Abstract
Background
Hyperglycemia during critical illness is common and associated with increased mortality. Intensive insulin therapy has improved outcomes in some, but not all, intervention trials. It is currently unclear whether the benefits of treatment differ among specific patient populations.
Objective
To determine the association between hyperglycemia and risk-adjusted mortality in critically ill patients and in separate groups stratified by admission diagnosis. Secondarily, determine if mortality risk from hyperglycemia varies with ICU type, length of stay, or diagnosed diabetes.
Design
Retrospective cohort study
Setting
173 U.S. medical, surgical, and cardiac ICUs
Patients
259,040 admissions from 10/2002–9/2005; unadjusted mortality, 11.2%
Measurements
A two-level logistic regression model determined the relationship between glycemia and mortality. Age, diagnosis, co-morbidities and laboratory variables were used to calculate a predicted mortality, which was then analyzed with mean glucose to determine the association of hyperglycemia with hospital mortality.
Results
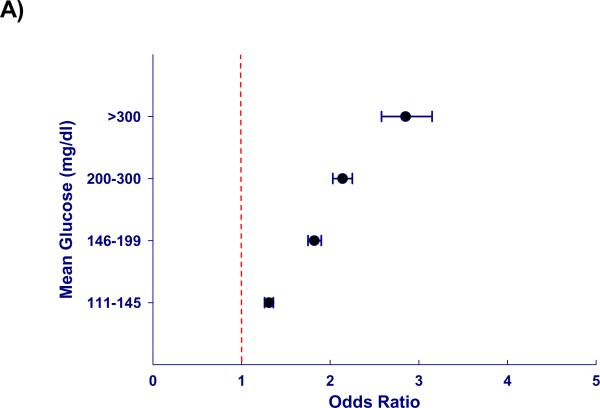
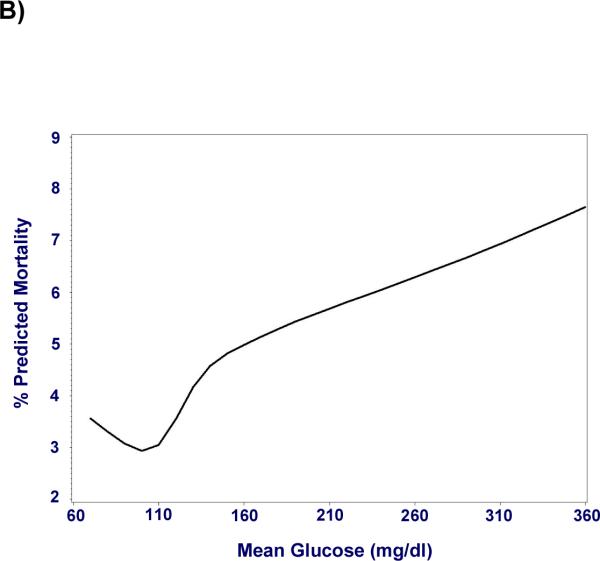
Hyperglycemia was associated with increased mortality independent of illness severity. When compared to normoglycemic individuals (70–110 mg/dl), adjusted odds of mortality (odds ratio, [95% CI]) for mean glucose 111–145, 146–199, 200–300, > 300 mg/dl was, 1.31(1.26–1.36), 1.82(1.74–1.90), 2.13(2.03–2.25), and 2.85(2.58–3.14) respectively. Moreover, the adjusted odds of mortality related to hyperglycemia varied with admission diagnosis, demonstrating a clear association in some (acute myocardial infarction, arrhythmia, unstable angina, pulmonary embolism) and little or no association in others. Hyperglycemia was associated with increased mortality independent of ICU type, length of stay and diabetes.
Conclusions
The association between hyperglycemia and mortality implicates hyperglycemia as a potentially harmful and correctable abnormality in critically ill patients. The finding that hyperglycemia-related risk varied with admission diagnosis suggests differences in the interaction between specific medical conditions and injury from hyperglycemia. The design and interpretation of future trials should consider the primary disease states of patients and the balance of medical conditions in the ICU studied.
Keywords: Hyperglycemia, Critical Illness, Mortality, Intensive Care Unit, Diabetes, Blood Glucose
INTRODUCTION
Hyperglycemia is common during critical illness, and occurs in individuals with and without a previous history of diabetes. Observational studies demonstrating that elevated blood glucose is associated with morbidity and mortality in hospitalized patients (1–13) are supported by the finding that correction of hyperglycemia can improve outcomes (14–20). In the most widely recognized of these intervention studies, mechanically ventilated patients admitted to a surgical intensive care unit (ICU) experienced significantly reduced morbidity and mortality when blood glucose levels were normalized with intensive insulin therapy (14). Subsequent trials have largely been unable to demonstrate similar success (15, 21–28), although many have faced difficulties in achieving statistical power, establishing significant differences in glucose levels between control and treated subjects, and from early study cessation due to excessive hypoglycemia.
An important issue in considering the conflicting findings from prospective trials of inpatient hyperglycemia treatment (14, 15, 20–23, 27, 28) is whether the benefits of intensive insulin therapy is observed in all patients. It is not known whether hyperglycemia contributes equally to mortality in all critically ill patients or whether specific individuals or groups are disproportionately affected. One possibility is that the deleterious effects of hyperglycemia are greater in certain primary medical conditions and as such, some of the discrepancies among intervention trials are due to the differences in the case-mix of the various ICUs examined. No study has directly compared the risk from hyperglycemia among the many diagnoses responsible for admission to the ICU. However, addressing this question could provide valuable insights into the interpretation of past studies, as well as help direct the design of future trials. To test this hypothesis, we examined the association between hyperglycemia and mortality in 259,040 critically ill patients with a broad range of medical and surgical conditions.
METHODS
This retrospective cohort study was based on data from patients admitted to 173 medical, cardiac, surgical, and mixed (medical, cardiac, surgical) ICUs in 113 Veterans Health Administration hospitals. The study was approved by the University of Cincinnati Institutional Review Board and the Cincinnati Veterans Affairs (VA) Research and Development Committee.
Case Selection
The 259,040 cases included in the analysis were selected from a total cohort of 425,853 consecutive first admissions to VA ICUs during a 3-year period from October 2002 through September 2005. We excluded from the analysis cases from hospitals lacking computerized records of arterial blood gas data (n=37,535) and those patients who did not have at least 2 serum glucose measures during their ICU stay (n=126, 220). Patients whose mean glucose throughout their ICU stay was less than 70 mg/dl (n=3,058) were also excluded based on the study's focus on hyperglycemia and the assumption that the pathophysiology of hypoglycemia-related mortality is entirely different. Data on hypoglycemia and mortality will be reported separately.
Data Collection
This study used de-identified data collected for the VA Inpatient Evaluation Center (IPEC), a national VA program that measures and reports risk adjusted outcomes in VA ICUs and implements evidenced-based practices to improve patient safety and quality. A customized program identifies patients with ICU stays at each VA and then extracts data from the Patient File, the Patient Treatment File and the Laboratory File at each VA hospital. Extracted data include demographic information, diagnostic coding from the index and prior hospitalization(s), laboratory data from the ICU, age, source of admission to the ICU, and outcome data (survival at discharge from the hospital and ICU length of stay). The data are scrambled, center specific identifiers are applied, and the dataset is sent electronically to the VA IPEC.
Definitions
We defined diabetes as the presence of ICD-9-CM codes, 250.xx, in the index or prior admission discharge abstract (29, 30). The mean glucose for each patient was calculated from all values measured during their ICU stay excluding admission glucose (included in the risk-adjustment model). The glucose measurements used in the analysis were obtained as part of a multi-sample biochemistry laboratory panel and not point-of-care (POC) testing specifically aimed at glucose monitoring. Excluding POC assessments results in fewer glucose measurements per patient; however, the laboratory glucose assessment provides a precise measurement of blood glucose while the accuracy of POC glucose testing during critical illness may be limited, particularly for hyper- and hypoglycemia (31). Mean glucose was chosen as the measure for glycemia in order to assess glucose values throughout the entire ICU stay (32). Patients were stratified into 5 categorical groups of mean glucose based on the distribution of data and findings from prior studies (6, 11, 14, 17): 70–110, 111–145, 146–199, 200–300, and greater than 300 mg/dl. Patients were also categorized by their length of stay (1 day, 2, 3, 3–6, >6 days) and by severity of illness, represented by predicted mortality (< 2.5, 2.5–5, 5–10, 10–30, and > 30% chance of death).
STATISTICAL ANALYSIS
Risk adjustment
A logistic regression model that predicts death at hospital discharge was used to account for variation in patient characteristics and control for severity of illness. This model has been previously validated and demonstrated to have excellent discrimination and calibration (11,866 patient validation set, AUROC 0.885 (33, 34). The model uses age, laboratory data from the ICU stay (sodium, blood urea nitrogen, creatinine, glucose, bilirubin, albumin, hematocrit, white blood count, partial pressure of oxygen and a combined variable partial pressure of carbon dioxide and pH), source of admission, diagnosis, and co-morbid disease burden as predictors. For each laboratory variable, the value deviating most from the normal range in the 24 hours surrounding the ICU admission is selected (35, 36). Patients are each assigned to one of five mutually exclusive groups defining the patient's location prior to ICU admission (emergency room/outpatient clinic, operating room, nursing home, other hospital, ward) and one of 83 mutually exclusive admission diagnoses (35) based on ICU bed-section ICD-9-CM coding from the index hospitalization. Co-morbid disease burden is determined using the index hospitalization ICD-9-CM codes grouped into 31 co-morbid diseases, including diabetes, applying a validated method (33, 37) modified from Elixhauser (38). The logistic regression model uses binary variables to represent diagnostic groups, comorbid conditions, and source of admission, and restricted cubic splines for continuous variables such as laboratory values, and age.
Association of blood glucose with mortality
We used a two level logistic regression model to determine the independent contribution of mean glucose to mortality risk in all ICU patients. The first level calculated the predicted hospital mortality for each patient, using the independent variables described above. The second level regression model used the predicted mortality risk for each patient from the first regression analysis together with the assigned categorical mean glucose for each patient to predict hospital mortality. To compare the mortality risk from hyperglycemia against normoglycemia, we used as a reference group those individuals with mean glucose levels in the normoglycemic range (70 – 111 mg/dl). To examine if admission diagnosis influenced the estimated impact from mean ICU glucose concentration on mortality risk, we constructed separate logistic regression models for all diagnostic groups with at least 400 cases, again using the predicted mortality and mean glucose as independent variables. In assessing the statistical significance of the relationship between mean glucose and mortality for each diagnostic group, we accounted for planned multiple comparisons by defining statistical significance as p-value < 0.0006 (p< 0.05 divided by the 78 comparisons). Separate, similar models were also constructed for each category of length of stay and severity of illness, and for patients with and without a diagnosis of diabetes. Analyses were conducted with SAS 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
Patient characteristics (Table 1)
Table 1.
Characteristics of the Study Population
| N | % | |
|---|---|---|
|
Age
| ||
| <40 | 3,313 | 1.3 |
| 40–59 | 84,481 | 32.6 |
| 60–79 | 136,124 | 52.5 |
| >79 | 35,122 | 13.6 |
|
Gender
| ||
| Male | 252,564 | 97.5 |
| Female | 6,476 | 2.5 |
|
Mean glucose group
| ||
| 70 – 110 mg/dl | 77,376 | 29.9 |
| 111– 145 mg/dl | 96,399 | 37.2 |
| 146 – 199 mg/dl | 57,437 | 22.2 |
| 200 – 300 mg/dl | 23,821 | 9.2 |
| >300 mg/dl | 4,007 | 1.6 |
|
Severity of Illness (% Likelihood of Death)
| ||
| <2.5 | 116319 | 44.9 |
| 2.5 – 5.0 | 42765 | 16.5 |
| 5.0 – 10.0 | 34978 | 13.5 |
| 10 – 30 | 38214 | 14.8 |
| >30 | 26764 | 10.3 |
|
Most common admission diagnoses
| ||
| Unstable angina | 22,020 | 8.5 |
| Acute myocardial infarct | 17,644 | 6.8 |
| CABG | 14,513 | 5.6 |
| CHF | 12,163 | 4.7 |
| Arrhythmia | 11,928 | 4.6 |
| Colectomy | 8,710 | 3.4 |
| Respiratory Failure | 8,283 | 3.2 |
| GI Bleed: ulcer/laceration | 8,175 | 3.2 |
| Pneumonia | 8,122 | 3.1 |
| PVD Bypass | 7,209 | 2.8 |
| COPD | 6,319 | 2.4 |
| Diabetes | 78,142 | 30.2 |
| No diabetes | 180,898 | 69.8 |
| Surgical | 85,945 | 33.2 |
| Non-surgical | 173,095 | 66.8 |
|
ICU Type
| ||
| Cardiac | 20,359 | 7.9 |
| Medical | 19,501 | 7.5 |
| Surgical | 71,672 | 27.7 |
| Medical/cardiac | 60,212 | 23.2 |
| Mixed | 87,296 | 33.7 |
Of the patients studied, 66% were greater than 60 years of age and 98% were male. Thirty three percent of the cohort was admitted to the ICU within 24 hours of surgery. The most common operative and non-operative diagnoses were cardiovascular, gastrointestinal, and respiratory disorders. Thirty percent of the cohort had ICD-9-CM codes identifying diabetes as a co-morbidity. The rate of unadjusted mortality at discharge from hospitalization was 11.2%, similar to other reports of risk-adjusted mortality in U.S. hospitals (39). Unadjusted mortality for each of the mean glucose categories was: 7.3% (70–110 mg/dl), 10.2% (111 – 145 mg/dl), 14.8% (146 – 199 mg/dl), 17.3% (200 – 300 mg/dl), and 21.9% (>300 mg/dl). The mean and median number of glucose measurements per patient was 7 and 3 respectively and was similar for all mean glucose categories indicating that there was no bias from oversampling of glucose in hyperglycemic individuals.
Hyperglycemia and increased mortality risk
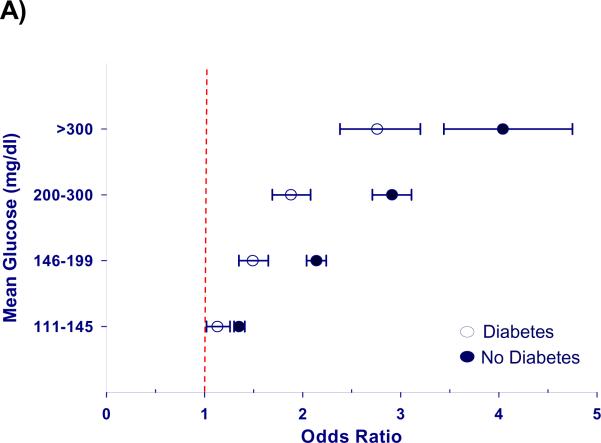
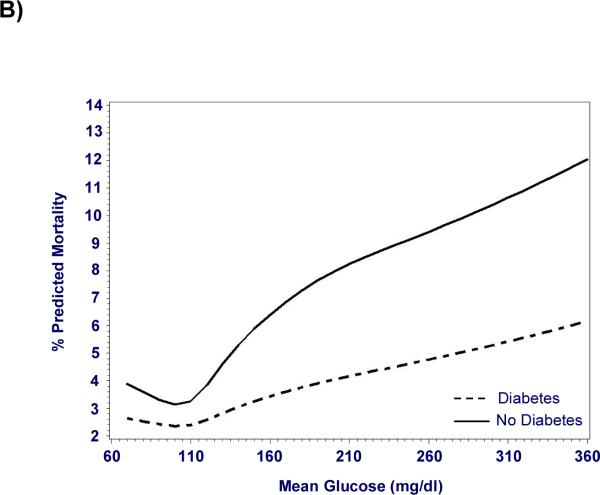
Hyperglycemia was independently associated with increased mortality after adjustment for severity of illness (p < 0.0001), and the risk of death increased in proportion to blood glucose levels (Figure 1). When compared to normoglycemic individuals, the adjusted odds ratios for hospital mortality ranged from 1.31 in cases with mean glucose levels of 111–145 mg/dl to 2.85 in individuals with mean glucose > 300mg/dl (Table 2). The association between hyperglycemia and increased mortality was significant in all types of ICUs, for all levels of severity of illness, and in those with and without a diagnosis of diabetes (Table 2). However, mortality was greater for hyperglycemic patients without a diagnosis of diabetes (p < 0.01, Figure 2). Hyperglycemia-related mortality appeared independent of length of stay in the ICU, with similar adjusted odds ratios for each level of hyperglycemia (Table 2). The relationship between mean glucose and risk adjusted mortality was not influenced by inclusion of admission glucose in the risk-adjustment model as a prior analysis excluding admission glucose produced similar results (40).
Figure 1. Hyperglycemia is associated with increased mortality in ICU patients, independent of severity of illness.
Mortality risk increases with mean glucose across the entire cohort (n = 259,040) starting at mild hyperglycemia (p < 0.0001). A) Odds ratios for mortality after adjustment for severity of illness are represented as point estimates with 95% confidence intervals for each mean glucose category; exclusion of unity represents a significant association. B) Cubic splines depict this relationship using mean glucose as a continuous variable.
Table 2.
Hyperglycemia is Associated with Increased Mortality for the Entire Cohort, and across All ICU Types, Levels of Severity of Illness, and Length of Stay.
| Cohort | N | P-value | Adjusted Odds Ratio (95% CI)a Mean Glucose (mg/dl) | |||
|---|---|---|---|---|---|---|
| 111–145 | 146–199 | 200–300 | >300 | |||
| Total | 259,040 | <.0001 | 1.31 (1.26–1.36) | 1.82 (1.74–1.90) | 2.13 (2.03–2.25) | 2.85 (2.58–3.14) |
| Diabetes | 77,850 | <.0001 | 1.13 (1.02–1.26) | 1.49 (1.35–1.65) | 1.88 (1.69–2.08) | 2.76 (2.38–3.20) |
| No Diabetes | 180,084 | <.0001 | 1.35 (1.30–1.41) | 2.14 (2.04–2.24) | 2.91 (2.71–3.11) | 4.04 (3.44–4.75) |
| ICU | ||||||
| Cardiac | 20,359 | <.0001 | 1.68 (1.43–1.97) | 2.27 (1.91–2.69) | 2.42 (1.97–2.98) | 3.85 (2.63–5.62) |
| Medical | 19,501 | <.0001 | 1.40 (1.25–1.57) | 1.86 (1.65–2.10) | 2.08 (1.80–2.40) | 2.39 (1.79–3.18) |
| Surgical | 71,672 | <.0001 | 1.06 (0.96–1.16) | 1.56 (1.42–1.73) | 1.86 (1.63–2.12) | 2.92 (2.20–3.89) |
| Medical/cardiac | 60,212 | <.0001 | 1.38 (1.28–1.48) | 1.77 (1.64–1.91) | 2.01 (1.83–2.21) | 2.70 (2.26–3.23) |
| Mixed | 87,296 | <.0001 | 1.36 (1.27–1.45) | 1.96 (1.83–2.10) | 2.36 (2.16–2.56) | 2.98 (2.55–3.48) |
| Severity of Illness: Predicted Mortality (% Likelihood of Death) | ||||||
| < 2.5% | 116319 | <.0001 | 1.71 (1.41–2.08) | 2.32 (1.89–2.85) | 2.85 (2.22–3.65) | 3.10 (1.91–5.01) |
| 2.5–5% | 42765 | <.0001 | 1.46 (1.26–1.69) | 2.20 (1.89–2.56) | 2.38 (1.97–2.87) | 3.58 (2.57–4.98) |
| 5–10% | 34978 | <.0001 | 1.52 (1.37–1.69) | 2.00 (1.79–2.24) | 2.50 (2.18–2.86) | 2.72 (2.07–3.56) |
| 10–30% | 38214 | <.0001 | 1.43 (1.33–1.52) | 1.95 (1.82–2.09) | 2.21 (2.03–2.41) | 2.71 (2.30–3.19) |
| > 30% | 26764 | <.0001 | 1.05 (0.99–1.12) | 1.49 (1.39–1.59) | 1.79 (1.65–1.94) | 2.89 (2.44–3.43) |
| Length of Stay | ||||||
| 1 Day | 2,153 | <.0001 | 1.52 (1.02–2.27) | 1.56 (1.03–2.36) | 1.79 (1.18–2.71) | 4.08 (2.52–6.59) |
| 2 Days | 51,171 | <.0001 | 1.14 (1.02–1.28) | 1.58 (1.39–1.78) | 1.87 (1.62–2.16) | 2.67 (2.15–3.31) |
| 3 Days | 64,693 | <.0001 | 1.28 (1.16–1.41) | 1.65 (1.49–1.83) | 1.97 (1.74–2.23) | 2.89 (2.32–3.60) |
| 3–6 Days | 88,121 | <.0001 | 1.19 (1.11–1.27) | 1.73 (1.61–1.87) | 1.91 (1.74–2.10) | 2.33 (1.92–2.83) |
| > 6 Days | 52,902 | <.0001 | 1.27 (1.18–1.35) | 1.80 (1.68–1.93) | 2.31 (2.11–2.52) | 3.09 (2.44–3.92) |
Reference group is the mean glucose category with normoglycemia, (70–110 mg/dl)
Figure 2. Mortality risk from hyperglycemia is greater in patients without a diagnosis of diabetes.
Hyperglycemia is significantly associated with increased adjusted mortality in patients with (n = 78,142) and without (n =180,898) diagnosed diabetes, but the mortality risk is greater in those without diabetes (P < 0.01). A) Odds ratios for mortality after adjustment for severity of illness are represented as point estimates with 95% confidence intervals for each mean glucose category; exclusion of unity represents a significant association. B) Cubic splines depict this relationship using mean glucose as a continuous variable.
In separate analyses of patients grouped by admission diagnosis, marked differences were seen in the relationships between hyperglycemia and mortality (Table 3). In fact, while some diagnostic categories showed a significant association between hyperglycemia and death, in other disorders there was no relationship. A significant association between hyperglycemia and adjusted mortality was seen in unstable angina, acute myocardial infarction, congestive heart failure, arrhythmia, ischemic and hemorrhagic stroke, gastrointestinal bleeding, acute renal failure, pneumonia, pulmonary embolism and sepsis (Table 3). On the other hand, hyperglycemia was not associated with increased mortality in patients with chronic obstructive pulmonary disease (COPD), hepatic failure, and gastrointestinal neoplasm, or those admitted to the ICU after surgery for coronary artery bypass graft (CABG), peripheral vascular disease (PVD), and hip fracture (Table 3). Even among disorders with significant mortality risk from hyperglycemia, the magnitude of that risk varied. For instance, among patients with pneumonia, the adjusted odds ratios for mortality relating to increasing hyperglycemia ranged from 1.4 to 1.7, while in patients with acute myocardial infarction the odds ratio ranged from 1.7 to 6.1, and 1.5 to 10.3 in patients with variceal gastrointestinal bleed (Table 3). The sample size or number of deaths in any diagnostic group did not correlate with mortality risk from hyperglycemia (r = −0.08).
Table 3.
Mortality Related to Hyperglycemia Varies with Admission Diagnosis
| Cohort | N | P-valuea | Adjusted Odds Ratio (95% CI)b Mean Glucose (mg/dl) | |||
|---|---|---|---|---|---|---|
| 111–145 | 146–199 | 200–300 | >300 | |||
| Significant Association between Mean Glucose and Adjusted Mortality | ||||||
| Medical | ||||||
| Unstable angina | 22,020 | <.0001 | 1.40 (1.04–1.88) | 1.98 (1.46–2.68) | 2.91 (2.04–4.14) | 3.05 (1.46–6.38) |
| Acute MI | 17,644 | <.0001 | 1.74 (1.49–2.04) | 2.60 (2.21–3.06) | 3.01 (2.50–3.63) | 6.07 (4.40–8.38) |
| CHF | 12,163 | <.0001 | 1.83 (1.56–2.15) | 2.47 (2.08–2.94) | 3.02 (2.44–3.73) | 3.17 (1.98–5.08) |
| Arrhythmia | 11,928 | <.0001 | 1.67 (1.33–2.11) | 2.61 (2.03–3.35) | 2.39 (1.71–3.34) | 5.24 (2.85–9.62) |
| Respiratory failure | 8,283 | <.0001 | 1.20 (1.04–1.38) | 1.54 (1.33–1.79) | 1.74 (1.46–2.08) | 2.39 (1.66–3.44) |
| GI bleed: ulcer | 8,175 | <.0001 | 1.75 (1.40–2.18) | 2.22 (1.73–2.85) | 2.79 (2.01–3.86) | 5.39 (2.59–11.2) |
| Pneumonia | 8,122 | <.0001 | 1.29 (1.10–1.51) | 1.74 (1.48–2.04) | 1.64 (1.34–1.99) | 1.67 (1.11–2.52) |
| Sepsis | 3,933 | <.0001 | 1.06 (0.88–1.28) | 1.43 (1.17–1.75) | 2.14 (1.67–2.75) | 3.28 (1.99–5.39) |
| Acute renal failure | 3,764 | <.0001 | 1.10 (0.87–1.39) | 1.58 (1.23–2.03) | 2.10 (1.52–2.91) | 2.87 (1.60–5.18) |
| GI bleed: varices | 2,746 | <.0001 | 1.47 (1.05–2.07) | 1.68 (1.15–2.47) | 1.94 (1.19–3.18) | 10.30 (4.10–26.1) |
| Ischemic CVA | 2,558 | <.0001 | 1.85 (1.35–2.54) | 3.30 (2.39–4.57) | 3.84 (2.69–5.50) | 8.49 (4.46–16.2) |
| Pulmonary embolism | 945 | .0002 | 1.69 (1.00–2.86) | 3.41 (1.93–6.03) | 2.37 (1.05–5.31) | 6.89 (1.78–26.7) |
| Intracerebral hemorrhage | 797 | <.0001 | 2.61 (1.54–4.42) | 5.55 (3.20–9.62) | 11.6 (5.92–22.6) | 26.7 (7.94–89.8) |
| Operative | ||||||
| Colectomy | 8,710 | 0.0005 | 0.81 (0.61–1.08) | 1.20 (0.89–1.62) | 1.49 (1.02–2.19) | 1.33 (0.47–3.72) |
| GU neoplasm | 5,091 | <.0001 | 0.56 (0.31–1.02) | 1.11 (0.61–2.05) | 2.72 (1.37–5.38) | 2.54 (0.49–13.3) |
| Valvular heart | 3,485 | 0.0001 | 0.66 (0.32–1.35) | 0.81 (0.40–1.66) | 1.73 (0.76–3.96) | 6.62 (1.62–27.1) |
| Combined | 156,119 | <.0001 | 1.41 (1.34–1.48) | 2.04 (1.94–2.15) | 2.48 (2.33–2.65) | 3.76 (3.31–4.27) |
| No Significant Association between Mean Glucose and Adjusted Mortality | ||||||
| Medical | ||||||
| COPD | 6,319 | 0.0082 | 1.22 (0.93–1.59) | 1.46 (1.12–1.91) | 1.66 (1.23–2.26) | 1.29 (0.78–2.16) |
| Hepatic failure | 3,045 | 0.0128 | 1.13 (0.90–1.42) | 1.57 (1.20–2.04) | 1.21 (0.83–1.76) | 1.74 (0.86–3.54) |
| GI neoplasm | 1,841 | 0.1122 | 1.09 (0.80–1.49) | 1.39 (0.98–1.97) | 1.70 (1.05–2.75) | 1.82 (0.49–6.71) |
| GI perforation or obstruction | 1,615 | 0.0488 | 1.13 (0.79–1.62) | 1.53 (1.02–2.28) | 1.56 (0.90–2.70) | 3.13 (1.16–8.46) |
| Musculoskeletal | 1,056 | 0.0203 | 0.78 (0.44–1.36) | 0.92 (0.49–1.73) | 2.15 (1.11–4.18) | 2.30 (0.58–9.15) |
| Operative | ||||||
| CABG | 14,513 | 0.0066 | 0.63 (0.38–1.04) | 0.87 (0.53–1.42) | 1.12 (0.64–1.94) | 1.94 (0.58–6.55) |
| PVD bypass | 7,209 | 0.0109 | 0.73 (0.48–1.12) | 0.97 (0.62–1.53) | 1.66 (0.99–2.79) | 0.98 (0.16–6.01) |
| Hip fracture | 1,772 | 0.2765 | 0.95 (0.61–1.48) | 1.31 (0.81–2.12) | 1.52 (0.79–2.95) | 1.96 (0.54–7.10) |
| Amputation | 1,612 | 0.7800 | 1.19 (0.75–1.86) | 1.24 (0.79–1.94) | 1.32 (0.77–2.29) | 0.67 (0.14–3.31) |
| Combined | 102,921 | <.0001 | 1.13 (1.06–1.21) | 1.47 (1.38–1.58) | 1.64 (1.50–1.78) | 1.86 (1.59–2.18) |
Statistical significance after adjustment for multiple comparisons is defined as p value < 0.0006.
Reference group is the mean glucose category with normoglycemia, (70–110 mg/dl)
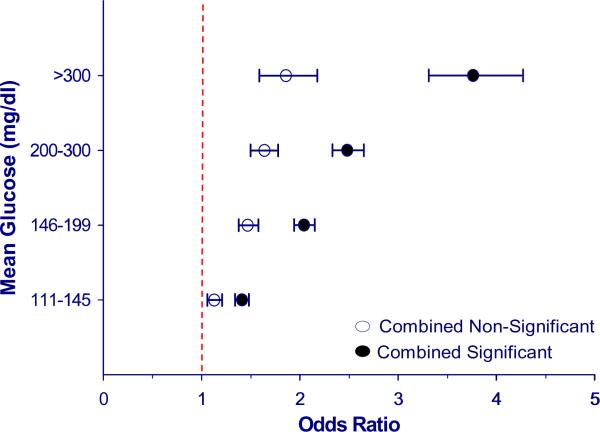
For several diagnostic groups the p-value indicated a statistically significant relationship between blood glucose and mortality risk overall, however inspection of individual point estimates indicated that most of the categorical mean glucose groups were not associated with mortality. Colectomy, and surgery for genito-urinary neoplasm or cardiac valve disease are examples of diagnostic categories where despite a significant p-value, the mortality estimates at individual stratified glucose levels are not different from one; in such cases it is difficult to conclude that a meaningful relationship truly exists between hyperglycemia and mortality (Table 3). When we aggregated those diagnostic groups lacking a significant association between hyperglycemia and mortality, a statistically significant and graded relationship between hyperglycemia and mortality was apparent, with adjusted odds ratios of 1.13, 1.47, 1.64, and 1.86 for the respective mean glucose (mg/dl) groups 111–145, 146–199, 200–300, and >300 (Table 3). As might be expected, the magnitude of mortality risk was more modest than what was found in the combined analysis of diagnostic groups where a significant relationship between mean glucose and mortality was detected (Figure 3), with adjusted odds ratios for mortality of 1.41, 2.04, 2.48, and 3.76 respectively (Table 3). Rates of co-morbid diabetes were identical (30%) between aggregate groups of admission diagnoses with significant and non-significant relationships of mean glucose and risk-adjusted mortality.
Figure 3. The association between hyperglycemia and mortality varies with admission diagnosis.
There is a significant association between hyperglycemia and increased mortality for aggregate groups of significant and non-significant diagnostic categories (p < 0.0001). However, the magnitude of mortality risk remains greater for the aggregate group of significant diagnoses (n = 156,119) vs. aggregate group of non-significant diagnoses (n = 102,921). Odds ratios for mortality after adjustment for severity of illness are represented as point estimates with 95% confidence intervals for each mean glucose category; exclusion of unity represents a significant association.
DISCUSSION
The effect of hyperglycemia on the outcomes of hospitalized patients has received considerable attention recently because of the potential benefits from intervention. In addition to numerous primary studies and reviews, expert committees have drafted position statements and treatment guidelines to define clinical approaches to this problem (41–44). However, the inconsistent findings from available intervention trials have raised several fundamental questions regarding glycemic management in patients with serious illness, fueling a debate about whether all individuals hospitalized in the ICU are equally susceptible to the harm from hyperglycemia and whether all would derive a similar benefit from treatment (6, 12, 21–26, 28, 45–55). We hypothesized that the effects of hyperglycemia are more deleterious in certain primary medical conditions and as such, differences in the case-mix of the various ICUs where intervention trials were conducted could contribute to their discrepant findings. Testing this hypothesis definitively with a randomized trial would require treating multiple large groups of patients with intensive and conventional approaches to compare the effects of glycemic control in populations of differing admission diagnoses. However, the challenges faced by recent prospective trials of intensive insulin therapy suggest that this may be prohibitively difficult (20–27) and therefore alternative strategies are warranted.
We have approached this question by analyzing a large and heterogeneous cohort of critically ill patients that compares mortality risk across a broad range of admission diagnoses, and thereby contribute several new findings that inform this discussion. First, we found that hyperglycemia-related mortality is disproportionately greater in some medical conditions than others, suggesting that the untoward effects of elevated blood glucose are specific to, or amplified in, particular conditions. Although variation in hyperglycemia-related mortality has been previously examined (12, 51, 53), the ability to analyze this relationship independent of severity of illness, in 78 distinct diagnostic groups, provides additional information over those prior studies that stratified patients more broadly by organ system or unit type. For example, we discovered marked differences in the presence or absence of hyperglycemia-related mortality among specific diagnoses within the same organ system, or that would be treated in the same unit, such as pneumonia (present) versus COPD (absent), gastrointestinal bleed (present) versus hepatic failure (absent), and acute myocardial infarction (present) versus valvular heart disease (absent). These are differences that would not be observed by examining hyperglycemia-related risk more generally, as has been done for instance, in respiratory, cardiovascular, and gastrointestinal disorders, or by comparing the risk in medical versus cardiac ICUs (12, 56, 57).
The finding that hyperglycemia-related mortality varies with admission diagnosis offers key insight into the variable results produced by prospective trials of intensive insulin therapy. For example, some interpretations of these studies suggest inherent differences in the effects of hyperglycemia for medical versus surgical ICUs, and distinct treatment paradigms for the two populations have even been proposed (49, 58). However, our results suggest that such strategies might be misguided since the differences in mortality risk between medical and surgical ICU populations are more likely due to the specific admission diagnoses of the patients admitted to these units. The case mix in any ICU varies not only by its specialization (e.g. cardiac, surgical, medical, mixed), but also the specific services provided at that hospital; such as dialysis, neurosurgery, trauma, coronary artery bypass graft surgery, chemotherapy, transplantation, and interventional radiology and cardiac services. Our results suggest that the distribution of medical conditions found in an ICU may influence the findings from trials examining glycemic control in that setting.
The fact that differences in the magnitude of mortality risk were evident even among disorders where a significant relationship was observed indicates that the effects from hyperglycemia constitute a spectrum in graded risk, as opposed to a dichotomous effect where the harm from hyperglycemia is either present or absent. Furthermore, for some medical conditions, a significant relationship between hyperglycemia and mortality may become apparent only with very large sample sizes. This possibility is supported by our finding that when the non-significant conditions are analyzed as one aggregate group, a significant and graded relationship appears but the magnitude of mortality risk is attenuated in comparison with the significant diseases (Figure 3). It is also possible that hyperglycemia may indeed be associated with mortality in those diagnoses where significance was not observed, but that in conditions where rates of death are already low, the influence of hyperglycemia on mortality becomes less important.
Our analysis extends previous findings of the relative risk from hyperglycemia in patients with and without antecedent diagnoses of diabetes (3, 4, 10, 12, 51, 59). Similar to what has been suggested previously by other investigators, we now demonstrate that the association of hyperglycemia and mortality was greater in individuals without known diabetes. Consistent with this finding is a recent sub-group analysis by Van den Berghe et al. of surgical and medical ICU patients demonstrating that while glucose lowering effectively reduced mortality in those without a previous history of diabetes, no benefit from treatment was observed in patients with diabetes (56). The statistical power achieved in our analysis of over 77,000 patients with a diagnosis of diabetes allows us to demonstrate that hyperglycemia is indeed associated with increased mortality in this group, but with lesser magnitude of risk than in those without diabetes. It is unlikely that severity of illness explains the difference in hyperglycemia-related mortality between those with and without a diabetes diagnosis since we adjust for this important confounder in our analysis. It is unclear why hyperglycemia would be more injurious in nondiabetic individuals than persons with known diabetes. We did not have access to information about which patients received insulin, but it is possible that individuals with diabetes received insulin more frequently and that this might have conferred benefits independent of glycemic control (60). Nevertheless, consideration of the differential effects of hyperglycemia in patients with diabetes is important, particularly in interpreting systematic reviews of insulin treatment during critical illness that are unable to adequately examine effects separately in individuals with and without diabetes (57), as well as randomized trials where a large percentage of patients with diabetes may attenuate the observed benefit from glucose lowering (27).
Another key finding from this analysis is that hyperglycemia is associated with increased mortality regardless of the length of time a patient is in the ICU. It has been debated whether the mortality risk from hyperglycemia is the same for patients who stay in the ICU for fewer than 3 days compared to individuals with longer ICU stays (46, 61, 62). This controversy, which has prompted some experts to propose delaying treatment of elevated blood glucose until later in the ICU course (58, 62), stems from the findings of Van den Berghe et al. who described mortality reduction with normalization of blood glucose in medical ICU patients staying greater than 3 days but not in those whose duration of stay was shorter (15). By comparing large groups of patients with differing lengths of ICU stay we found no difference in hyperglycemia-related mortality between patients with short and long duration in the ICU. In our cohort, hyperglycemia was similarly associated with mortality for all lengths of stay, even among patients in the ICU for a single day, indicating that elevated blood glucose is equally harmful in the initial days of hospitalization. These results are important because they address another question not easily amenable to randomized trials that are limited by the inability to predict length of stay at the time of admission.
Importantly, this study clearly demonstrates that hyperglycemia, even mild glucose elevation, is independently associated with mortality in a large and heterogeneous cohort after adjustment for severity of illness, making it unlikely that elevated glucose levels are simply a surrogate for critical illness. While observational studies demonstrating that hospital-related hyperglycemia is associated with increased morbidity and mortality have been published for a variety of disorders and settings (1–13), they are limited by relatively small sample sizes, restriction to specific subsets of patients, or the ability to adequately control for severity of illness. Finally, previous investigations commonly used only admission glucose as an index of glycemia, a measure that while practical for identification at admission, has been found to be inferior to mean glucose in predicting mortality (32). In contrast, our analysis includes each individual's mean glucose, and uses a robust method to adjust for severity of illness (33).
There were limitations to the approach taken in this study. First, as an observational study we can suggest a relationship between hyperglycemia and mortality independent of illness severity, but cannot establish causality. Second, since the risk adjustment model lacks clinical indicators of neurologic status (e.g. Glasgow Coma Scale), we cannot exclude that unmeasured severity of illness contributes to estimates of mortality risk in primary neurologic diagnoses. However, in previous applications the model was found to be robust, stable and reproducible (33). Third, interpretation of our findings should consider that the study population is mostly male and 66% are over the age of 60 (no pediatric subjects). Future analysis focusing specifically on the 6476 female patients from the database may provide some general insights on hyperglycemia and mortality in critically ill adult females. Finally, our study does not address the influence of treatment (insulin, steroids, nutrition) on hyperglycemia-related mortality. While the presence or absence of these agents may influence the development of hyperglycemia in critically ill patients, it is unlikely that the systematic variation we observed in mortality risk can be explained by treatment differences. Studies demonstrating that the benefits related to intensive insulin therapy in the ICU are dependent on the control of glucose (63) make it less likely that any non-glycemic effects of insulin therapy have biased our findings. Furthermore, methodologic limitations with the inclusion and interpretation of data on insulin treatment in similar studies have been reported (12).
In summary, hyperglycemia is significantly associated with increased mortality in critically ill individuals, independent of severity of illness, diabetes diagnosis or length of stay in the ICU. These results implicate hyperglycemia as a potentially harmful and correctable abnormality in critically ill patients and support ongoing consideration of hyperglycemia as a therapeutic target to improve outcomes in hospitalized patients. The finding that the magnitude of mortality risk from hyperglycemia depends on the underlying disease suggests that the degree of benefit from glucose-lowering may differ among patients with certain conditions. As such, the interpretation and design of trials examining the effects of inpatient glycemic control should consider the primary disease state of those individuals studied.
ACKNOWLEDGEMENTS
We thank Annette Christianson for statistical support.
Role of the funding source This work was supported by the Veterans Affairs Inpatient Evaluation Center, NIH DK K23 74440 (MF), and NIH DK R01 57900 (DD). The funding sources did not have a role in the study design; data collection, analysis or interpretation; or the decision to submit the manuscript for publication.
This work was supported by the Veterans Affairs Inpatient Evaluation Center and National Institutes of Health, NIH DK K23 74440 (Dr. Falciglia), and NIH DK R01 57900 (Dr. D'Alessio).
Appendix 1. Unadjusted Mortality Rates for the Overall Cohort and by Diabetes and ICU Type
| Cohort | N | Unadjusted Mortality Rate % Mean Glucose (mg/dl) | ||||
|---|---|---|---|---|---|---|
| 70–110 | 111–145 | 146–199 | 200–300 | >300 | ||
| Total | 259,040 | 7.3 | 10.2 | 14.8 | 17.3 | 21.9 |
| Diabetes | 77,850 | 5.7 | 6.3 | 8.8 | 11.1 | 15.4 |
| No Diabetes | 180,084 | 7.6 | 11.3 | 19.8 | 30.5 | 40.5 |
| ICU | ||||||
| Cardiac | 20,359 | 3.6 | 8.9 | 12.8 | 13.1 | 25.5 |
| Medical | 19,501 | 12.2 | 19.7 | 26.1 | 25.8 | 24.1 |
| Surgical | 71,672 | 5.4 | 5.2 | 7.8 | 9.6 | 17.7 |
| Medical/cardiac | 60,212 | 8.5 | 14.5 | 20.1 | 20.1 | 22.2 |
| Mixed | 87,296 | 7.4 | 11.0 | 16.4 | 18.9 | 21.9 |
Appendix 2. Unadjusted Mortality Rates by Admission Diagnosis
| Cohort | N | Unadjusted Mortality Rate % Mean Glucose (mg/dl) | ||||
|---|---|---|---|---|---|---|
| 70–110 | 111–145 | 146–199 | 200–300 | >300 | ||
| Medical | ||||||
| Unstable angina | 22,020 | 0.9 | 1.8 | 3.1 | 3.6 | 5.5 |
| Acute MI | 17,644 | 4.3 | 9.7 | 16.2 | 19.4 | 33.6 |
| CHF | 12,163 | 6.7 | 13.2 | 17.8 | 21.9 | 21.1 |
| Arrhythmia | 11,928 | 2.6 | 6.0 | 10.5 | 10.1 | 19.1 |
| Respiratory failure | 8,283 | 28.9 | 33.7 | 39.9 | 41.2 | 51.8 |
| GI bleed: ulcer | 8,175 | 4.6 | 9.8 | 14.4 | 18.7 | 23.8 |
| Pneumonia | 8,122 | 17.8 | 23.9 | 30.5 | 28.4 | 24.8 |
| Sepsis | 3,933 | 37.5 | 40.3 | 50.4 | 57.9 | 72.6 |
| Acute renal failure | 3,764 | 13.6 | 17.2 | 25.3 | 30.0 | 38.6 |
| GI bleed: varices | 2,746 | 7.2 | 13.5 | 17.2 | 21.1 | 51.5 |
| Ischemic CVA | 2,558 | 10.2 | 18.9 | 32.7 | 36.3 | 51.9 |
| Pulmonary embolism | 945 | 8.4 | 14.9 | 28.7 | 24.3 | 50.0 |
| Intracerebral hemorrhage | 797 | 11.2 | 27.5 | 43.0 | 63.6 | 80.0 |
| COPD | 6,319 | 9.8 | 11.8 | 13.8 | 15.0 | 11.8 |
| Hepatic failure | 3,045 | 26.5 | 28.9 | 38.1 | 32.0 | 41.3 |
| GI neoplasm | 1,841 | 20.1 | 21.7 | 31.7 | 36.0 | 46.2 |
| GI perforation or obstruction | 1,615 | 15.2 | 18.6 | 24.3 | 24.2 | 37.5 |
| Musculoskeletal | 1,056 | 9.4 | 9.9 | 13.6 | 23.4 | 15.8 |
| Operative | ||||||
| Colectomy | 8,710 | 5.6 | 5.5 | 8.7 | 11.1 | 13.2 |
| GU neoplasm | 5,091 | 2.0 | 1.3 | 2.9 | 6.7 | 9.5 |
| Valvular heart | 3,485 | 5.0 | 3.8 | 5.2 | 10.2 | 50.0 |
| CABG | 14,513 | 2.2 | 1.6 | 2.4 | 3.3 | 7.5 |
| PVD bypass | 7,209 | 2.4 | 2.2 | 3.5 | 5.6 | 3.6 |
| Hip fracture | 1,772 | 10.2 | 9.3 | 13.0 | 13.3 | 20.0 |
| Amputation | 1,612 | 10.1 | 11.9 | 14.3 | 14.6 | 9.1 |
Footnotes
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006;166(15):1613–1619. doi: 10.1001/archinte.166.15.1613. [DOI] [PubMed] [Google Scholar]
- 2.Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58(5):921–924. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 3.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 5.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146(1):30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 6.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290(15):2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 7.Golden SH, Peart-Vigilance C, Kao WH, et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22(9):1408–1414. doi: 10.2337/diacare.22.9.1408. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara M, Kojima S, Sakamoto T, et al. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005;150(4):814–820. doi: 10.1016/j.ahj.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Kornum JB, Thomsen RW, Riis A, et al. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. 2007;30(9):2251–2257. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 10.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111(23):3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 12.Whitcomb BW, Pradhan EK, Pittas AG, et al. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Critical care medicine. 2005;33(12):2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 13.Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–289. doi: 10.1136/thx.2005.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 15.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 16.Malmberg K, Norhammar A, Wedel H, et al. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 17.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 18.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 19.Reed CC, Stewart RM, Sherman M, et al. Intensive insulin protocol improves glucose control and is associated with a reduction in intensive care unit mortality. Journal of the American College of Surgeons. 2007;204(5):1048–1054. doi: 10.1016/j.jamcollsurg.2006.12.047. discussion 1054-1045. [DOI] [PubMed] [Google Scholar]
- 20.Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009 doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 21.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 22.Malmberg K, Ryden L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26(7):650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 23.Pittas AG, Siegel RD, Lau J. Insulin therapy and in-hospital mortality in critically ill patients: systematic review and meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2006;30(2):164–172. doi: 10.1177/0148607106030002164. [DOI] [PubMed] [Google Scholar]
- 24.Cheung NW, Wong VW, McLean M. The hyperglycemia: intensive insulin infusion in infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care. 2006;29(4):765–770. doi: 10.2337/diacare.29.04.06.dc05-1894. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 26.Mehta SR, Yusuf S, Diaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293(4):437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 27.Arabi YM, Dabbagh OC, Tamim HM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Critical care medicine. 2008;36(12):3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 28.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 29.Hebert PL, Geiss LS, Tierney EF, et al. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 30.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 31.Kanji S, Buffie J, Hutton B, et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. Critical care medicine. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 33.Render ML, Kim HM, Welsh DE, et al. Automated intensive care unit risk adjustment: results from a National Veterans Affairs study. Critical care medicine. 2003;31(6):1638–1646. doi: 10.1097/01.CCM.0000055372.08235.09. [DOI] [PubMed] [Google Scholar]
- 34.Render ML, Deddens J, Freyberg R, et al. Veterans Affairs intensive care unit risk adjustment model: validation, updating, recalibration. Critical care medicine. 2008;36(4):1031–1042. doi: 10.1097/CCM.0b013e318169f290. [DOI] [PubMed] [Google Scholar]
- 35.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 36.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228(4):491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston JA, Wagner DP, Timmons S, et al. Impact of different measures of comorbid disease on predicted mortality of intensive care unit patients. Med Care. 2002;40(10):929–940. doi: 10.1097/00005650-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal GE, Harper DL, Quinn LM, et al. Severity-adjusted mortality and length of stay in teaching and nonteaching hospitals. Results of a regional study. JAMA. 1997;278(6):485–490. [PubMed] [Google Scholar]
- 40.Falciglia M, D'Alessio D, Freyberg R, et al. Hyperglycemia Increases the Risk of Mortality in Critically Ill Patients: Differential Effect in Diabetic and Non-diabetic Patients. Endocrine Society 86th Annual Meeting; New Orleans, LA. 2004 June 16–19.2004. [Google Scholar]
- 41.Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–597. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 42.Garber AJ, Moghissi ES, Bransome ED, Jr., et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(Suppl 2):4–9. doi: 10.4158/EP.10.S2.4. [DOI] [PubMed] [Google Scholar]
- 43.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355(18):1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 44.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. The Annals of thoracic surgery. 2009;87(2):663–669. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Bellomo R, Egi M. Glycemic control in the intensive care unit: why we should wait for NICE-SUGAR. Mayo Clin Proc. 2005;80(12):1546–1548. doi: 10.4065/80.12.1546. [DOI] [PubMed] [Google Scholar]
- 46.Devos P, Preiser JC. Is it time for implementation of tight glycaemia control by intensive insulin therapy in every ICU? Crit Care. 2006;10(2):130. doi: 10.1186/cc4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egi M, Bellomo R, Stachowski E, et al. Intensive insulin therapy in postoperative intensive care unit patients: a decision analysis. American journal of respiratory and critical care medicine. 2006;173(4):407–413. doi: 10.1164/rccm.200506-961OC. [DOI] [PubMed] [Google Scholar]
- 48.Fowler RA, Annane D. The highs and lows of intensive insulin therapy. American journal of respiratory and critical care medicine. 2006;173(4):367–369. doi: 10.1164/rccm.2510006. [DOI] [PubMed] [Google Scholar]
- 49.Hammer L, Dessertaine G, Timsit J-F, et al. Intensive Insulin Therapy in the Medical ICU. N Engl J Med. 2006;354(19):2069–2071. doi: 10.1056/NEJMc060566. [DOI] [PubMed] [Google Scholar]
- 50.Ligtenberg JJ, Meijering S, Stienstra Y, et al. Mean glucose level is not an independent risk factor for mortality in mixed ICU patients. Intensive Care Med. 2006;32(3):435–438. doi: 10.1007/s00134-005-0052-y. [DOI] [PubMed] [Google Scholar]
- 51.Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80(12):1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 52.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132(1):268–278. doi: 10.1378/chest.06-3121. [DOI] [PubMed] [Google Scholar]
- 53.Vogelzang M, Nijboer JM, van der Horst IC, et al. Hyperglycemia has a stronger relation with outcome in trauma patients than in other critically ill patients. J Trauma. 2006;60(4):873–877. doi: 10.1097/01.ta.0000195715.63978.80. discussion 878–879. [DOI] [PubMed] [Google Scholar]
- 54.van den Heuvel I, Ellger B. A sweet debate: glycemic control in the intensive care unit. Critical care medicine. 2008;36(12):3271–3272. doi: 10.1097/CCM.0b013e31818f2831. [DOI] [PubMed] [Google Scholar]
- 55.Levy MM, Rhodes A. The ongoing enigma of tight glucose control. Lancet. 2009 doi: 10.1016/S0140-6736(09)60045-3. [DOI] [PubMed] [Google Scholar]
- 56.Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 57.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. Jama. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 58.Osborne M. Update in critical care. Ann Intern Med. 2007;147(6):412–416. doi: 10.7326/0003-4819-147-6-200709180-00011. [DOI] [PubMed] [Google Scholar]
- 59.Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Critical care medicine. 2008;36(8):2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 60.Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109(7):849–854. doi: 10.1161/01.CIR.0000116762.77804.FC. [DOI] [PubMed] [Google Scholar]
- 61.Hafidh SA, Reuter MD, Chassels LJ, et al. Effect of intravenous insulin therapy on clinical outcomes in critically ill patients. The American journal of the medical sciences. 2007;333(6):354–361. doi: 10.1097/MAJ.0b013e318065a940. [DOI] [PubMed] [Google Scholar]
- 62.Malhotra A. Intensive insulin in intensive care. N Engl J Med. 2006;354(5):516–518. doi: 10.1056/NEJMe058304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Critical care medicine. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]