (as judged in western blots) was also very low (Fig. 12A and B), it is reasonable to predict that the NCBE transporter activity of the oocytes expressing those mutants will also be undetectable. Because even the single mutants do not traffic efficiently to the plasma membrane, it is presently impossible to know if the NCBE mutants themselves are active. We suggest that all three NCBE N-glycosylation sites are necessary for the efficient folding and subsequent trafficking (Helenius and Aebi, 2004; Ruddock and Molinari, 2006), as well as for efficient remodeling of and additions to the glycan in the Golgi apparatus. Disruption of glycosylation sites can cause protein misfolding by reducing local hydrophilicity and access to the chaperones of the endoplasmic reticulum. The quality-control machinery recognizes such misfolded proteins and targets them to an active protein-degradation process called endoplasmic reticulum-associated degradation or ERAD (Ellgaard and Helenius, 2003; van Anken and Braakman, 2005)—presumably accounting for the decreased expression of NCBE mutants that we observed in oocytes. Because the quality-control machinery is highly conserved (van Anken and Braakman, 2005), it is reasonable to suggest that full glycosylation is also essential for the functional expression of NCBE in mammalian neurons.
Mutational analysis of NBCe1 demonstrates that disrupting all three consensus N-glycosylation sites of NBCe1 yields a protein that is totally unglycosylated, and yet is expressed at high levels in oocytes and maintains electrogenicity, as well as Na+ and dependence (Choi et al., 2003). Like NBCe1, unglycosylated AE1 (SLC4A1) is still functional in Xenopus oocytes (Groves and Tanner, 1994). Thus, compared to these two other SLC4 family members, NCBE is particularly dependent upon glycosylation for efficient folding.
Summary and conclusions
We generated a polyclonal antibody directed against the Nt of NCBE, and confirmed the specificity of the antibody using western blots of purified Nt peptides as well as western blots of heterologously expressed full-length proteins. Using this antibody we studied the distribution of NCBE protein in rodent CNS, showed that NCBE protein is present in brain tissue devoid of choroid plexus, and demonstrated for the first time at the protein level that NCBE is expressed in both cultured and freshly dissociated hippocampal neurons. Finally, by using the new antibody, we demonstrated that the three consensus N-glycosylation sites on the NCBE are essential for the efficient folding and correct trafficking of NCBE protein to membrane.
ACKNOWLEDGEMENTS
We thank Dr. Erlend Nagelhus (Nordic Centre for Water Imbalance Related Disorders and Centre for Molecular Biology and Neuroscience, Institute of Basic Medical Sciences, University of Oslo, P.O. Box 1105, Blindern, N-0317 Oslo, Norway) for his suggestion in tissue dissection.
DISCLOSURES This work was supported by grants NS18400 and HD32573.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol (Lond ) 1996;494:315–328. doi: 10.1113/jphysiol.1996.sp021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J Gen Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer P, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J Physiol (Lond ) 2004;559:85–101. doi: 10.1113/jphysiol.2004.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer P, Zhou Y, Boron WF. An increase in intracellular calcium concentration that is induced by basolateral CO2 in rabbit renal proximal tubule. Am J Physiol Renal Physiol. 2003;285:F674–F687. doi: 10.1152/ajprenal.00107.2003. [DOI] [PubMed] [Google Scholar]
- Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjær C. Na+-dependent HCO3− uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol Cell Physiol. 2005;289:C1448–C1456. doi: 10.1152/ajpcell.00313.2005. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel B, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3. Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1) Am J Physiol Renal Physiol. 2003;284:F1199–F1206. doi: 10.1152/ajprenal.00131.2002. [DOI] [PubMed] [Google Scholar]
- Choi I, Rojas JD, Kobayashi C, Boron WF. Functional charaterization of “NCBE”, an electroneutral Na/HCO3 cotransporter. FASEB J. 2002;16:A796. [Google Scholar]
- Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. Eur J Neurosci. 2003;18:2935–2945. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods in Enzymology. 1992;207:266–279. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
- Groves JD, Tanner MJ. Role of N-glycosylation in the expression of human band 3-mediated anion transport. Mol Membr Biol. 1994;11:31–38. doi: 10.3109/09687689409161027. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Hübner CA, Hentschke M, Jacobs S, Hermans-Borgmeyer I. Expression of the sodium-driven chloride bicarbonate exchanger NCBE during prenatal mouse development. Gene Expression Patterns. 2004;5:219–223. doi: 10.1016/j.modgep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lee YS, Ouyang YB, Giffard RG. Regulation of the rat brain Na+ -driven Cl−/HCO3− exchanger involves protein kinase A and a multiprotein signaling complex. FEBS Lett. 2006;580:4865–4871. doi: 10.1016/j.febslet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neumann M, Gabel D. Simple method for reduction of autofluorescence in fluorescence microscopy. J Histochem Cytochem. 2002;50:437–439. doi: 10.1177/002215540205000315. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol. 2004;286:C601–C610. doi: 10.1152/ajpcell.00240.2003. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol. 2006;291:C59–C67. doi: 10.1152/ajpcell.00433.2005. [DOI] [PubMed] [Google Scholar]
- Romero MF. Molecular pathophysiology of SLC4 bicarbonate transporters. Curr Opin Nephrol Hypertens. 2005;14:495–501. doi: 10.1097/01.mnh.0000168333.01831.2c. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Ruddock LW, Molinari M. N-glycan processing in ER quality control. J Cell Sci. 2006;119:4373–4380. doi: 10.1242/jcs.03225. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurons from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. J Physiol (Lond ) 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006;291:C788–C801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier on the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger: Cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci. 2001;21:2630–2639. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhou Y, Boron WF. Effect of isolated removal of either basolateral HCO3− or basolateral CO2 on HCO3− reabsorption by rabbit S2 proximal tubule. Am J Physiol Renal Physiol. 2003;285:F359–F369. doi: 10.1152/ajprenal.00013.2003. [DOI] [PubMed] [Google Scholar]