Abstract
Large-conductance Ca2+-activated K+ (BKCa, MaxiK) channels are important for the regulation of neuronal excitability. Peripheral nerve injury causes plasticity of primary afferent neurons and spinal dorsal horn neurons, leading to central sensitization and neuropathic pain. However, little is known about changes in the BKCa channels in the dorsal root ganglion (DRG) and spinal dorsal horn and their role in the control of nociception in neuropathic pain. Here we show that L5 and L6 spinal nerve ligation in rats resulted in a substantial reduction in both the mRNA and protein levels of BKCa channels in the DRG but not in the spinal cord. Nerve injury primarily reduced the BKCa channel immunoreactivity in small- and medium-sized DRG neurons. Furthermore, although the BKCa channel immunoreactivity was decreased in the lateral dorsal horn, there was an increase in the BKCa channel immunoreactivity present on dorsal horn neurons near the dorsal root entry zone. Blocking the BKCa channel with iberiotoxin at the spinal level significantly reduced the mechanical nociceptive withdrawal threshold in control and nerve-injured rats. Intrathecal injection of the BKCa channel opener NS1619 dose dependently reversed allodynia and hyperalgesia in nerve-ligated rats but it had no significant effect on nociception in control rats. Our study provides novel information that nerve injury suppresses BKCa channel expression in the DRG and induces a redistribution of BKCa channels in the spinal dorsal horn. BKCa channels are increasingly involved in the control of sensory input in neuropathic pain and may represent a new target for neuropathic pain treatment.
Keywords: Calcium-activated potassium channels, neuropathic pain, synaptic transmission, dorsal root ganglion, analgesia, spinal cord
Introduction
Neuropathic pain remains a significant clinical problem and is often resistant to conventional analgesics. Peripheral nerve injury elicits several changes in the nervous system, including the spontaneous activity from the injured peripheral nerve and dorsal root ganglion (DRG) neurons (Wall and Devor 1983; Kajander and Bennett 1992; Matzner and Devor 1994) and hypersensitivity of spinal dorsal horn neurons (Laird and Bennett 1993; Leem et al. 1996). These abnormal neuronal activities play an important role in the development of hyperalgesia and allodynia associated with neuropathic pain. However, the molecular mechanisms underlying the increased excitability of neurons in the DRG and spinal dorsal horn after nerve injury are not fully understood.
Ca2+-activated K+ channels are expressed in many different types of neurons and are involved in the regulation of neuronal excitability and function (Poolos and Johnston 1999; Martinez-Pinna et al. 2000; Pedarzani et al. 2000). These channels can be divided into three main subfamilies on the basis of their electrophysiological, pharmacological, and molecular profiles: large-conductance Ca2+-activated K+ (BKCa, MaxiK) channels, intermediate-conductance Ca2+-activated K+ (IKCa) channels, and small-conductance Ca2+-activated K+ (SKCa) channels (Sah 1996; Vergara et al. 1998). All these three types are present in DRG neurons (Zhang et al. 2003; Bahia et al. 2005; Mongan et al. 2005). Ca2+-activated K+ channels couple the membrane potential to the intracellular Ca2+ concentration in such a way that an increase in internal Ca2+ leads to an efflux of K+ and a subsequent hyperpolarization of the membrane. Because of this property, these channels can link intracellular Ca2+ and membrane excitability and thus are important for regulating action potential frequency, duration, and inter-burst intervals in neurons (Faber and Sah 2003a).
BKCa channels represent a unique superfamily of K+ channels that are both voltage- and intracellular Ca2+-dependent (Gribkoff et al. 2001; Xia et al. 2002; Zhang et al. 2003). The main functions of BKCa channels in DRG neurons are to shorten the action potential duration, enhance the rate of repolarization, and contribute to rapid after-hyperpolarization, which could lead to reduced repetitive firing (Scholz et al. 1998; Zhang et al. 2003). Activation of BKCa channels can reduce depolarization- and 4-aminopyridine-induced neuronal hyperexcitability in DRG neurons (Zhang et al. 2003). However, little is known about how nerve injury affects the expression and distribution of BKCa channels in DRG neurons and the spinal dorsal horn. Furthermore, the functional significance of BKCa channels in the control of nociceptive processing in chronic neuropathic pain is not known. Therefore, in the present study, we examined the expression and spatial distribution of BKCa channels in DRG neurons and the spinal dorsal horn after nerve injury in rats. We also determined the functional role of BKCa channels at the spinal level in the control of nociceptive input in a rat model of neuropathic pain.
Materials and Methods
Induction of neuropathic pain and implantation of intrathecal catheters
Male Harlan Sprague-Dawley rats weighing 150–180 g were used for neuropathic pain induction. Ligation of the left L5 and L6 spinal nerves in rats was used in this study as an experimental model of neuropathic pain because it produces profound and sustained tactile allodynia, which resembles the condition observed in patients with neuropathic pain (Kim and Chung 1992). Under isoflurane (2–3%) anesthesia, the left L5 and L6 spinal nerves were isolated and ligated tightly with 4.0 silk suture, as described previously (Kim and Chung 1992). The animals were allowed to recover for 7–10 days before intrathecal cannulation. Age-matched sham control rats were prepared as described above except that the nerve was not ligated.
Intrathecal catheters (PE-10 polyethylene tubing) were advanced 8 cm caudal through an incision in the cisternal membrane and secured to the musculature at the incision site. Only animals with no evidence of neurologic deficits after catheter insertion were studied. Final experiments were conducted 3–4 weeks after spinal nerve ligation. The surgical preparation and experimental protocols were approved by the Animal Care and Use Committee of the University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Real-time RT-PCR analysis of BKCa channel mRNA
Total RNA was extracted from the L5 and L6 DRGs and lumbar dorsal spinal cord in nerve-ligated and control rats using Purelink total RNA purification system (Invitrogen, Carlsbad, CA) with on-column DNase I digestion according to the manufacturer’s instruction. For each sample, 2 µg of total RNA was reverse transcribed for 60 min at 37EC with random primers and M-MLV reverse transcriptase. cDNA was prepared by using Superscript III first strand synthesis kit (Invitrogen, Carlsbad, CA). Quantitative PCR was performed by using the iQ5 real-time PCR detection system with SYBR Green PCR kit (Bio-Rad, Hercules, CA). All samples were run in duplicate using an annealing temperature of 60EC, and the same experiment was repeated at least once. The primer pairs used were as follows: BKCa-α1 (NM_031828), 5'-GATGGGCCCTTTGCGGACTTAG-3' (3835–3856) and 5'-CTGGCTGGGGGTGCT GAGGT-3' (3966-3947); rat ribosomal protein 18 (S18) (NM_213557), 5'-ACTTTTGGGGCCTTCGTGTC-3' (419–438) and 5'-GCCCAAAGACTCATTTCTTCTTG-3' (514-492). To calculate the relative expression level of BKCa-α1 mRNA in each sample, standard curves were generated by a two-fold dilution of the cDNA sample of the spinal cord or DRGs as the PCR templates. The relative amount of BKCa-α1 mRNA in each sample was normalized to the housekeeping gene S18 (the mRNA level of the BKCa channel in the control rat was considered as 1). The PCR specificity was verified by the melting curve analysis and agarose gel electrophoresis.
Quantification of BKCa channel proteins using Western blotting
To quantify the BKCa channel changes induced by nerve injury, rats underwent L5 and L6 spinal nerve ligation or sham surgery under isoflurane-induced anesthesia. After 3 weeks of recovery, the mechanical allodynia threshold was confirmed with von Frey filaments. Rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, ip). The rats were then decapitated, and the left L5 and L6 DRGs and lumbar spinal cords were removed. The spinal cords at the L5 and L6 levels were dissected and sectioned into quadrants to obtain the ipsilateral dorsal spinal cord. All tissues were individually frozen on dry ice and stored at −70EC.
Isolated DRG and spinal cord tissues were homogenized in an ice-cold buffer containing 10 mM HEPES, 1 mM EGTA, 0.1 mM EDTA, 10% sucrose, and protease inhibitor cocktail (Sigma, St. Louis, MO). The homogenate was centrifuged at 1,000 g for 10 min at 4EC, and the supernatant was then centrifuged 100,000 g for 1 hour at 4EC. The resulting pellet was resuspended with 100 µl of lysis buffer containing 1% triton X-100, 20 mM MOPS, 4.5 mM Mg(CH3COO)2, 150 mM KCl, and protease inhibitor cocktail. The suspension was centrifuged at 1,000 g for 5 min at 4EC. The supernatant was collected and the protein concentration was determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Thirty µg of proteins were mixed with an equal volume of sodium dodecyl sulfate (SDS) sample buffer (125 mM Tris, pH: 6.8; 4% SDS, 0.02% bromophenol blue, 20% glycerol, and 5% β-mercaptoethanol). The samples were heated for 10 min at 70EC.
The samples were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane (Chen and Pan 2003). The membrane was blocked for 2 hours in 5% nonfat milk in PBS and then incubated with the rabbit anti-BKCa channel α subunit primary antibody (APC-021, Alomone Labs, Israel; dilution:1:300) for 24 hours at 4EC. The specificity of this antibody has been confirmed by preadsorption (Adamson et al. 2002a; Douglas et al. 2006) and further validated using brain tissues from the BKCa knockout mice (Ruttiger et al. 2004; Misonou et al. 2006). For the protein loading control, some membranes were incubated with a rabbit anti-β-actin antibody (1:2000, Sigma; St. Louis, MO). The membrane was rinsed and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) at 1:5,000 dilution for 2 hours at room temperature. The membrane was developed with an enhanced chemoluminescence kit according to the manufacturer's instructions. The intensity of bands was captured digitally and analyzed quantitatively with AIS software (Imaging Research Inc., London, ON, Canada).
Immunofluorescence labeling of BKCa channels in the DRG and spinal dorsal horn
Under deep anesthesia with sodium pentobarbital (60 mg/kg, ip), rats were perfused intracardially with 4% paraformaldehyde and 10% sucrose in PBS. The L5 and L6 DRGs and spinal cords were removed quickly, postfixed for 2 hours in the same fixative solution and cryoprotected in 30% sucrose in PBS for 48 hours at 4EC. The sections were cut to 30 µm for the DRG tissue and 25 µm for the spinal cord and collected free-floating in 0.1 M PBS. For BKCa channel immunofluorescence staining, the sections were incubated with the rabbit anti-BKCa channel α subunit primary antibody (1:500 dilution) in PBS containing 0.3% TX-100 for 2 hours at room temperature and 48 hours at 4EC. Sections were then rinsed and incubated with the secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, dilution: 1:100; Jackson ImmunoResearch Laboratories, West Grove, PA). The sections were then incubated with fluorescein tyramide (PerkinElmer Life Sciences, dilution: 1:100) for 10 min, and incubated with IB4-Alexa Fluor-546 (Molecular probers, Eugene, OR) at 2 µg/ml for 2 hours at room temperature. Finally, the sections were rinsed, mounted on slides, dried, and coverslipped. The negative controls were included by omitting the primary antibody in the above labeling procedures. All sections were examined using a laser scanning confocal microscope (Zeiss, Germany), and the areas of interest were photo-documented. To examine the cell profile of BKCa channel-expressing DRG neurons in the control and nerve-ligated rats, three sections from the L5 DRG (one DRG per rat, n = 5 rats per group) were sampled. Neurons with evident nucleus localization near the center of the cells were measured for the diameter and counted.
Double immunofluorescence labeling of BKCa and NeuN in the spinal cord sections
To determine whether BKCa channels are expressed in spinal dorsal horn neurons after nerve injury, we performed double immunofluorescence labeling of the BKCa channel and NeuN, a neuronal marker, in the spinal cord sections. The tissue sections were first incubated with 1% H2O2 for 30 min to quench the endogenous peroxidase. Sections were blocked with 5% blocking reagent (PerkinElmer Life Sciences, Inc., Boston, MA) in 0.1 M Tris-HCl for 1 hour at room temperature. Then the sections were incubated with the primary antibody mixture, including rabbit anti-BKCa α subunit (Alomone) and mouse anti-NeuN (Chemicon, Temecula, CA; dilution:1:50), for 2 hours at room temperature and overnight at 4EC. Subsequently, sections were incubated with the secondary antibody mixture [peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.; dilution: 1:100) and Alexa Fluor-594-conjugated goat anti-mouse IgG (Molecular Probes; dilution 1:400)] for 2 hours at room temperature. The sections then were rinsed and incubated with fluorescein tyramide (PerkinElmer Life Sciences, Inc; dilution: 1:100) for 10 min. After rinsing, the sections were mounted on slides, dried, and coverslipped. The negative controls were included by omitting the primary antibodies.
Behavioral tests of nociception
Thermal nociception
Rats were placed on the glass surface of a thermal plantar testing apparatus (IITC Life Science, Woodland Hills, CA). The rats were allowed to acclimate for 30 min before testing. The temperature of the glass surface was maintained at a constant 30EC. A mobile radiant heat source located under the glass was focused onto the hindpaws of the rats. The paw withdrawal latency was recorded by a digital timer. The withdrawal latencies for the left and right paws were averaged, and the mean value was used to indicate the sensitivity to noxious heat stimulation. A cut-off of 30 s was used to prevent potential tissue damage (Chen and Pan 2006).
Mechanical nociception
The nociceptive mechanical withdrawal thresholds, expressed in grams, were measured with the Randall-Selitto test using an Ugo Basil Analgesimeter (Varese, Italy). The test was performed by applying noxious pressure to the hindpaw. We pressed a pedal that activated a motor, the force of which increased at a constant rate on a linear scale. When the animal displayed pain by paw withdrawal or vocalization, the pedal was immediately released and the nociceptive threshold was read on the scale. A cut-off of 400 g was used to avoid potential tissue injury (Chen and Pan 2006). Both hindpaws were tested on each rat, and the mean value was used as the withdrawal threshold in response to the noxious pressure.
Tactile allodynia
The tactile threshold was determined by the application of von Frey filaments to the left hindpaw before and after spinal nerve ligation in all animals. To quantify mechanical sensitivity of the paw, rats were placed in individual plastic boxes on a mesh floor and allowed to acclimate for 30 min. A series of calibrated von Frey filaments (Stoelting Co., Wood Dale, IL) were applied perpendicularly to the plantar surface of the left paw with sufficient force to bend the filaments for 6 s. Brisk withdrawal or paw flinching was considered a positive response, and a filament of the next lower force was applied. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the “up-down” calculation method, as described in detail previously (Chaplan et al. 1994). Each trial was repeated 2–3 times at approximately 2-min intervals, and the mean value was used as the force needed to produce a paw withdrawal response.
In the behavioral tests described above, motor function was evaluated by the placing/stepping and the righting reflexes. The former was evoked by drawing the dorsum of either hindpaw across the edge of the table. The latter was assessed by placing the rat horizontally with its back on the table, which normally gives rise to an immediate coordinated twisting of the body into an upright position. Changes in motor function were scored as follows: 0, normal; 1, slight deficit; 2, moderate deficit; and 3, severe deficit.
Drug administration
After the baseline was measured, rats were treated with intrathecal 5, 10, or 20 µg of NS1619. The doses of the specific BKCa channel opener NS1619 used in this study were determined in the pilot study. The paw withdrawal latency in response to thermal stimulation and the paw withdrawal thresholds in response to the pressure or von Frey filaments applied to the hindpaws were determined individually for different groups of rats. The effects of NS1619 were determined every 15 or 30 min for 120–180 min after intrathecal injection. To determine the specific effect of NS1619, the highly selective BKCa channel blocker iberiotoxin (10 ng) (Lee et al. 1995; Gribkoff et al. 2001) was administered 15 min before 20 µg of NS1619 was injected in another group of nerve-ligated rats. Drugs for intrathecal injections were dissolved in normal saline and administered in a volume of 5 µl, followed by a 10-µl flush with normal saline. NS1619 and iberiotoxin were purchased from Sigma (St. Louis, MO).
Statistical analysis
Data are presented as means ± SEM. The differences in the BKCa channel mRNA and protein levels in the spinal cords and DRGs between the control and nerve-injured groups were determined by paired or unpaired Student t-test. Paw withdrawal thresholds in response to thermal and pressure stimuli before and after drug treatment were compared using repeated measures analyses of variance followed by Tukey’s post hoc test. The drug effects on the tactile withdrawal threshold were determined by using Kruskal-Wallis test. Differences were considered to be statistically significant when P < 0.05.
Results
BKCa protein levels in the DRG and dorsal spinal cord in nerve-ligated rats
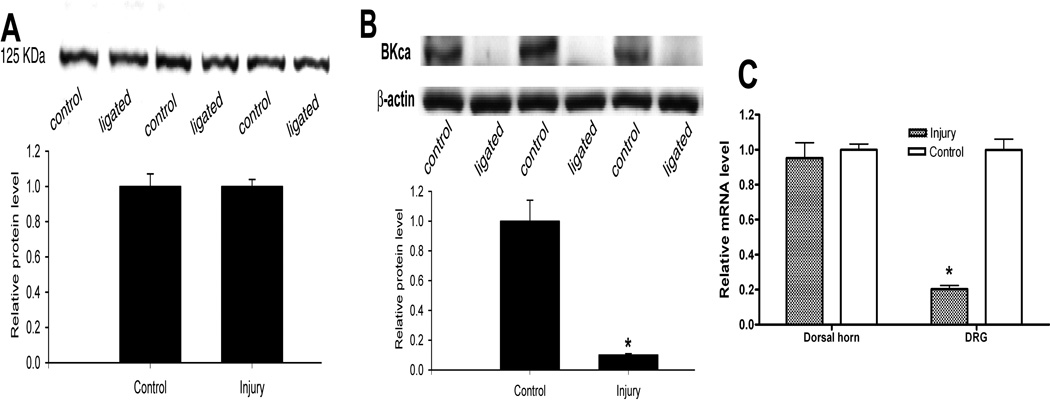
The BKCa channel protein in the sham and nerve-injured rats was quantified using Western blot. There was a robust expression of a single protein band at 125 kDa, corresponding to the size of the BKCa channel protein. To determine the nerve injury-induced changes in the spinal cord, the protein band density in the injured group was normalized to the sham control (the protein amount of BKCa channels from the sham rats was considered as 1). The protein level of BKCa channels in the dorsal spinal cord was not significantly different between the sham control and nerve-ligated groups (n = 9 rats in each group, Fig. 1A). However, there was a large reduction in the protein amount of BKCa channels in the L5 and L6 DRGs in the nerve-injured group, compared with that in the sham control group (n = 9 in each group, Fig. 1B).
Fig. 1.
Quantification of the protein and mRNA levels of BKCa channels in the DRG and dorsal spinal cord in control and nerve-injured rats. A: representative gel images and group data show the lack of significant changes in the BKCa channel protein level in the dorsal spinal cord between the control and nerve-ligated rats (n = 9 rats in each group). B: representative gel images and summary data show the changes in the BKCa channel protein level in L5 and L6 DRGs between the control and nerve-ligated rats (n = 9 rats in each group). Note that β-actin was used as a loading control. C: group data show a differential change in the mRNA levels of BKCa channels in the L5 and L6 DRGs and dorsal spinal cord in nerve-injured and control rats. * P < 0.05 compared with the value in the control group.
BKCa mRNA levels in the spinal cord and DRGs in nerve-injured rats
To investigate whether peripheral nerve injury changes the expression level of BKCa channel mRNA, we used real-time PCR to measure the mRNA level of the pore forming BKCa channel α-subunit. The mRNA level of BKCa channels in the DRGs of nerve-injured rats was substantially reduced compared with that in the control rats (Fig. 1C). However, there was no significant difference in the mRNA level of BKCa channels in the spinal cord between the control and nerve-injured groups.
Changes in BKCa channel immunoreactivity in injured DRG neurons
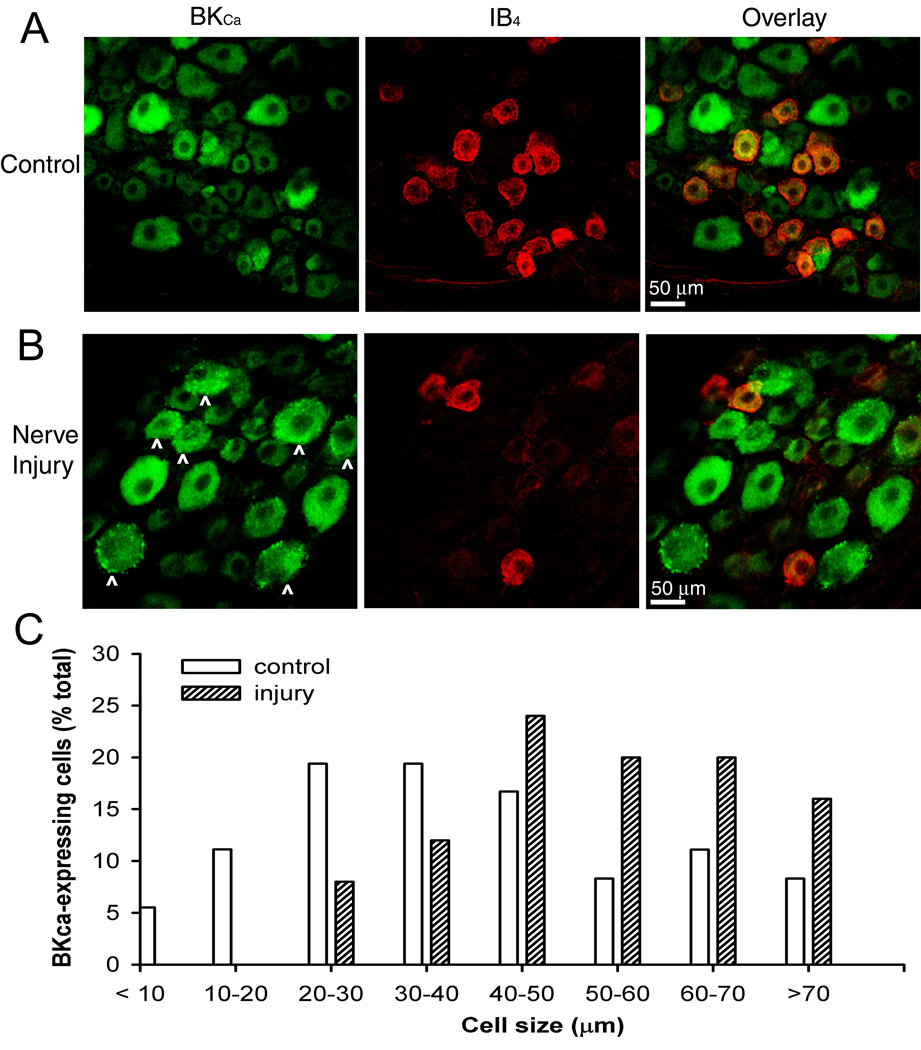
We determined whether the spatial distribution of BKCa channels in the spinal cord and DRG was altered by nerve injury. In the DRGs of sham-operated rats, BKCa channel immunoreactivity was present in different sizes of neurons (Fig. 2A). Approximately 43% of IB4-positive DRG neurons showed BKCa channel immunoreactivity. In addition, BKCa channel immunoreactivity in the cytoplasm was largely uniform in DRG neurons from control rats. By contrast, in the ipsilateral DRGs removed from nerve-ligated rats, there was a substantial loss of IB4-positive DRG neurons (approximately 67%; 1,124 cells in the control group vs. 368 cells in the nerve-ligated group) (Fig. 2B). Notably, BKCa channel immunoreactivity was dense and appeared aggregated in the cytoplasm and cell membrane of the ipsilateral DRG neurons in nerve-injured rats (Fig. 2B). Analysis of distribution of BKCa-immunoreactive neurons revealed that BKCa channel immunoreactivity was primarily present in large-diameter (> 50 µm) DRG neurons in nerve-injured rats (Fig. 2C). Omitting the primary BKCa antibody or preadsorption of the BKCa antibody with the control antigen did not result in evident BKCa labeling (data not shown). Considering our findings from the real-time RT-PCR and Western blot analysis, these immunocytochemical data suggest that decreased expression of BKCa channels by spinal nerve injury primarily occurs to small- and medium-sized DRG neurons (Fig. 2C).
Fig. 2.
Representative confocal images show changes in BKCa channel immunoreactive- and isolectin B4 (IB4)-positive dorsal root ganglion (DRG) neurons in a control rat (A) and a nerve-injured rat (B). Co-localization of the BKCa channel immunoreactivity and IB4 is indicated in yellow when the two images are digitally merged. Note that dense and aggregated BKCa channel immunoreactivity appears in medium- and large-sized DRG neurons (marked with ^) in the nerve-injured rat. All images are single confocal optical sections. C, histograms show the somatic size distribution of BKCa channel immunoreactive-neurons in the left L5 DRGs from the control and nerve-ligated rats (n = 5 rats in each group, one section per DRG). A total of 262 and 253 neurons was counted from the control and nerve-injured rats, respectively.
Nerve injury-induced changes in BKCa channel immunoreactivity in the spinal dorsal horn
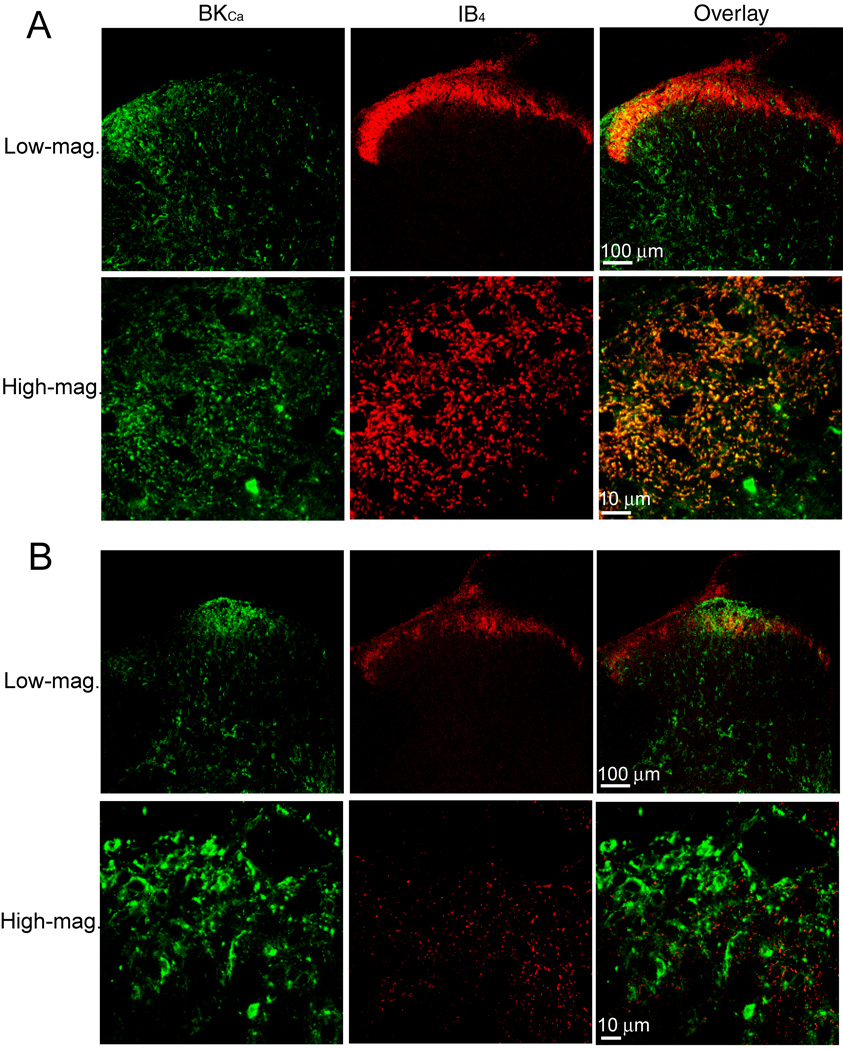
In the spinal cords of sham control rats, the BKCa channel immunoreactivity was distributed throughout the spinal dorsal horn, especially in the lateral side of laminae I-III. Dense IB4-labeling was restricted to laminae I and II in the spinal cords of control rats. BKCa channel immunoreactivity was particularly dense in the lateral superficial dorsal horn and was co-localized with IB4-positive nerve terminals in this region (Fig. 3A). By contrast, a large reduction in IB4-labeling in the superficial dorsal horn was observed in the ipsilateral spinal cord from nerve-ligated rats. Strikingly, there was a distinct redistribution of BKCa channel immunoreactivity in the superficial dorsal horn of the ipsilateral spinal cord in nerve-ligated rats. There was a marked reduction in BKCa channel immunoreactivity in the lateral dorsal horn of the ipsilateral spinal cord. But dense labeling of BKCa channel immunoreactivity appeared near the center of the superficial dorsal horn, especially at the dorsal root entry zone in nerve-ligated rats (Fig. 3B). However, increased BKCa channel immunoreactivity was not co-localized with the remaining IB4-positive afferent terminals in the spinal cord of nerve-injured rats. High-magnification confocal images show that increased BKCa channel immunoreactivity appeared in many interneuron-like structures in this region (Fig. 3B).
Fig. 3.
Representative confocal images show the distribution of BKCa channel immunoreactivity and isolectin B4 (IB4) labeling in the spinal dorsal horn of a control rat and a nerve-injured rat. A: confocal images showing BKCa channel immunoreactivity (green) and IB4-positive afferent terminals (red) in the spinal dorsal horn from a control rat. Co-localization of the BKCa channel immunoreactivity and IB4 in the lateral dorsal horn is shown in high-magnification confocal images. B: confocal images showing changes in BKCa channel immunoreactivity and IB4-positive afferent terminals in the spinal dorsal horn from a nerve-injured rat. Note that the BKCa channel immunoreactivity is decreased in the lateral horn but increased in the central region near the dorsal root entry zone in the nerve-injured rat. Co-localization is indicated in yellow when the two images are digitally merged. All images are single confocal optical sections.
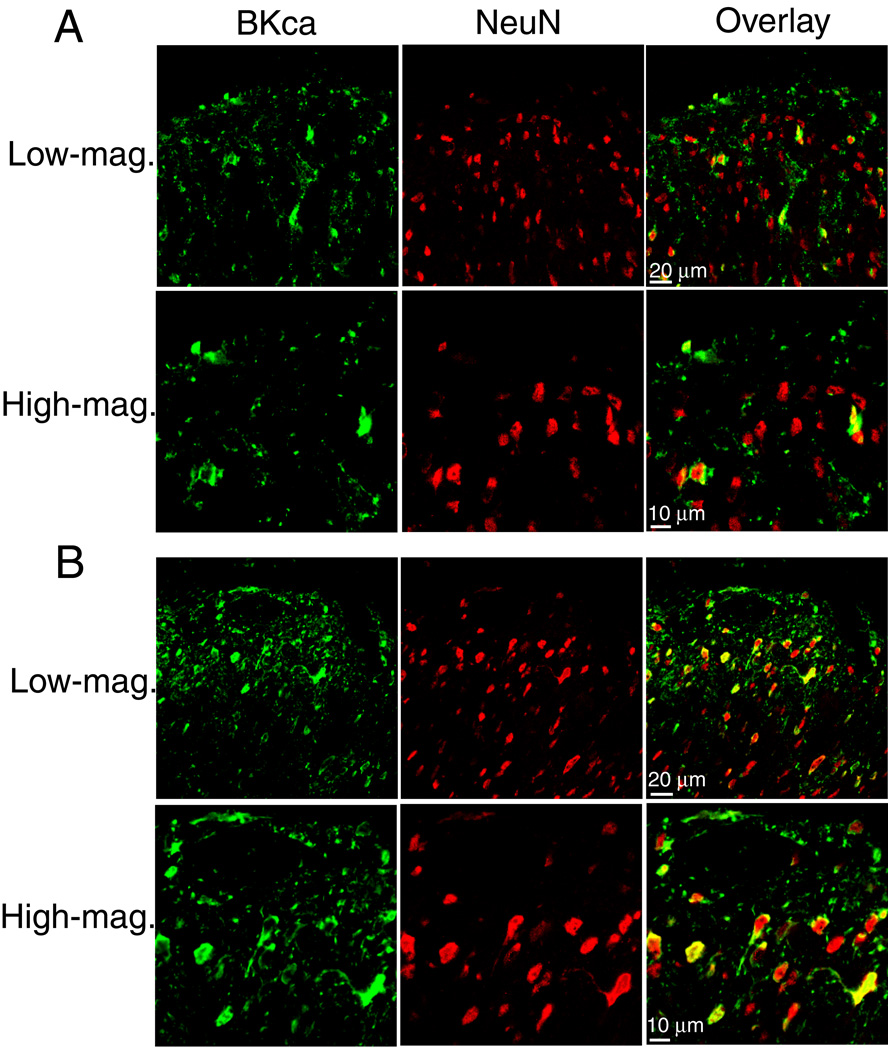
Double immunofluorescence labeling of NeuN and BKCa channels was performed to determine whether the increased BKCa channel immunoreactivity is expressed in dorsal horn neurons in nerve-injured rats. Colocalization of NeuN and BKCa channel immunoreactivities appeared in only a few neurons in laminae I-III of sham control rats. In contrast, the majority of NeuN-positive dorsal horn neurons near the dorsal root entry zone exhibited the BKCa channel immunoreactivity in the ipsilateral spinal cord of nerve-injured rats (Fig. 4). To quantify the number of BKCa− and NeuN-immunoreactive neurons in the dorsal horn, 9 confocal images were randomly selected from spinal cord tissue sections in three control and three nerve-ligated rats, and the total number of double-labeled neurons was counted from 9 sections in each group. This analysis showed that there was a three-fold increase (197 in the control group vs. 602 in the nerve-injured group) in the number of dorsal horn neurons that had both BKCa− and NeuN-immunoreactivities in nerve-injured animals.
Fig. 4.
Representative confocal images show the distribution of BKCa channel immunoreactive and the neuronal marker NeuN in the spinal dorsal horn of a control rat and a nerve-injured rat. A: confocal images showing BKCa channel immunoreactivity (green) and NeuN immunoreactivity (red) in the spinal dorsal horn from a control rat. Co-localization of the BKCa channel and NeuN immunoreactivities in the dorsal horn is shown in high-magnification confocal images. B: confocal images showing changes in BKCa channel immunoreactivity and NeuN immunoreactivity in the dorsal horn from a nerve-injured rat. Note that high-magnification confocal images revealed that BKCa channel immunoreactivity appeared in the majority of NeuN-immunoreactive neurons in the superficial dorsal horn in the nerve-injured rat. All images are single confocal optical sections.
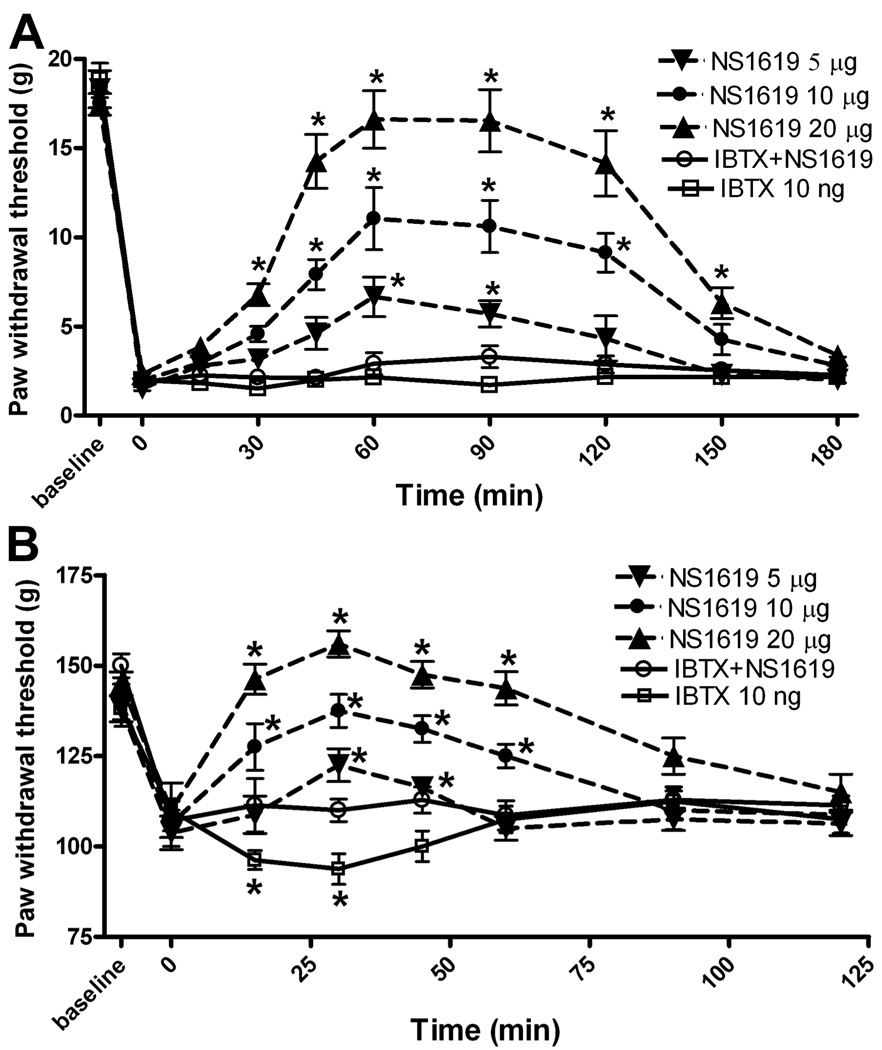
Effect of intrathecal NS1619 on hyperalgesia and allodynia in nerve-ligated rats
The hindpaw withdrawal threshold tested with von Frey filaments before spinal nerve ligation was 19.6 ± 1.4 g. The tactile threshold decreased significantly (2.1 ± 0.3 g, Fig. 5A) 2 weeks after nerve ligation and was stable for the duration of the study in all animals tested. The nociceptive mechanical threshold, measured with the Randall-Selitto pressure device, was also significantly lower in nerve-injured rats than in sham control rats 2 weeks after surgery (107.1 ± 5.3 g vs. 147.3 ± 8.5 g, Fig. 5B).
Fig. 5.
Effects of intrathecal injection of NS1619 on allodynia and hyperalgesia in nerve-injured rats. A: time course of the effect of intrathecal injection of 5 (n = 8 rats), 10 (n = 8 rats), and 20 (n = 7 rats) µg of NS1619 on the tactile withdrawal threshold, tested with von Frey filaments, in nerve-injured rats. B: time course of the effect of intrathecal injection of 5 (n = 7 rats), 10 (n = 8 rats), and 20 (n = 8 rats) µg of NS1619 on the mechanical threshold, measured with nociceptive pressure stimulus, in nerve-injured rats. Intrathecal injection of 10 ng iberiotoxin alone (IBTX, n = 7 rats) significantly decreased the pressure withdrawal threshold in nerve-injured rats. Data are presented as means ± SEM. *, P < 0.05 compared with the respective pre-drug control (time 0).
NS1619 is a benzimidazolone analog that has been shown to activate BKCa channels in neurons in the DRG and the ventromedial hypothalamus (Sellers and Ashford 1994; Zhang et al. 2003). Intrathecal injection of 5, 10, and 20 µg of NS1619 caused a dose-dependent increase in the paw withdrawal threshold in response to the application of von Frey filaments. This effect appeared within 45 min and lasted for more than 120 min after intrathecal injection. Intrathecal injection of 10 ng of iberiotoxin, a highly selective blocker of BKCa channels (Lee et al. 1995; Gribkoff et al. 2001), 15 min before injection of 20 µg NS1619 abolished the antiallodynic effect of intrathecal NS1619 (Fig. 5A). Intrathecal administration of 10 ng of iberiotoxin alone did not significantly change the tactile withdrawal threshold.
In another group of nerve-injured rats, intrathecal injection of 5, 10, and 20 µg of NS1619 significantly increased the paw withdrawal threshold, as measured by the application of a noxious pressure stimulus, in a dose-dependent manner (Fig. 5B). The maximal effect appeared within 30 min and lasted 60–90 min. Notably, intrathecal injection of 10 ng of iberiotoxin alone caused a transient but significant decrease in the nociceptive withdrawal threshold. Pretreatment with 10 ng of iberiotoxin abolished the effect of intrathecal administration of 20 µg of NS1619 on the pressure withdrawal threshold in nerve-injured rats (Fig. 5B). No animals that had been given an intrathecal injection of NS1619 or iberiotoxin exhibited signs of sedation or motor dysfunction, as assessed by the placing/stepping and the righting reflexes.
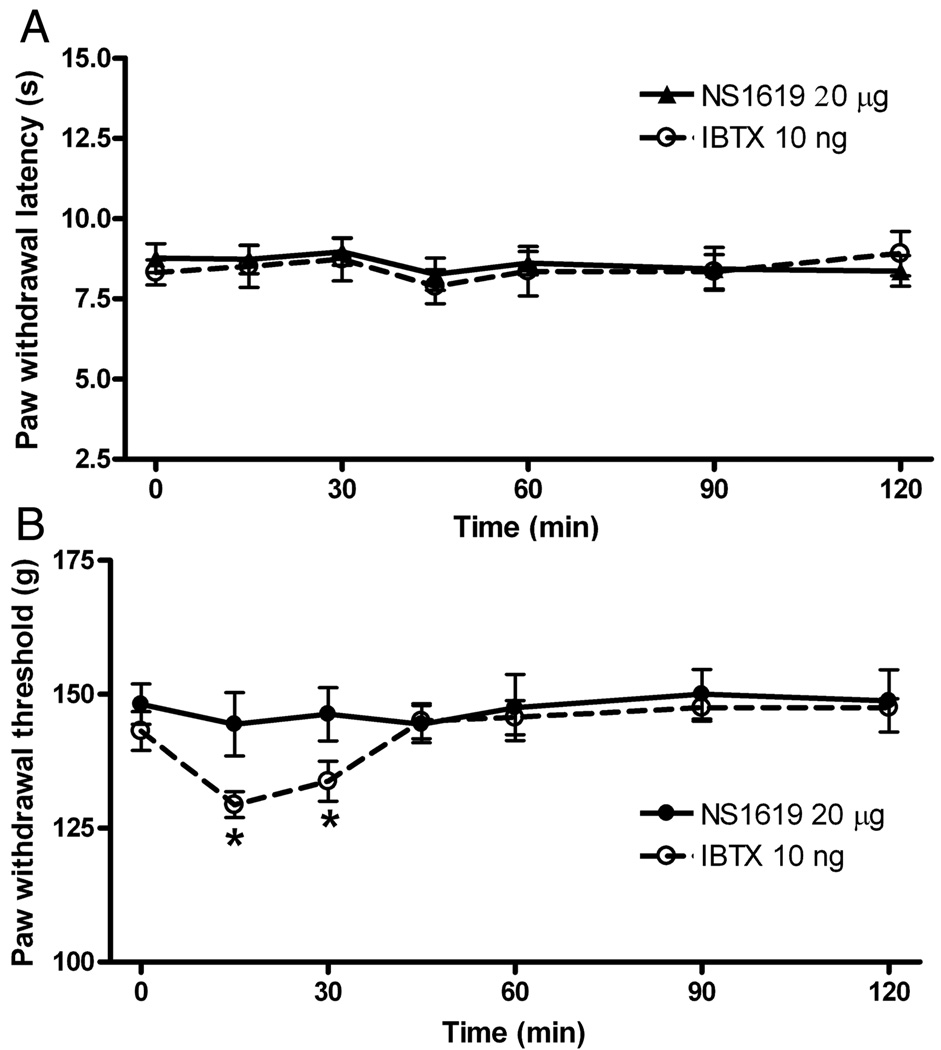
Effect of intrathecal NS1619 on nociception in control rats
The effect of NS1619 on the thermal nociceptive threshold was tested only in control rats because thermal hyperalgesia is not an evident feature in this rat model of neuropathic pain (Kim and Chung 1992). In age-matched control rats, the intrathecal injection of NS1619 up to 20 µg did not significantly increase the paw withdrawal latency in response to the noxious heat stimulus (Fig. 6A). Furthermore, intrathecal administration of 20 µg of NS1619 did not significantly alter the paw withdrawal threshold in response to the noxious pressure stimulus in control rats (Fig. 6B). Interestingly, intrathecal injection of 10 ng of iberiotoxin significantly decreased the nociceptive mechanical, but not the thermal, threshold in control rats (Fig. 6).
Fig. 6.
Effects of intrathecal injection of NS1619 on nociception in control rats. A: lack of effect of intrathecal injection of 20 µg of NS1619 on the withdrawal latency, tested with a noxious heat stimulus, in 8 sham control rats. B: lack of effect of intrathecal injection of 20 µg of NS1619 on the mechanical threshold, measured with nociceptive pressure stimulus, in another 8 control rats. Note that intrathecal injection of 10 ng iberiotoxin alone (IBTX, n = 7 rats) significantly decreased the pressure withdrawal threshold. Data are presented as means ± SEM. *, P < 0.05 compared with the respective pre-drug control (time 0).
Discussion
The major objective of our study was to determine the plasticity and functional role of BKCa channels in the regulation of nociception in chronic neuropathic pain. Using quantitative RT-PCR and Western blots, we found that both the mRNA and protein levels of BKCa channels were significantly reduced in the DRG, but not in the dorsal spinal cord, after nerve ligation. Our immunocytochemical analysis revealed a substantial loss of IB4-positive DRG neurons and a shift in BKCa expression to large DRG neurons in nerve-injured rats, suggesting that decreased BKCa expression in the DRG after nerve injury mainly involves small- and medium-sized neurons. Furthermore, we found that intrathecal administration of the specific BKCa channel activator NS1619 had no significant effect on nociceptive thresholds in control rats but dose dependently attenuated mechanical allodynia and hyperalgesia in nerve-ligated rats. Therefore, our findings suggest that peripheral nerve injury suppresses the expression of BKCa channels in small- and medium-sized DRG neurons and causes a redistribution of BKCa channels within the spinal dorsal horn. Activation of BKCa channels at the spinal level can effectively reverse neuropathic pain.
All three major calcium-activated potassium channels are present in DRG neurons (Boettger et al. 2002; Mongan et al. 2005). Peripheral nerve injury reduces SKCa and IKCa channels in the DRG neurons (Boettger et al. 2002). However, the nerve injury-induced changes in BKCa channels in the DRG and spinal cord are not clear. In the present study, we found that both the mRNA and protein levels of BKCa channels were profoundly reduced in the DRG of nerve-injured rats. The complementary immunohistochemical analysis revealed a large reduction in IB4-positive DRG neurons and a shift in BKca expression to large DRG neurons, suggesting that decreased BKCa expression in the DRG by nerve injury primarily occurs to small- and medium-sized neurons. Consistent with our histologic findings, it has been shown that BKCa channel activity is decreased in axotomized L5 DRG neurons (Sarantopoulos et al. 2007). The loss of DRG neurons is probably due to Wallerian degeneration induced by spinal nerve ligation (Peyronnard et al. 1989; Tandrup et al. 2000). Interestingly, in large-diameter DRG neurons in nerve-ligated rats, we found that BKCa channel immunoreactivity was aggregated in the cytoplasm and cell membrane. In our study, BKCa immunoreactivity was detected in the cytoplasm of DRG neurons, suggesting that the BKCa protein is not restricted only to the membrane surface of DRG neurons. Consistent with this observation, the BKCa protein is present in the cytoplasm of different types of neurons (Adamson et al. 2002b; Martin et al. 2004; Ruttiger et al. 2004; Misonou et al. 2006; Sailer et al. 2006), such as the mitochondria (Douglas et al. 2006). Furthermore, functional BKCa channels have been documented in the mitochondrial membrane of human glioma cells (Siemen et al. 1999), skeletal muscle fibers (Skalska et al. 2008), and cardiac myocytes (Xu et al. 2002; Sato et al. 2005). Additionally, the BKCa channel mRNA is present in the cytoplasm of hippocampal neurons, and the cytoplasmic BKCa channel mRNA level can influence the surface expression of the BKCa channel protein and neuronal firing properties (Bell et al. 2008). The development of neuropathic pain induced by peripheral nerve injury is associated with the increased excitability of primary afferent neurons and central sensitization (Wall and Devor 1983; Laird and Bennett 1993; Harris et al. 1996; Leem et al. 1996). Our findings suggest that reduced expression of BKCa channels in small- and medium-sized DRG neurons after nerve injury could contribute to increased excitability of sensory neurons in this animal model of neuropathic pain.
At the spinal cord level, we found that the overall mRNA and protein levels of BKCa channels did not differ significantly between the control and nerve-injured groups. Strikingly, our immunocytochemical labeling revealed a substantial reduction in BKCa channel immunoreactivity in the lateral spinal dorsal horn and a large increase in BKCa channel immunoreactivity at the dorsal root entry zone in spinal nerve-ligated rats. Our immunocytochemical study revealed that many neurons in the superficial dorsal horn expressed dense BKCa channel immunoreactivity after spinal nerve ligation. Thus, the loss of BKCa channels in the lateral dorsal horn after nerve ligation may be compensated for by the upregulation of BKCa channels on dorsal horn neurons near the dorsal root entry zone. This topographical redistribution of BKCa channels in the spinal dorsal horn probably explains why no significant global changes were found in the BKCa channel expression in the spinal dorsal horn after nerve injury. It has been shown that the BKCa mRNA level in the mouse spinal cord is significantly reduced after nerve injury (Furukawa et al. 2008). However, in the study by Furukawa et al, only the superficial dorsal horn was sampled for the RT-PCR experiment. We used the entire dorsal half of the rat spinal cord for both the RT-PCR and Western blots. Because the nerve injury-induced changes in BKca expression in the spinal dorsal horn are highly localized, this could explain the different results between the work by Furukawa et al. and our own study. The mechanisms underlying the plasticity of BKCa channel expression after nerve injury are not clear. Neurotrophin-3 and nerve growth factor can increase the number of BKCa channels in central neurons (Holm et al. 1997). In addition, glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor stimulate the functional activity of BKCa channels in neurons (Martin-Caraballo and Dryer 2002; Mizoguchi et al. 2002). In the spinal dorsal horn, the level of brain-derived neurotrophic factor is increased after nerve ligation injury (Miletic and Miletic 2002). Thus, the increased neurotrophin level may play a role in the upregulation of BKCa channels in some dorsal horn neurons after nerve injury.
Another salient finding of our study is that intrathecal injection of the specific BKCa channel activator NS1619 dose dependently reduced mechanical allodynia and hyperalgesia in nerve-ligated rats. Pretreatment with the selective BKCa channel blocker iberiotoxin abolished the effects of NS1619. BKCa channels are involved in afterhyperpolarization in excitable cells (Poolos and Johnston 1999; Pedarzani et al. 2000). The suppression of neuronal firing activity by NS1619 can be achieved through an increase in the threshold for action potential firing and an increase in the amplitude of afterhyperpolarization (Zhang et al. 2003). Reduced BKCa channel expression in injured DRG neurons and in the lateral dorsal horn of the spinal cord may contribute to the hypersensitivity of these neurons after nerve injury. Furthermore, activation of BKCa channels requires simultaneous depolarization and elevated cytosolic Ca2+, which necessitates that electrical activity be coupled to changes in intracellular Ca2+ (Sah 1996; Xia et al. 2002). Activation of BKCa channels can also reduce the amount of neurotransmitters released to the spinal dorsal horn neurons by shortening the duration of depolarization that allow Ca2+ entry through voltage-gated Ca2+ channels (Hu et al. 2001; Faber and Sah 2003b; Raffaelli et al. 2004). As a result, opening of BKCa channels could suppress the hyperactivity of spinal dorsal horn neurons. We observed that intrathecal injection of NS1619 had no significant effect on the nociceptive threshold in control rats, suggesting that further BKCa channel activation does not affect nociceptive transmission under normal conditions. In nerve-ligated rats, the nociceptive input may be primarily transmitted by remaining DRG neurons and afferent terminals, in which BKCa channels remained functional. This could explain why opening BKCa channels with NS1619 is effective at reducing neuropathic pain caused by nerve injury. It has been shown that the intracellular Ca2+ level is decreased in DRG neurons in nerve-injured rats (Fuchs et al. 2005), which is not favorable for BKCa channel activation. Notably, in both control and nerve-injured rats, the specific BKCa channel blocker iberiotoxin significantly decreased the nociceptive mechanical threshold. This could be due to that BKCa channels are mainly present on the mechano-sensitive primary afferent neurons. We observed that iberiotoxin alone had no significant effect on the tactile threshold in nerve-ligated rats. This is because the tactile threshold in nerve-injured rats was already very low, and the lack of effect of iberiotoxin alone could be explained by a 'floor' effect. Since the drugs were given intrathecally in the behavioral experiments, it is difficult to determine the relative roles of BKCa channels in the spinal cord and DRG in the control of nociceptive transmission in neuropathic pain. Nevertheless, activation of BKCa channels with NS1619 significantly attenuated neuropathic pain, suggesting that reduced BKCa channel function at the spinal level could contribute to the central sensitization and chronic pain induced by nerve injury.
In summary, our study demonstrates a profound reduction in the expression level of BKCa channels in the DRG and a distinct redistribution of BKCa channels within the spinal dorsal horn after nerve injury. These changes could contribute to increased nociceptive input and central sensitization in neuropathic pain. Because activation of BKCa channels can reverse allodynia and hyperalgesia caused by nerve injury, the BKCa channel at the spinal level represents a new therapeutic target for neuropathic pain treatment.
Acknowledgments
This work was supported by grants GM64830 and NS45602 from the National Institutes of Health and by the MD Anderson’s core grant CA16672 from the National Cancer Institute.
List of abbreviations
- DRG
Dorsal root ganglion
- IB4
Griffonia simplicifolia isolectin B4
- BKCa
Large-conductance Ca2+-activated K+ channels
- NS1619
[1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one]
References
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002a;22:1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002b;447:331–350. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Bahia PK, Suzuki R, Benton DC, Jowett AJ, Chen MX, Trezise DJ, Dickenson AH, Moss GW. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J Neurosci. 2005;25:3489–3498. doi: 10.1523/JNEUROSCI.0597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell TJ, Miyashiro KY, Sul JY, McCullough R, Buckley PT, Jochems J, Meaney DF, Haydon P, Cantor C, Parsons TD, Eberwine J. Cytoplasmic BK(Ca) channel intron-containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:1901–1906. doi: 10.1073/pnas.0711796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger MK, Till S, Chen MX, Anand U, Otto WR, Plumpton C, Trezise DJ, Tate SN, Bountra C, Coward K, Birch R, Anand P. Calcium-activated potassium channel SK1- and IK1-like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain. 2002;125:252–263. doi: 10.1093/brain/awf026. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Antinociceptive effect of morphine, but not mu opioid receptor number, is attenuated in the spinal cord of diabetic rats. Anesthesiology. 2003;99:1409–1414. doi: 10.1097/00000542-200312000-00026. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006;1081:119–125. doi: 10.1016/j.brainres.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139:1249–1261. doi: 10.1016/j.neuroscience.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 2003a;9:181–194. doi: 10.1177/1073858403009003011. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J Physiol. 2003b;552:483–497. doi: 10.1113/jphysiol.2003.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102:1217–1225. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Takasusuki T, Fukushima T, Hori Y. Presynaptic large-conductance calcium-activated potassium channels control synaptic transmission in the superficial dorsal horn of the mouse. Neurosci Lett. 2008;444:79–82. doi: 10.1016/j.neulet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Jr, Dworetzky SI. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 2001;7:166–177. doi: 10.1177/107385840100700211. [DOI] [PubMed] [Google Scholar]
- Harris JA, Corsi M, Quartaroli M, Arban R, Bentivoglio M. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience. 1996;74:7–12. doi: 10.1016/0306-4522(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Holm NR, Christophersen P, Olesen SP, Gammeltoft S. Activation of calcium-dependent potassium channels in mouse [correction of rat] brain neurons by neurotrophin-3 and nerve growth factor. Proc Natl Acad Sci U S A. 1997;94:1002–1006. doi: 10.1073/pnas.94.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Laird JM, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol. 1993;69:2072–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- Lee K, Rowe IC, Ashford ML. NS 1619 activates BKCa channel activity in rat cortical neurones. Eur J Pharmacol. 1995;280:215–219. doi: 10.1016/0014-2999(95)00251-f. [DOI] [PubMed] [Google Scholar]
- Leem JW, Choi EJ, Park ES, Paik KS. N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett. 1996;211:37–40. doi: 10.1016/0304-3940(96)12714-2. [DOI] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, Treistman S. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. J Neurosci. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Caraballo M, Dryer SE. Glial cell line-derived neurotrophic factor and target-dependent regulation of large-conductance KCa channels in developing chick lumbar motoneurons. J Neurosci. 2002;22:10201–10208. doi: 10.1523/JNEUROSCI.22-23-10201.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinna J, Davies PJ, McLachlan EM. Diversity of channels involved in Ca(2+) activation of K(+) channels during the prolonged AHP in guinea-pig sympathetic neurons. J Neurophysiol. 2000;84:1346–1354. doi: 10.1152/jn.2000.84.3.1346. [DOI] [PubMed] [Google Scholar]
- Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- Miletic G, Miletic V. Increases in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci Lett. 2002;319:137–140. doi: 10.1016/s0304-3940(01)02576-9. [DOI] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi Y, Monji A, Nabekura J. Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons. Eur J Neurosci. 2002;16:1417–1424. doi: 10.1046/j.1460-9568.2002.02198.x. [DOI] [PubMed] [Google Scholar]
- Mongan LC, Hill MJ, Chen MX, Tate SN, Collins SD, Buckby L, Grubb BD. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience. 2005;131:161–175. doi: 10.1016/j.neuroscience.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Kulik A, Muller M, Ballanyi K, Stocker M. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J Physiol. 2000;527:283–290. doi: 10.1111/j.1469-7793.2000.t01-1-00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronnard JM, Charron L, Messier JP, Lavoie J, Leger C, Faraco-Cantin F. Changes in lectin binding of lumbar dorsal root ganglia neurons and peripheral axons after sciatic and spinal nerve injury in the rat. Cell Tissue Res. 1989;257:379–388. doi: 10.1007/BF00261840. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:5205–5212. doi: 10.1523/JNEUROSCI.19-13-05205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Kogler M, Chen L, Sausbier U, Ottersen OP, Ruth P, Shipston MJ, Knaus HG. Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur J Neurosci. 2006;24:442–454. doi: 10.1111/j.1460-9568.2006.04936.x. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos CD, McCallum JB, Rigaud M, Fuchs A, Kwok WM, Hogan QH. Opposing effects of spinal nerve ligation on calcium-activated potassium currents in axotomized and adjacent mammalian primary afferent neurons. Brain Res. 2007;1132:84–99. doi: 10.1016/j.brainres.2006.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers AJ, Ashford ML. Activation of BKCa channels in acutely dissociated neurones from the rat ventromedial hypothalamus by NS 1619. Br J Pharmacol. 1994;113:659–661. doi: 10.1111/j.1476-5381.1994.tb17041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- Skalska J, Piwonska M, Wyroba E, Surmacz L, Wieczorek R, Koszela-Piotrowska I, Zielinska J, Bednarczyk P, Dolowy K, Wilczynski GM, Szewczyk A, Kunz WS. A novel potassium channel in skeletal muscle mitochondria. Biochim Biophys Acta. 2008;1777:651–659. doi: 10.1016/j.bbabio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol. 2000;422:172–180. doi: 10.1002/(sici)1096-9861(20000626)422:2<172::aid-cne2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]