Abstract
The goal of this murine investigation was to evaluate the effect of an antivascular ultrasound treatment on the growth of an implanted melanoma and the consequent survival rate. Following the intravenous injection of 0.2 mL ultrasound contrast agent (Definity), therapy (n = 15) was performed on 1 mL tumors for 3 minutes with low intensity, continuous ultrasound (3 MHz; 2.4 ± 0.1 W.cm−2 [ISATA]); control mice (n = 17) received a sham treatment. Mice were euthanized once the tumor had reached 3 mL and survival percentage versus time curves were plotted. The median survival time (time for tumor to reach 3 mL) for the treated group was 23 days and for the control group was 18 days; the difference was statistically significant (P ≤ 0.0001). Antivascular ultrasound therapy reduced the growth rate of an implanted melanoma and increased survival time. The ultrasound therapy provides a further example of tumor vascular disruption and its future clinical potential should be investigated.
Keywords: Low-intensity ultrasound therapy, Ultrasound contrast agent, Antivascular, Angiogenesis, Melanoma
INTRODUCTION
Since the early observations that obstruction of tumor blood vessels lead to tumor regression (Denekamp 1982; Denekamp et al.1983), the use of vasculature targeting for cancer therapy has been studied extensively (Baguley 2003; Hinnen and Eskens 2007; Lippert 2007). Most of the research to date, however, has concentrated on the use of pharmacological agents including tubulin-binding agents (Rustin et al. 2003a; Beerport et al. 2006; Hande et al. 2006) and flavonoids for vascular disruption [Chabot et al. 1989; Rustin et al. 2003b). In a series of reports our studies in mice demonstrated that a physical agent, low-intensity ultrasound, also disrupted the neovasculature of a tumor in the presence of a circulating microbubble-containing ultrasound contrast agent (Bunte et al. 2006; Wood et al. 2005, 2007, 2008). It was hypothesized, that in the lumen of tumor blood vessels, the impinging ultrasound beam interacted with the circulating microbubble, which could act as a localized heat source as it absorbed the acoustic power from the ultrasound beam (Wood et al. 2008). The weakly structured tumor vessels, formed as a result of angiogenesis, were irreparably damaged during the insonation and histologic studies showed dilation of tumor capillaries with associated hemorrhage and intercellular fluid. These histologic changes encompassed the entire tumor and were not limited to the more central regions, as found following therapy with vascular disrupting agents such as the combretastatins (Chaplin and Hill 2002; Salmon and Siemann 2007). For each minute of insonation at about 2 W.cm−2 there was up to 25% reduction in tumor vascular perfusion (Wood et al. 2005). Histology also demonstrated, that although the cancer cells were initially unaffected by the insonation, liquefactive necrosis, secondary to ischemia, followed 24 hours later (Bunte et al. 2006). Of particular interest was an associated observation in ultrasound imaging studies that, although the neovasculature in all regions of the neoplasm was damaged, the vascular structures in the contiguous normal tissues were unaffected by the therapy. Histology also demonstrated that pre-existing vascular, neural and other normal structures enveloped by the growing neoplasm were unaffected by the therapy (Bunte et al. 2006). These post-antivascular therapy findings are quite different to those described after tumor therapy with high intensity focused ultrasound, where power levels of 1,000 to 20,000 W.cm−2 (Briones et al. 2008, Van Leenders et al. 2000) lead to localized temperature rises of 85–100°C (Maestroni et al. 2008). There is instantaneous coagulative necrosis of all tissues in the therapeutic zone (Susani et al. 1993; Van Leenders et al. 2000) the histologic changes may, however, not be confined to the target structure and involve adjacent normal tissues (Van Leenders et al. 2000).
To date, data has only been available from observations made up to a day following tumor treatment with low-intensity ultrasound, and it is not known whether such an antivascular therapy has a long lasting effect and whether it could reduce tumor volume and prolong the life of the animal. Other tumor vascular disrupting agents, including the combretastatins, do not typically induce tumor regression (Chaplin and Hill 2002; Nielsen et al. 2008) or eliminate the tumor mass (Salmon and Siemann 2007). The central regions undergo necrosis but viable tumor cells remain at the edges (Salmon and Siemann 2007) and rapid regrowth occurs from the periphery of the tumor (Chaplin and Hill 2002). Further, the resultant hypoxia in the central areas can lead to the release of factors stimulating further angiogenesis and the regrowing tumor can be particularly aggressive (Tozer et al 2008). The aim of this Technical Note is to present the results of a longitudinal study in which the growth of an implanted murine melanoma was followed over time, prior to and following, a single three minute episode of low-intensity antivascular ultrasound therapy.
METHODS AND MATERIALS
Animal studies
The studies, approved by the University’s Institutional Animal Care and Use Committee, were performed in 32 mice (6–8 weeks of age; C3HV/HeN strain; Charles River Laboratories, Wilmington, MA, USA), randomly placed into treated (n = 15) or control (n = 17) groups. In each mouse two million murine melanoma cells (K173522) were injected subcutaneously in the right flank. About a week later the mouse was anesthetized with isoflurane and oxygen, and the hair coat overlying the injection site was removed by clipping and applying a depilation cream. As soon as the tumor was visually detected, the mouse was re-anesthetized and a B-mode ultrasound examination was performed (7–15 MHz broad-band probe [HDI 5000 SonoCT, Philips, Bothell, WA]). In each of two orthogonal B-mode images, the length (L), width (W) and depth (D) of the tumor were measured and its volume (mL) was calculated by the formula V = 0.5 LWD, where D was measured in the two image planes and averaged (Fig. 1). Each mouse was then re-anesthetized every two to three days and the tumor volume was again measured. Once the tumor had grown to about 1 mL in volume, a catheter (26 gauge Abbocath, Abbot Ireland, Sligo, Republic of Ireland) was inserted into the tail vein, the mouse was anesthetized as described above, and 0.2 mL microbubble-containing, ultrasound contrast agent (Definity, Lantheus Medical Imaging, North Billerica, MA) was injected. The contrast agent was injected in both control and treatment groups. The sonographer making the tumor volume measurements and the contrast injection was blinded of the control and the treatment group.
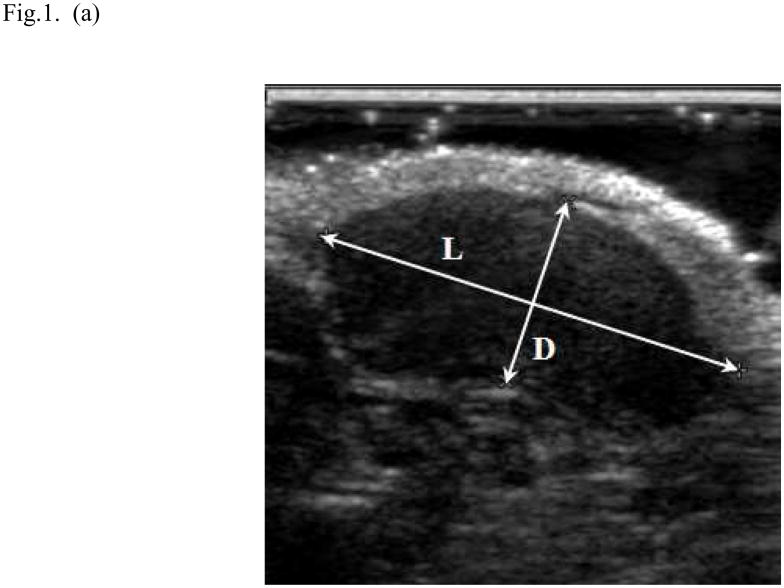
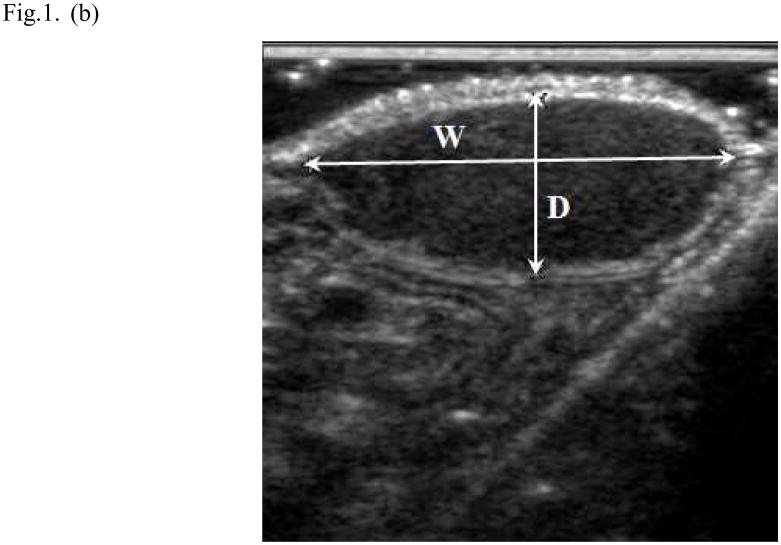
Fig. 1.
Ultrasound method for measuring tumor volume. In the longitudinal image (a) the length (L) of the tumor was 1.73cm and its depth (D) was 0.75cm; in the transverse image (b) the width (W) of the tumor was 1.65cm and its depth was 0.68cm. The volume (V) of the tumor was 1.02 mL was calculated (V = 0.5 LWD; where the depth measured in the two image planes was averaged).
In the treated group, tumor therapy was performed with low intensity ultrasound (3 MHz; continuous output; power level = 2; D150 Plus, Dynatronics Corp., Salt Lake City, UT); the transducer had an effective radiating area of 7 cm2. The effective intensity of the beam, measured using a radiation force balance (UPM 30, Ultrasound Power Meter, Ohmic Instruments Co, St Michaels, MD), was 2.4 ± 0.1 W.cm−2 (ISATA). Therapy commenced within two minutes of the completion of the injection of the contrast agent. Ultrasound gel was applied to the tumor, and the face of the therapy probe was directly applied to the skin overlying the tumor and centered to the center of the tumor. As the tumor was located subcutaneously on the animal’s flank, it was possible to direct the therapy beam parallel to, rather than towards, the lateral abdominal wall. Three one minute treatments were given with a one minute gap between each treatment (to ensure that the face of the probe remained cool, it was placed in iced water during the gap time). The transducer was moved gently back and forth over a distance of 2–3 mm during the treatment to average the exposure of the tumor spatially. In the control group of mice, the physiotherapy ultrasound probe was applied to the tumor in an identical fashion to that described above, but the machine was not switched on (a sham-treatment was performed). B-mode ultrasound measurements of the growth of the tumor continued every two to three days. Once the tumor had reached about 3 mL in volume, corresponding to 10% of body weight, each anesthetized mouse was euthanized by cervical dislocation. The time of euthanasia was used in plotting the survival curves.
Data analyses
In each mouse the time (days) from the injection of cancer cells to first visual detection of a tumor was recorded, and expressed as a mean and standard deviation across all mice. In the control and treated groups, the tumor size on the day of treatment and day of euthanasia was recorded and expressed as a mean ± standard deviation; a 2-tailed T-test (MedCalc Software, Mariakerke, Belgium) was performed to look for differences in tumor volume between the two groups. A P - value of ≤0.05 was considered statistically significant. The time (days) from the first measurement of the tumor size until euthanasia was recorded for each animal, and the survival percentage versus time curve was plotted for each group of mice (Kaplan-Meier survival curves; MedCalc Software, Mariakerke, Belgium). The log rank test was used to analyze the differences between the two survival curves, and 95% confidence limits were also calculated.
RESULTS
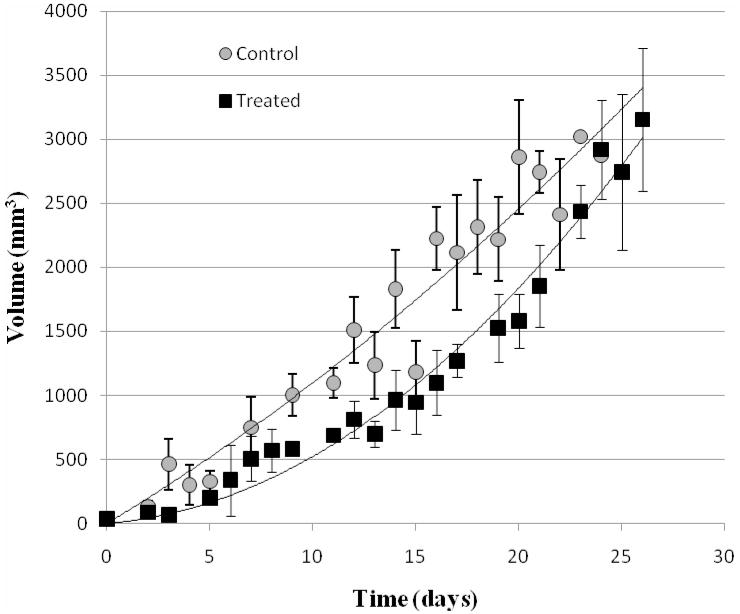
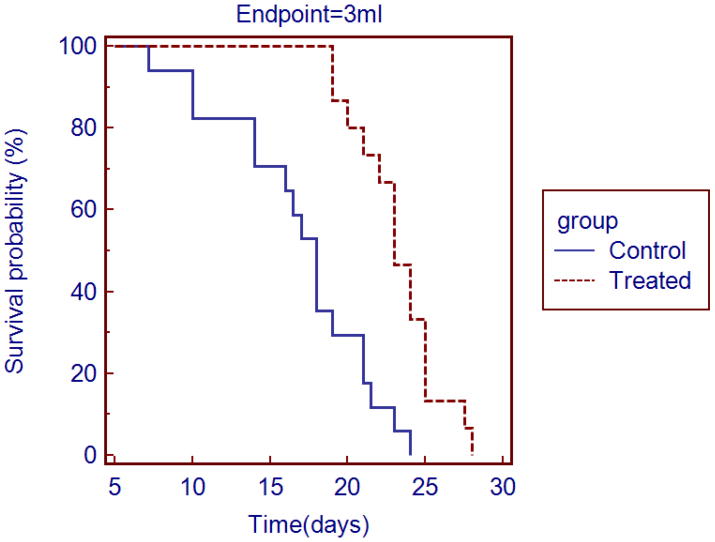
10.3 ± 4.7 days after the injection of cells, the tumor was visually detected and its volume was easily measured in the B-mode ultrasound examinations; the tumor was hypoechoic to the surrounding tissues and had distinct margins (Fig. 1). The mice recovered normally from each of the general anesthetics, including that used for treatment. At the time of therapy, there was no statistically significant difference (P = 0.36) between the sizes of the tumors of the treated and control groups of mice (treated group: tumor volume = 0.88 ± 0.38 mL; control group: tumor volume = 1.00 ± 0.35 mL). In the treated mice there was an increase in tumor volume immediately following therapy (Fig. 2). Throughout the duration of the experiment the mice exhibited normal behavior i.e. each was active, and ate and groomed itself normally. At euthanasia, there was no statistically significant difference (P = 0.67) between the size of the tumor in the treated and control groups (treated group: tumor volume = 2.88 ± 0.52 mL; control group: tumor volume = 2.78 ± 0.54 mL). In two mice, a significant cutaneous ulcer developed on the surface of the tumor and euthanasia was performed prior to the tumor reaching 3 mL in size (mouse 46 – treated group, tumor volume at euthanasia = 2.1 mL; mouse 51 – control group, tumor volume at euthanasia = 1.2 mL). The growth of the tumors continued after treatment in four mice. In the remaining 11 mice tumor growth decreased immediately after therapy but later resumed; there was no such interruption to growth in the tumors of the sham-treated mice (Fig. 2). The growth of the tumor in the treated group was slower than the control group (Fig 3). The median survival time for the treated group was 23 days and for the control group was 18 days, and survival curves were plotted (Fig. 4). The 28% increase in survival time for the treated group was statistically significant (P ≤ 0.0001); the 95% confidence interval was 2.5 to 14.7. At necropsy all tumors had distinct boundaries and there was no evidence of local invasion.
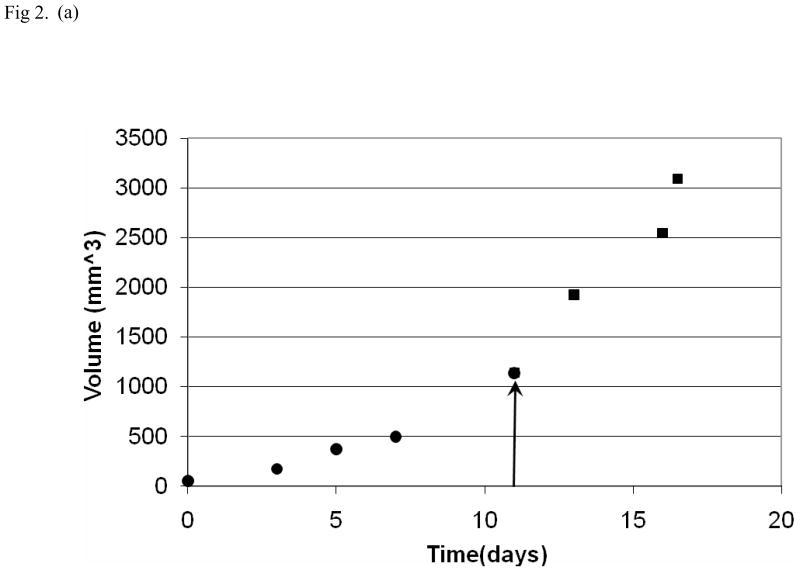
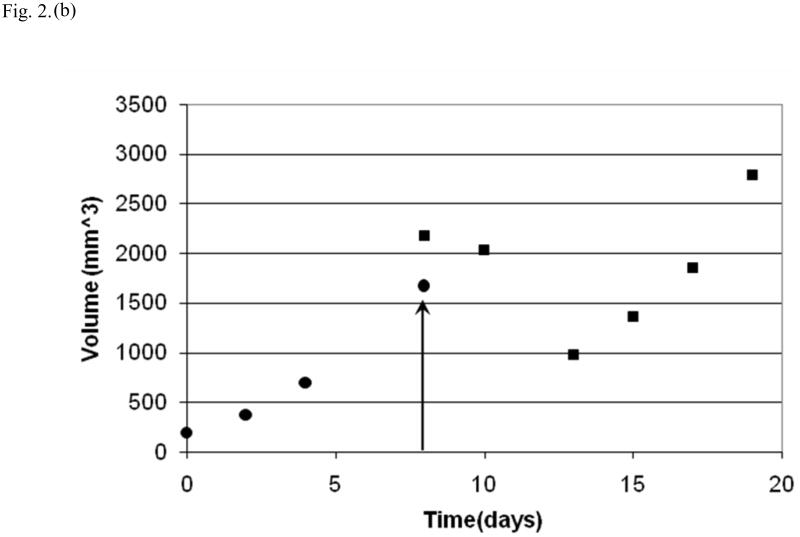
Fig. 2.
Tumor growth curves for individual mice (black dots = tumor volume before therapy; black squares = tumor volume after therapy; day of therapy indicated by arrow). In a mouse from the control group (a) there is continued growth of tumor following the sham-treatment. After low-intensity ultrasound therapy in another mouse (b), there was an immediate initial increase in tumor volume followed by a decline over the next five days and then a further increase.
Fig. 3.
Tumor growth curves for the control and the treated groups. Each point represents the average volume of all mice in the group. Bars represent the standard error of the mean.
Fig. 4.
Survival percentage versus time curves. Effect of therapy on survival percentage (days after first measurement of tumor volume to euthanasia when the volume was 3 mL) - the apparent difference between the control and treated groups was statistically significant (P ≤ 0.0001).
DISCUSSION
In this study of the growth of a primary cancer, it was demonstrated that animal survival time was increased by a single three minutes episode of antivascular ultrasound treatment. Such a finding has not been reported following therapy with the combretastatins, another form of tumor vascular disrupting agent (Chaplin and Hill 2002; Nielsen et al. 2008; Salmon and Siemann 2007). Previous contrast-enhanced ultrasound studies have shown that the murine melanoma used in this study was well perfused and that the low-intensity ultrasound therapy had a prominent antivascular effect on the tumor neovasculature (Bunte et al. 2006; Sehgal et al. 2009; Wood et al 2005, 2007, 2008). It is probable that the increased survival time found in this study was again related to the disruption of the tumor vasculature, formed as a result of angiogenesis.
Histologic studies have shown that the neoplastic cells were initially unaffected by the ultrasound therapy but they underwent liquefactive necrosis secondary to an ischemia (related to the damaged tumor neovasculature; Bunte et al. 2006). The immediate increase in tumor size after therapy was related to the production of hemorrhage and intercellular fluid. The decrease in tumor size over the next day will be related to the absorption of the intercellular fluid (Bunte et al. 2006). The subsequent reduction in tumor size has not been followed histologically but the liquefactive necrosis of the tumor cells will lead to the release of cellular fluids and necrotic cell debris, which will washed away by the remaining functional vascular system. The debris is likely to be scavenged by macrophages in the spleen and liver, as no such cells were found in the tumor in the first 24 hours following therapy (Bunte et al. 2006).
In the single episode of therapy, the low-intensity ultrasound beam covered the entire surface of the tumor and its frequency of 3 MHz ensured that it easily penetrated the full depth of the mass. Thus it would be expected that the function of much of the tumor neovasculature would have been rendered ineffective – following three minutes therapy, the overall perfusion of the tumor would have been reduced by the order of 75% (Wood et al 2008). As not all of the neovasculature was disrupted by the single episode of therapy, the surviving vessels would maintain the vascular supply to some of the cancer cells and tumor growth would continue. In contrast to the antivascular actions of the combretastatins, which preferentially targeted the vessels in the central regions of the tumor (Salmon and Siemann 2007), low intensity ultrasound therapy affected all regions of this neoplasm (Bunte et al. 2006; Sehgal et al. 2009; Wood et al. 2005, 2007, 2008); whether this observation is only characteristic of murine melanomas, or applies to antivascular ultrasound therapy of all tumors, requires further investigation. There is also a need for future studies in which multiple episodes of ultrasound treatment are given on a regular and/or fractionated basis, as it may be possible to further increase survival times and further delay tumor growth. It would be of interest to also perform such longitudinal studies on metastatic tumors to determine whether their neovasculature is as sensitive to the antivascular actions of low-intensity ultrasound therapy as that of a primary tumor.
It has been proposed that a neoplasm can be divided into two compartments of cells – one compartment related to the vascular structures and the other to the neoplastic cells (Folkman 2001). The low-intensity ultrasound therapy is aimed primarily at the vascular compartment which raises the possibility of combination therapy in which a second therapy, primarily aimed at the neoplastic cells, is also used. Such a combination therapy could utilize one of a broad range of chemotherapeutic agents (Chaplin and Hill 2002) which could be injected intravenously immediate prior to the insonation of the tumor. The chemotherapeutic agent could be trapped in the tumor as the tumor neovasculature is disrupted by the ultrasound therapy – such sonographic trapping may enhance the efficacy of the therapeutic agent in destroying the neoplastic cells and it may also be possible to utilize lower doses of the chemotherapeutic agent so minimizing their side effects on the animal as a whole. The role of such a combination of therapies would be to further increase cell death leading to decreased tumor growth and to increased survival times.
In conclusion, this study demonstrated that antivascular ultrasound therapy reduced the growth rate of an implanted murine melanoma and increased the animal’s survival time. The therapy provides a further example of tumor vascular disruption and its future clinical potential should be investigated.
Acknowledgments
This work was supported by a grant from the Center for Technology Transfer, University of Pennsylvania, and NIH grant no. CA139657.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baguley BC. Antivascualr therapy of cancer: DMXAA. Lancet Oncol. 2003;4:141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- Beerepoot LV, Radema SA, Witteveen EO, Thomas T, Wheeler C, Kempin S, Voest EE. Phase I clinical evaluation of weekly administration of the novel vascular-targeting agent, ZD6126, in patients with solid tumors. J Clin Oncol. 2006;24:1491–1498. doi: 10.1200/JCO.2005.02.7458. [DOI] [PubMed] [Google Scholar]
- Briones JR, Serra AC, Lozano AG, Ramón-Borja JC, Juan II, Narbón ES. High-intensity focused ultrasound in small renal masses. Adv Urol. 2008 doi: 10.1155/2008/809845. article ID 809845, Epub 2008 Dec 31, 5 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte RM, Ansaloni S, Sehgal CM, Lee WM-F, Wood AKW. Histpathological observations of the antivascular effects of physiotherapy ultrasound on a murine neoplasm. Ultrasound Med Biol. 2006;32:453–61. doi: 10.1016/j.ultrasmedbio.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Chabot GG, Bissery MC, Corbett TH, Rutkowski K, Baker LH. Pharmacodynamics and causes of dose-dependent pharmacokinetics of flavone-8-acetic acid (LM-975; NSC-347512) in mice. Cancer Chemother Pharmacol. 1989;24:15–22. doi: 10.1007/BF00254099. [DOI] [PubMed] [Google Scholar]
- Chaplin DJ, Hill SA. The development of combretastatin A4 phosphate as a vascular targeting agent. Int J Radiat Oncol Biol Phys. 2002;54:1491–1496. doi: 10.1016/s0360-3016(02)03924-x. [DOI] [PubMed] [Google Scholar]
- Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumor therapy. Br J Cancer. 1982;45:136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J, Hill SA, Hobson B. Vascular occlusion and tumour cell death. European J Cancer Clin Oncol. 1983;19:271–275. doi: 10.1016/0277-5379(83)90426-1. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. In: Braunwald E, Fauci AS, Kasper DL, Longo DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison’s textbook of internal medicine. New York: McGraw-Hill; 2001. pp. 517–530. [Google Scholar]
- Hande KR, Hagey A, Berlin J, Cai Y, Meek K, Kobayashi H, Lockhart AC, Medina D, Sosman J, Gordon GB, Rothenberg ML. The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: results of a phase 1 study. Clin Cancer Res. 2006;12:2834–2840. doi: 10.1158/1078-0432.CCR-05-2159. [DOI] [PubMed] [Google Scholar]
- Hinnen P, Eskens FA. Vascular disrupting agents in clinical development. Br J Cancer. 2007;96:1159–65. doi: 10.1038/sj.bjc.6603694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert JW. Vascular disrupting agents. Bioorg Med Chem. 2007;15:605–615. doi: 10.1016/j.bmc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Maestroni U, Ziveri M, Azzolini N, Dinale F, Ziglioli F, Campaniello G, Frattini A, Ferretti S. High intensity focused ultrasound (HIFU): a useful alternative choice in prostate cancer treatment. Preliminary results. Acta Biomed. 2008;79:211–216. [PubMed] [Google Scholar]
- Nielsen T, Murata R, Maxwell RJ, Stødkilde-Jørgensen H, Ostergaard L, Horsman MR. Preclinical studies to predict efficacy of vascular changes induced by combretastatin A-4 disodium phosphate in patients. Int J Radiat Oncol Biol Phys. 2008;70:859–866. doi: 10.1016/j.ijrobp.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Rustin GJ, Galbraith SM, Anderson H, Stratford M, Folkes LK, Sena L, Gumbrell L, Price PM. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol. 2003a;21:2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
- Rustin GJ, Bradley C, Galbraith S, Stratford M, Loadman P, Waller S, Bellenger K, Gumbrell L, Folkes L, Halbert G. 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent: phase I clinical and pharmacokinetic study. Br J Cancer. 2003b;88:1160–1167. doi: 10.1038/sj.bjc.6600885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon BA, Siemann DW. Characterizing the tumor response to treatment with combretastatin A4 phosphate. Int J Radiat Oncol Biol Phys. 2007;68:211–217. doi: 10.1016/j.ijrobp.2006.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal CM, Cary TW, Arger PH, Wood AKW. Delta projection imaging on contrast-enhanced ultrasound to quantify tumor microvasculature and perfusion. Acad Radiol. 2009;16:71–78. doi: 10.1016/j.acra.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susani M, Madersbacher S, Kratzik C, Vingers L, Marberger M. Morphology of tissue destruction induced by focused ultrasound. Eur Urol. 1993;23 (Suppl 1):34–38. doi: 10.1159/000474677. [DOI] [PubMed] [Google Scholar]
- Tozer GM, Kanthou C, Lewis G, Prise VE, Vojnovic B, Hill SA. Tumour vascular disrupting agents: combating treatment resistance. Br J Radiol. 2008;81(Spec No 1):S12–20. doi: 10.1259/bjr/36205483. [DOI] [PubMed] [Google Scholar]
- Van Leenders GJLH, Beerlage HP, Ruijter ET, de la Rosette JJ, van de Kaa CA. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localized adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391–394. doi: 10.1136/jcp.53.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AKW, Ansaloni S, Ziemer LS, Lee WM-F, Feldman MD, Sehgal CM. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med Biol. 2005;31:1403–10. doi: 10.1016/j.ultrasmedbio.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AKW, Bunte RM, Cohen J, Tsai JH, Lee WM-F, Sehgal CM. The antivascular action of physiotherapy ultrasound on a murine tumor: role of a microbubble contrast agent. Ultrasound Med Biol. 2007;33:1901–10. doi: 10.1016/j.ultrasmedbio.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AKW, Bunte RM, Price H, Deitz M, Tsai JH, Lee WM-F, Sehgal CM. The disruption of murine tumor neovasculature by low-intensity ultrasound – comparison between 1 MHz and 3 MHz sonication frequencies. Acad Radiol. 2008;15:1133–41. doi: 10.1016/j.acra.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]