Abstract
Carnitines are involved in mitochondrial transport of fatty acids and are of critical importance for maintaining normal mitochondrial function. This review summarizes recent experimental and clinical studies showing that mitochondrial dysfunction secondary to a disruption of carnitine homeostasis may play a role in decreased NO signaling and the development of endothelial dysfunction. Future challenges include development of agents that can positively modulate L-carnitine homeostasis which may have high therapeutic potential.
1. Introduction
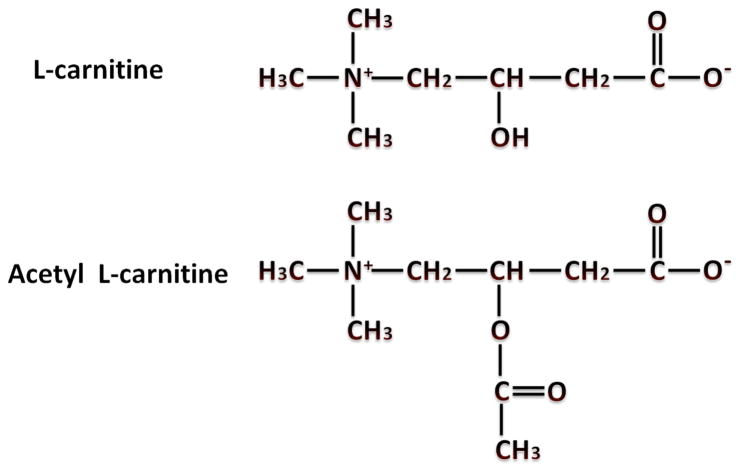
L-carnitine is a ubiquitously occurring trimethylated amino acid that plays an important role in the transport of long chain fatty acids across the inner mitochondrial membrane [1]. Carnitines exist either as free carnitines or as acylcarnitines (Figure 1 A). The acylcarnitines are products of the reaction in which acyl moieties are transferred to carnitine from acyl-CoA. These acyl groups vary in length from short chain (acetyl) to long chain (palmitoyl).
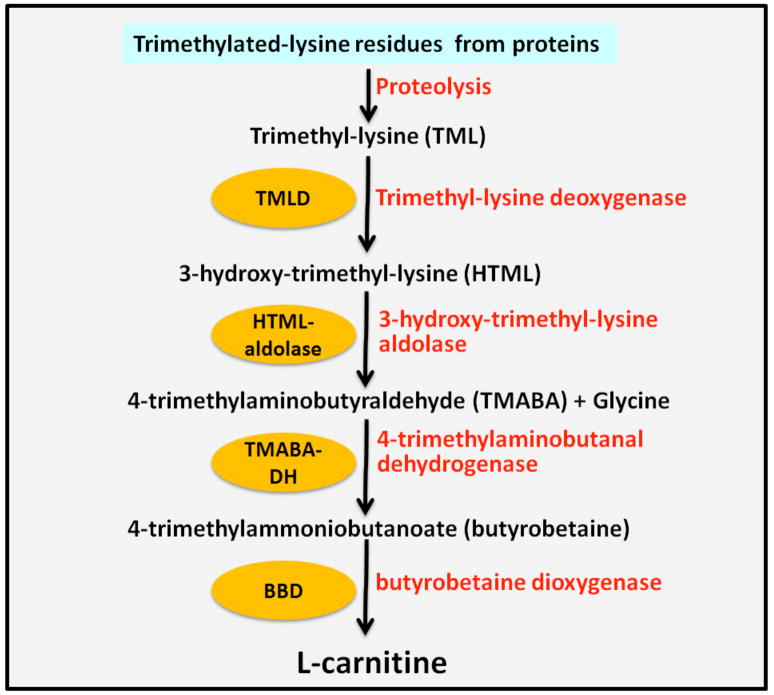
Figure 1.
Chemical structures of L-carnitine and Acetyl L-carnitine (Panel A) and the Carnitine Biosynthetic Pathway (Panel B). Abbreviations: TML, trimethyl-lysine; TMLD, trimethyl-lysine deoxygenase; HTML, 3-hydroxy-trimethyl-lysine; TMABA, 4-trimethylaminobutyraldehyde; TMABA-DH, 4-timethylaminobutanal dehydrogenase, BBD, butyrobetaine dioxygenase.
This reaction is catalyzed by a family of enzymes known as acyltransferases. These enzymes differ on the basis of the structural specificity of the acyl group and their sub-cellular localization. Carnitine deficiency or abnormalities in the carnitine acyltransferase systems results in a reduced β-oxidation of fatty acids and therefore, reduced energy (ATP) production. Recent studies suggest that mitochondrial dysfunction, secondary to a disruption of carnitine homeostasis, may also play a role in the loss of nitric oxide (NO) signaling and the development of endothelial dysfunction associated with a variety of cardiovascular diseases [2].
2. Carnitine Biosynthesis
Carnitine in humans is derived from diet and de novo biosynthesis using lysine and methionine. The main dietary sources of carnitine are red meat, fish, and dairy products which can supply 2 to 12 μmols/day/kg of body weight, whereas 1–2 μmols of carnitine is endogenously synthesized [3]. The main sites of L-carnitine synthesis are the kidney, liver and brain. After synthesis, carnitine is transported through the circulation and is then taken up by other tissues through active transport systems. In the kidney, carnitine and butyrobetaine are reabsorbed efficiently to minimize the urinary loss. Sodium dependent cationic transporter (OCTN2) [4] is the primary known transporter responsible both for transport of carnitine to other tissues and reabsorption in kidney.
Carnitine biosynthesis is a multi-step process (Figure 1 B). Trimethyl-lysine residues are the primary precursors for carnitine biosynthesis, which are hydroxylated on the third carbon by the enzyme trimethyl-lysine deoxygenase (TMLD) in mitochondria of liver, kidney, heart, muscle and brain [5]. The product of this reaction is 3-hydroxy-trimethyl-lysine which is converted to 4-trimethylaminobutyraldehyde and glycine by a reaction catalyzed by 3-hydroxy-trimethyl-lysine aldolase. 4-trimethylaminobutyraldehyde is then dehydrogenated by 4-trimethylaminobutanal dehydrogenase (TMABA-DH) results in the formation of 4-trimethylammoniobutanoate, also known as butyrobetaine. Finally, butyrobetaine is hydroxylated on the third carbon by butyrobetaine dioxygenase (BBD) to form L-carnitine.
3. Functions of Carnitine
It is well established that carnitines play an important role as carriers of activated fatty acids across the inner mitochondrial membrane and are essential for energy production through fatty acid metabolism. Carnitines are also involved in the removal of accumulated toxic fatty acyl-CoA metabolites, and help in buffering the balance between free and acyl-CoA. Carnitine is present in the form of either free carnitine (nonesterified molecule; FC), or acylcarnitines (esterified form; AC). Thus, the AC/FC ratio is a measure of acylated carnitines versus the free carnitines. L-carnitine deficiency can raise the AC/FC ratio resulting in a form of functional carnitine deficiency. Other factors such as enzymatic alterations in carnitine metabolism can also result in higher levels of acylated carnitines and hence an elevated AC/FC ratio. Thus, a low AC/FC ratio suggests healthy mitochondria whereas a high AC/FC ratio suggests a decreased mitochondrial capacity for energy production.
4. Carnitine Acyltransferases
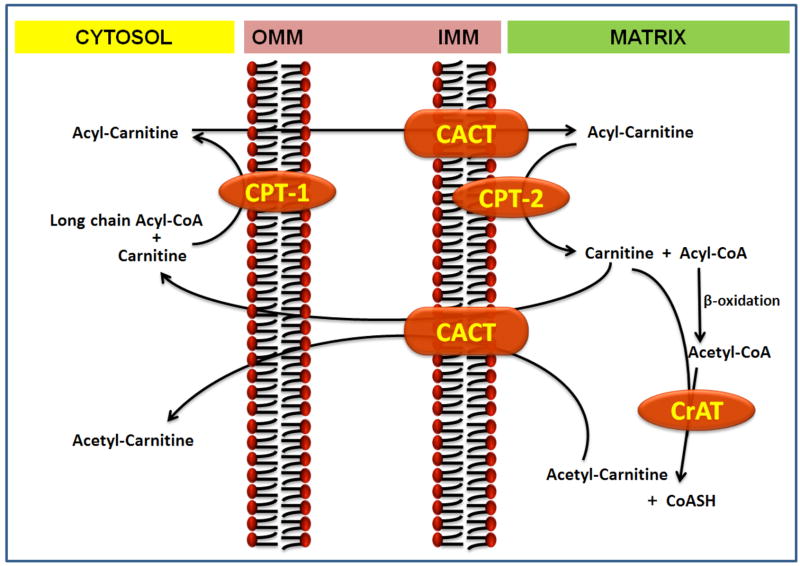
Carnitine acyltransferases reversibly transfer the acyl group from acyl-CoA to carnitine. These can be divided into 3 types: carnitine palmitoyl transferase (CPT), carnitine octanoyltransferase (COT), and carnitine acetyltransferase (CrAT), based upon their specificity to acyl groups and their cellular localization. CrAT uses short-chain acyl groups (C1-C4) as substrate, whereas COT uses both medium and long-chain fatty acids (C5-C12) having a preference for medium chain acyl-CoA. CPT transfers medium and long chain acyl-CoA (>C12) to carnitine with a preference for long chain acyl-CoA, at least at physiological concentrations of carnitines. The CPT system is an important component of the “carnitine shuttle” which facilitates the transport of long-chain fatty acids from cytosol into the mitochondrial matrix, the site of β-oxidation (Figure 2). This transport system consists of carnitine palmitoyl transferase 1 (CPT1) localized in the outer mitochondrial membrane, the carnitine-acylcarnitine translocase (CACT), an integral inner membrane protein, and carnitine palmitoyltransferase 2 (CPT2) localized on the inner mitochondrial membrane [6]. CPT1 conjugates carnitine with long chain fatty acids, carnitine acylcarnitine translocase (CACT) transfers the acylcarnitine across the inner plasma membrane and CPT2 conjugates the fatty acid back to Coenzyme A for subsequent β-oxidation. CPT-1 has different isoforms with tissue specific expression, CPT-1A (liver), CPT-1B (muscle), and CPT-1C (brain) [7]. Finally, carnitine acetyl transferase (CrAT), which resides in the matrix, is able to reconvert the short- and medium chain acyl-CoAs into acylcarnitines using intra-mitochondrial carnitine. Decreased CrAT activity leads to increased levels of acyl-CoA, which leads to inhibition of multiple enzymatic processes involved in oxidative metabolism.
Figure 2.
The Carnitine Shuttle and role of carnitine in the mitochondrial oxidation of fatty acids. Abbreviations: CPT-I, carnitine palmitoyltransferase-1; CPT-II, carnitine palmitoyltransferase-2; CACT, carnitine acylcarnitine translocase; CrAT, carnitine acetyl transferase; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane.
5. Carnitine Transporters
Carnitine transport is mediated by a family of organic cation transporters (OCTs). OCTs play an important role in the intracellular carnitine homeostasis. Three main members of this family are OCTN1, OCTN2, and OCTN3. OCTN1 is a lower-affinity mitochondrial carnitine transporter. It is a pH-dependent and sodium-independent multispecific organic cation carrier [8] which may be energized by the proton antiport mechanism in renal epithelial cells [9]. Newly synthesized L-carnitine is also transported through the circulation and effectively absorbed by other tissues by active sodium-dependent transport. OCTN2 is the sodium-dependent organic cation transporter [4] and this is the most important controlling factor for carnitine pools in the plasma membrane [10]. It also mediates cellular uptake and absorption by the kidneys of both carnitine as well as its precursor, 4-trimethylaminobutyric acid (butyrobetaine, BB) [11]. OCTN2 has a Km of 2–6 μM for carnitine and is expressed in a variety of tissues including muscle, heart, kidney, and fibroblasts [12,13]. OCTN3 is a mammalian peroxisomal membrane carnitine transporter which is uniquely involved in intracellular carnitine-dependent transport [14]. It is expressed exclusively in testes, kidney, and intestine and has the highest specificity for carnitine [14].
6. Inborn Errors of Metabolism
Inborn errors of metabolism are one of the largest groups of genetic diseases that lead to cardiomyopathy which has been associated with high morbidity and mortality. Inborn errors of metabolism mainly include diseases such as fatty acid oxidation defects, mitochondrial dysfunctions, organic acidurias, as well as lysosomal and glycogen storage disorders. In the last few years mouse models have been developed to study the inborn errors of fatty acid oxidation with phenotypes representative of the human diseases (as shown in Table 1). These diseases will be discussed briefly below.
Table 1.
Mouse models of Inborn Errors of Metabolism
| Human disease | Phenotype of mouse model | References |
|---|---|---|
| VLCAD Deficiency | Mild hepatic steatosis, cold intolerance, increased C16- C18-acylcarnitines in bile and serum of VLCAD−/− mice. | Cox et al. 2001 [51] Exil et al. 2003 [52] |
| MCAD deficiency | MCAD−/− mice developed an organic aciduria and fatty liver, sporadic cardiac lesions and showed profound cold intolerance. | Tolwani et al. 2005 [54] |
| LCAD Deficiency | Sudden death, fasting intolerance, cold intolerance, hypoketotic-hypoglycemia in LCAD−/− mouse. | Kurtz et al. 1998 [53] |
| SCAD deficiency | Mitochondrial swelling, micro-vesicular fatty changes in hepatocytes, non-ketotic hypoglycemia, and cold intolerance in SCAD deficient BALB/cByJ mice. | Armstrong et al. 1993 [55] |
| OCTN2 deficiency | OCTN2-type carnitine transporter is dysfunctional in JVS mice, exhibiting hereditary systemic carnitine deficiency. | Yokogawa et al. 1999 [57] |
| CPT-1a deficiency | Decreased Cpt-1a mRNA expression in liver, heart, brain, testis, kidney, and white fat in CPT-1a +/− mice. | Nyman et al., 2005 [50] |
| MTP deficiency | Fetal growth retardation, neonatal hypoglycemia, and sudden death after birth in Mtpa(−/−) mice. | Ibdah et al. 2001 [56] |
VLCAD: very long-chain acyl-CoA dehydrogenase; MCAD: medium-chain acyl-CoA dehydrogenase; LCAD: long-chain acyl-CoA dehydrogenase; SCAD: short-chain acyl-CoA dehydrogenase; MTP: mitochondrial trifunctional protein
-
Fatty oxidation disorders (FODs) are genetic defects in which body is unable to breakdown fatty acids to make energy. A common pathological factor of fatty acid oxidation disorder or mitochondrial dysfunction is decreased energy production.
-
Coenzyme A dehydrogenase deficiencies:
Very long-chain acyl-coenzyme A dehydrogenase deficiency (VLCAD) results from mutations in the acyl-Coenzyme A dehydrogenase, very long chain (ACADVL) gene. These mutations lead to inadequate levels of the enzyme, very long-chain acyl-coenzyme A dehydrogenase resulting in the accumulation of long-chain fatty acids and patients are unable to make ketone bodies for energy. Characteristic symptoms of this disorder are lethargy and hypoglycemia. During infancy or childhood, common symptoms are cardiomegaly or skeletal myopathy in addition to the common Reye-like illness [15].
Medium Chain Acyl-CoA Dehydrogenase (MCAD) deficiency is an autosomal recessive disorder of fatty acid oxidation. Therefore, individuals who are homozygous or compound heterozygous for an MCAD mutation can also generate abnormal MCAD proteins and inefficient enzymatic activity to metabolize medium-chain fatty acids. This disorder was not described until 1983 and the estimated incidence of MCAD is approximately 1:12,000 live births. The gene for MCAD is located on chromosome 1p31. Over 25 MCAD gene variants have been reported. However, only one of these mutations, K304E accounts for the majority of MCAD mutations identified to date. MCAD presents with an increase in C6-C10 dicarboxylics in the plasma. If undiagnosed, the disorder can lead to metabolic collapse, coma and even death. Infants who survive run the risk of severe brain damage. Treatment involves strict diet without medium-chain fats. It is a life-long disorder. MCAD-deficient patients can exhibit a number of conditions: hypoglycemia, vomiting, lethargy, encephalopathy, respiratory arrest, hepatomegaly, seizures, apnea, cardiac arrest, coma, and sudden death. Long-term outcomes may include developmental and behavioral disability, chronic muscle weakness, failure to thrive, cerebral palsy, and attention deficit hyperactivity disorder (ADHD). MCAD deficiency is the cause of 1–3% of sudden infant death syndrome.
Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) deficiency is a genetic syndrome with many similarities to MCAD deficency. LCHAD is 1 of 3 enzymatic activities that make up the trifunctional protein of the inner mitochondrial membrane. The other 2 activities of the protein are 2-enoyl CoA hydratase (LCEH) and long-chain 3-ketoacyl CoA thiolase (LCKT). This enzyme complex metabolizes long-chain fatty acids, and the LCHAD activity is specific for compounds of C12-C16 chain length. The genes for the alpha and beta subunits have been localized to chromosome 2. LCHAD deficiency is an autosomal recessive trait and affected infants present in infancy with acute hypoketotic hypoglycemia. These episodes typically appear for the first time after a fast. The clinical manifestations of LCHAD deficiency result from direct toxicity of the build up of long-chain acyl-CoA esters causing cardiomyopathy and cardiac arrhythmias or from a block in long-chain fatty acid oxidation that leads to an inability to synthesize ketone bodies and/or adenosine triphosphate from long-chain fatty acids. The LCHAD gene has been cloned and a common mutation, G1528C, has been identified in 87% of mutant alleles. LCHAD deficiency affects a number of organ systems: the CNS (secondary to hypoketotic hypoglycemia), the heart (cardiomyopathy develops due to the underlying energy deficiency), and the liver (hepatomegaly due to fat accumulation and fibrosis).
Short-chain acyl-coenzyme A dehydrogenase deficiency (SCAD): The SCAD enzyme is an intra-mitochondrial enzyme of beta oxidation that catalyzes the oxidation of C4-C6 carbon acyl-CoA fatty acid esters to produce 2 trans-enoyl-CoA fatty acid esters. SCAD was initially thought to produce severe problems including progressive muscle weakness, hypotonia, acidemia, developmental delay, and even early death. However, this SCAD deficiency appears to be both more common and, in many cases, less severe than initially thought. The use of tandem mass spectrometry technology has identified many more SCAD many of whom show no obvious signs of disease. Symptoms include developmental delay, metabolic acidosis and cardiomyopathy [16,17].
3-hydroxyacyl-coenzyme A dehydrogenase deficiency (HADH) is caused by the mutation in the HADH gene. Inadequate level of the enzyme prevents the metabolization of short and medium-chain fatty acids. Mutations in the HADH gene have been reported in a rare disorder known as familial hyperinsulinemic hypoglycemia that is characterized by abnormally high levels of insulin and unusually low levels of blood sugar. Three HADH mutations have been identified: one that is thought to alter the 3-dimensional structure of the protein destabilizing it. Another mutation deletes a small section of the HADH gene, which likely leads to a truncated enzyme that is again unstable. The third type of mutation is a splicing mutant. Initial symptoms of this disorder occur during infancy or early childhood and include lethargy, hypoglycemia, hypotonia, liver problems, and hyperinsulinism. The gene has been localized to chromosome 4.
Primary carnitine deficiency or carnitine transporter defect is an autosomal recessive disorder of fatty acid oxidation caused by heterozygous mutations in the SLC22A5 gene that encodes the high-affinity carnitine transporter, OCTN2. Metabolic cardiomyopathy is the hallmark symptom of this disease. In addition, patients also suffer from central nervous system abnormalities and gastrointestinal symptoms such as recurring abdominal pain and diarrhoea. There is increased loss of carnitine in the urine, low serum carnitine levels (0–5 μM, normal 25–50 μM) and decreased carnitine accumulation in tissues.
Carnitine palmitoyl transferase 1 (CPT-1) deficiency: is a fatty acid oxidation defect that results in accumulation of toxic long chain substrates and impedes mitochondrial β-oxidation. Diagnosis is suspected from the elevation of free and short chain acylcarnitine, with low levels of long-chain acylcarnitine. CPT1 deficiency can be identified by newborn screening using MS/MS [7]. The disease symptoms include hypoketotic hypoglycemia, hepatomegaly, muscle weakness, and elevated levels of carnitine in the blood. CPT-1 deficient patients are treated with dietary management strategies such as frequent feedings with medium-chain triglycerides and strict avoidance of fasting [15].
Carnitine palmitoyl transferase 2 (CPT-2) deficiency: involves the ineffective conversion of long-chain to acyl-CoAs. The prominent long-chain acylcarnitines found in plasma indicates that the accumulating acylcarnitines may be transported out of mitochondria. A mild adult and severe infantile/neonatal form of deficiency have been reported though more prevalent in adolescents and young adults. Main symptoms are hyperammonemia, metabolic acidosis, hypoketotic hypoglycemia with elevated creatine kinase and reduced carnitine levels [18].
Carnitine-acylcarnitine translocase (CACT) deficiency: is a disorder of fatty acid and carnitine transport. Two types have been described, one with neonatal onset and the other with onset later in infancy/early childhood. In neonatal onset CACT, there is a metabolic crisis that often results in death from cardiopulmonary complications and/or liver failure. When the onset is later in infancy/early childhood, the affected person shows hypoglycemia, but not cardiomyopathy. CACT deficiency is most prevalent in the neonatal period accompanied with seizures, irregular heart beat, and apnea. Diagnostic markers of CACT deficiency are extremely low carnitine levels (<5 μM) and plasma acylcarnitine profile showing a marked increase in long-chain acylcarnitines and decreased levels of free carnitines. Symptoms include clinical encephalopathy, hepatomegaly, and arrhythmias as well as hyperammonaemia and elevation of creatine kinase levels.
-
-
Mitochondrial diseases: These conditions are inherited via maternal transmission. It is estimated that mitochondrial disease affects between 40,000 and 70,000 Americans with an occurrence of 1: 2,500 to 1: 4,000 births. Although not directly related to this review it is worth briefly describing the most common syndromes:
Leigh syndrome: is a mitochondrial-DNA associated disorder caused by abnormalities of mitochondrial energy generation. The main symptoms are progressive neurological disease with motor and intellectual developmental delay and increased lactate concentration in blood and/or cerebrospinal fluid. Approximately 10%–20% of individuals with Leigh syndrome have either the T8993G or the T8993C mutation in MT-ATP6 gene.
Myoclonic epilepsy with ragged red fibers (MERRF): is a maternally inherited disorder of oxidative phosphorylation caused by specific point mutations within the mitochondrial tRNA (Lys) gene. Mitochondrial dysfunction in the central nervous system of patients with MERRF accounts for the neurological manifestations of the disease. Other symptoms include epilepsy, ataxia, weakness, and dementia.
Pearson syndrome: is caused by large deletions within the mitochondrial genome. Main symptoms are pancreatic insufficiency, sideroblastic anemia due to bone marrow dysfunction, and renal tubular leak. Treatment consists of correcting electrolyte disturbances, regular blood transfusion, and iron chelation therapy [19].
Mitochondrial neurogastrointestinal encephalopathy (MNGIE): is caused by mutations in the gene encoding thymidine phosphorylase. Abnormalities in the digestive system are the most common feature of MNGIE. Diagnosis involves screening for the thymidine phosphorylase gene.
Leber congenital amaurosis (LCA): is the most severe form of the inherited retinal dystrophies causing blindness or severe visual impairment before one year of age. Linkage analysis, homozygosity mapping, and candidate gene analysis has led to the identification of 14 genes mutated in individuals with LCA.
Alpers syndrome: is a severe neurodegenerative disorder frequently associated with mitochondrial DNA polymerase γ gene (POLG1) mutations. It is a mitochondrial dysfunction disorder where intractable epilepsy is followed by liver failure.
Kearns-Sayre syndrome (KSS): is a rare genetic abnormality. Classified as a mitochondrial cytopathy, the primary pathology of this syndrome is a disturbance of the genes in the mitochondrial genome that encode the proteins required for the assembly of a functional respiratory chain. Onset of this disease occurs before age 20, and manifests as chronic progressive external ophthalmoplegia and retinal degeneration [20].
Myoclonic epilepsy lactic acidosis and stroke-like syndrome (MELAS): is a rare form of dementia affecting less than 20,000 people in USA. The underlying causes are mutations in the mitochondrial genome that lead to a defective mitochondrial respiratory chain resulting in impaired ATP production.
-
Lysosomal storage disorders (LSDs): are caused by the accumulation of macromolecules (proteins, polysaccharides, lipids) in the lysosomes because of a genetic failure to manufacture the lysosomal hydrolases. These are the lysosomal enzymes that are needed for the breakdown of macromolecules within the lysosome. LSDs can also occur due to the deficient activity of transporters or integral membrane proteins within the lysosome itself [21].
Pompe’s disease is a lysosomal glycogen storage disease caused by a recessively inherited deficiency of α-1,4-glucosidase (acid maltose). In infants it leads to severe hypertrophic cardiomyopathy and muscle weakness.
I-cell disease or mucolipidosis II is a rare inherited metabolic disorder characterized by defective mannose phosphorylation resulting in mistargeting of glycoproteins normally destined for lysosomes. The symptomology is coarse facial features, skeletal abnormalities, and mental retardation. These symptoms all become obvious during infancy.
Tay-Sachs disease is a fatal genetic lipid storage disorder in which harmful quantities of ganglioside GM2 build up in tissues and nerve cells in the brain. The condition is caused by insufficient activity of beta-hexosaminidase A that normally catalyzes the biodegradation of gangliosides. Infants with Tay-Sachs disease become blind, deaf, and are unable to swallow. As these symptoms advance they eventually lead to muscle atrophy and paralysis. Other neurological symptoms include dementia, seizures, and an increased startle reflex to noise.
Sphingolipids mucopolysaccharidosis I (MPS-I) is caused by failure to synthesize an enzyme needed to break down proteoglycans.
Organic acidurias disorder results from a lack of mitochondrial enzymes that metabolize CoA activated carboxylic acids. This disorder results in the accumulation of fatty acids in the plasma and tissues. It leads to long term neurological effects, encephalopathy, and disturbance in mitochondrial energy production. Therapy involves protein restriction, carnitine supplementation, and treating acute decompensation with a combination of high-energy fluids and drugs to remove toxic metabolites [19].
7. Role of Carnitines in Mitochondrial Function and Apoptosis
Disruption of mitochondrial function is acknowledged as a critical event in a number of pathological conditions, including hypoxia-ischemic injuries, stroke, and diabetes. The carnitine acyltransferase pathway has been shown to be of critical importance for maintaining normal mitochondrial function. Fatty acids are transported via carnitine into mitochondria for their subsequent oxidation to generate ATP. Carnitines also remove the acyl groups from the mitochondria as acylcarnitines. Increase in free fatty acid levels can induce mitochondrial dysfunction resulting in cell death and/or enhanced secondary generation of reactive oxygen species. These effects can be attenuated with L-carnitine treatment [22]. In addition, L-carnitine can suppress the palmitoyl-CoA induced dysfunction, membrane permeability transition (MPT), and cytochrome c release of isolated mitochondria as well as reduce the mitochondrial swelling and depolarization induced by long chain fatty acids (LCFAs) and palmitoyl-CoA through a protective mechanism thought to occur in and around the mitochondrial membrane [23]. L-carnitine also suppresses oleic acid-mediated MPT by accelerating β-oxidation [24]. Studies have also shown that carnitine has a protective effect both on mitochondria and in whole cells by inhibiting free fatty acid-induced mitochondrial membrane damage and/or its secondary effects [23,25]. The protective effects of carnitine and related metabolites have been postulated to be due to improved energy metabolism and the inhibition of electron leakage from mitochondrial electron transport systems [26]. Administration of carnitine has also been shown to decrease free fatty acids in serum and tissues and prevent tissue injury in juvenile visceral steatosis (JVS) mice that lack a carnitine transporter [27]. Mitochondria are also emerging as important signaling transducers in the apoptotic pathway in a variety of disease states. Previous studies have shown a close relationship between carnitine and mitochondria. Cultured neonatal rat cardiac myocytes were studied to assess mitochondrial alterations during apoptosis. It was shown that CPT1 inhibition, ceramide accumulation, and complex III inhibition were downstream events in cardiac apoptosis mediated by long-chain fatty acids, such as palmitate [28]. L-carnitine has also been shown to inhibit mitochondria-dependent apoptosis both in vivo and in vitro [29,30].
8. Mitochondrial Dysfunction and Nitric Oxide Signaling
The role of mitochondria in cardiovascular pathologies is being extensively scrutinized. In cardiovascular diseases, there is increased production of reactive oxygen species (ROS). This leads to endothelial dysfunction and impaired endothelium dependent vasodilation by reducing the bioavailability of nitric oxide (NO). Studies on the mechanism(s) underlying the effects of L-carnitine in cardiovascular diseases have demonstrated that the chronic administration of L-carnitine can reduce blood pressure and attenuate the inflammatory process associated with arterial hypertension [31]. Carnitine treatment has also been shown to increase post-ischemic blood flow in patients with peripheral vascular disease, indicative of an improvement in functional circulatory reserve [32]. L-carnitine has been shown to limit ischemia reperfusion injury by preventing long-chain acyl-CoA accumulation and subsequent production of ROS by damaged mitochondria. L-carnitine also improves repair mechanisms for oxidative-induced damage to membrane phospholipids and reduces the ischemia-induced apoptosis and the consequent remodeling of the left ventricle [32]. L-carnitine restores endothelial dysfunction present in spontaneously hypertensive rats (SHR) and has the ability to increase the release of the vasodilator PGI2 and enhance the production of TXA2 in normotensive rats. An acute L-carnitine treatment to SHR was also able to restore endothelium-dependent relaxations of aortic rings [33].
NO production and availability plays a major role in determining endothelial cell functions. Decreases in endothelial nitric oxide synthase (eNOS) expression and/or activity leading to decreased bioavailable NO generation has been linked to endothelial dysfunction and vascular remodeling. We have observed reduced mitochondrial function as measured by increased lactate/pyruvate ratios, increased uncoupling protein-2 (UCP-2) and decreased mitochondrial superoxide dismutase (SOD-2) protein levels in a lamb model of pulmonary hypertension [2]. Further, the mitochondrial dysfunction was associated with decrease in interaction of HSP90 with eNOS, which correlated with a progressive decrease in relative eNOS activity, and increased NOS-dependent superoxide levels [2]. Thus, L-carnitine supplementation may help to maintain eNOS-HSP90 interactions, NO signaling, and normal endothelial function. Finally, GPLC (a novel form of carnitine) supplementation with exercise has been shown to elevate NO levels in human subjects. Besides, decreasing oxidative stress, GPLC supplementation was associated with normalization/enhancement of circulating NO levels [34].
9. Potential of Carnitine as a Therapeutic Agent
In recent years, L-carnitine has become more prevalent in therapies aimed at improving mitochondrial energy metabolism and has been shown to be beneficial in cardiovascular pathologies. The therapeutic potential of L-carnitine in the management of diseases such as peripheral vascular diseases, congestive heart failure, angina, and anthracycline-induced cardiotoxicity has been frequently reported [35,36]. Carnitine availability becomes a limiting step for β-oxidation in certain physiological and pathological diseases, and carnitine supplementation enhances fatty acid metabolism in the mitochondria, restoring normal mitochondrial function by maintaining the equilibrium between acyl-CoA and free CoA [37]. L-carnitine has also been shown to be an effective cardioprotective agent in different models of experimental ischemia. The supplementation of the myocardium with carnitine resulted in an increased tissue carnitine content, a stimulation of pyruvate oxidation, lessening of the severity of ischemic injury, and improvement in the recovery of heart function during reperfusion [38]. L-carnitine supplementation has been shown to improve CoA recycling and increase the mitochondrial-free CoA levels by shuttling the short-chain acyl groups from within mitochondria to the cytosol. A multi-central study with 472 patients was conducted to evaluate the effects of L-carnitine administration on long-term left ventricular dilation in patients with acute anterior myocardial infarction. Carnitine treatment attenuated left ventricular dilation and resulted in smaller left ventricular volumes [39]. Some clinical studies have also suggested that L-carnitine can be employed as a treatment drug in patients with essential hypertension and diabetes [40]. In a recently published study involving newborn screening for MCAD deficiency medium chain length acylcarnitines, octanoylcarnitine (C8) and decanoylcarnitine (C10) were measured on newborn screening blood spot cards [41]. Out of 121,000 live births, 17 newborns had C8 values above the screening cut-off of 0.38 μmol/L. Ten newborns had elevated C8 on repeat cards and were investigated further. Both C8 and C8/C10 ratios remained abnormal in all confirmed MCAD cases. Upon frequent feeding and carnitine supplementation, none of the patients had metabolic crises or adverse outcomes. Another study in patients undergoing hemodialysis has shown that L-carnitine supplementation decreases the left ventricular mass [42]. The cardiac morphology and function of 10 patients given 10 mg/kg of L-carnitine orally, immediately after hemodialysis sessions, 3 times per week for a 12-month period were compared with 10 untreated control patients. Therapy resulted in an increase in serum-free carnitine levels from 28.4 ± 4.7 to 58.5 ± 12.1 micromol/L and the regression of left ventricular hypertrophy, even in those with normal systolic function.
L-carnitine supplementation in CACT deficiency has also shown therapeutic benefits by improving the acylcarnitine profile and preventing further attacks of hypoglycemia and arrhythmia [43–45]. Although only a study with 10 CACT deficiency patients suffering from cardiomyopathy, therapy with L-carnitine resulted in improvement in cardiac function. The effect of carnitine supplementation on endothelial dysfunction in hypertension has also been studied in spontaneously hypertensive rats (SHR). L-carnitine was able to restore endothelium-dependent relaxations of aortic rings from SHR and reduce ROS as well as increase NO participation in endothelium-dependent relaxations in SHR [33]. Further, a recently published study in rats has shown nutritional supplementation with L-carnitine and moderate physical exercise could improve the mitochondrial oxidative metabolism and subsequently limit the side-effects of aging [46]. L-carnitine supplementation has been shown to influence the influx of fatty acids into the mitochondria, alter lipid accumulation in the skeletal muscle [47], and also increase the oxidation of dietary fatty acids in healthy humans [48,49].
10. Conclusions
There is a great need for therapies to provide more effective treatments for cardiovascular disorders. Carnitines have an essential role in the regulation of mitochondrial function and recent studies both in animal models and human subjects have emphasized the importance of mitochondrial function in regulating NO signaling. Thus, as appears likely, the disruption of carnitine metabolism is an important contributing factor in the development of endothelial dysfunction, then preventing the disruption of carnitine homeostasis may attenuate, at least in part, the endothelial injury associated with cardiovascular disease. Therefore, we propose that the development of agents that can positively modulate L-carnitine homeostasis may have high therapeutic potential.
Acknowledgments
This research was supported in part by grants HL60190, HL67841, HL72121, HL70061, HD039110, and HD057406 all from the National Institutes of Health to SMB, and by a grant from the Fondation Leducq to SMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L46–56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahajwalla CG, et al. Multiple-dose pharmacokinetics and bioequivalence of L-carnitine 330-mg tablet versus 1-g chewable tablet versus enteral solution in healthy adult male volunteers. J Pharm Sci. 1995;84(5):627–633. doi: 10.1002/jps.2600840520. [DOI] [PubMed] [Google Scholar]
- 4.Tamai I, et al. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273(32):20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 5.Sachan DS, Hoppel CL. Carnitine biosynthesis. Hydroxylation of N6-trimethyl-lysine to 3-hydroxy-N6-trimethyl-lysine. Biochem J. 1980;188(2):529–534. doi: 10.1042/bj1880529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486(1):1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 7.Longo N, et al. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142C(2):77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, et al. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta. 2000;1466(1–2):315–327. doi: 10.1016/s0005-2736(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 9.Yabuuchi H, et al. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289(2):768–773. [PubMed] [Google Scholar]
- 10.Stanley CA. Carnitine deficiency disorders in children. Ann N Y Acad Sci. 2004;1033:42–51. doi: 10.1196/annals.1320.004. [DOI] [PubMed] [Google Scholar]
- 11.van Vlies N, et al. PPAR alpha-activation results in enhanced carnitine biosynthesis and OCTN2-mediated hepatic carnitine accumulation. Biochim Biophys Acta. 2007;1767(9):1134–1142. doi: 10.1016/j.bbabio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Pons R, et al. Deficient muscle carnitine transport in primary carnitine deficiency. Pediatr Res. 1997;42(5):583–587. doi: 10.1203/00006450-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Treem WR, et al. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle, and fibroblasts. N Engl J Med. 1988;319(20):1331–1336. doi: 10.1056/NEJM198811173192006. [DOI] [PubMed] [Google Scholar]
- 14.Lamhonwah AM, et al. A third human carnitine/organic cation transporter (OCTN3) as a candidate for the 5q31 Crohn’s disease locus (IBD5) Biochem Biophys Res Commun. 2003;301(1):98–101. doi: 10.1016/s0006-291x(02)02946-7. [DOI] [PubMed] [Google Scholar]
- 15.Roe CR. Inherited disorders of mitochondrial fatty acid oxidation: a new responsibility for the neonatologist. Semin Neonatol. 2002;7(1):37–47. doi: 10.1053/siny.2002.0097. [DOI] [PubMed] [Google Scholar]
- 16.Bhala A, et al. Clinical and biochemical characterization of short-chain acyl-coenzyme A dehydrogenase deficiency. J Pediatr. 1995;126(6):910–915. doi: 10.1016/s0022-3476(95)70207-5. [DOI] [PubMed] [Google Scholar]
- 17.Gregersen N, et al. Identification of four new mutations in the short-chain acyl-CoA dehydrogenase (SCAD) gene in two patients: one of the variant alleles, 511C-->T, is present at an unexpectedly high frequency in the general population, as was the case for 625G-->A, together conferring susceptibility to ethylmalonic aciduria. Hum Mol Genet. 1998;7(4):619–627. doi: 10.1093/hmg/7.4.619. [DOI] [PubMed] [Google Scholar]
- 18.Bonnefont JP, et al. Carnitine palmitoyltransferase deficiencies. Mol Genet Metab. 1999;68(4):424–440. doi: 10.1006/mgme.1999.2938. [DOI] [PubMed] [Google Scholar]
- 19.Hendriksz CJ. Inborn errors of metabolism for the diagnostic radiologist. Pediatr Radiol. 2008 doi: 10.1007/s00247-008-1072-x. [DOI] [PubMed] [Google Scholar]
- 20.Chu BC, et al. MRI of the brain in the Kearns-Sayre syndrome: report of four cases and a review. Neuroradiology. 1999;41(10):759–764. doi: 10.1007/s002340050838. [DOI] [PubMed] [Google Scholar]
- 21.Tardy C, et al. Lysosomal storage diseases: is impaired apoptosis a pathogenic mechanism? Neurochem Res. 2004;29(5):871–880. doi: 10.1023/b:nere.0000021232.05175.38. [DOI] [PubMed] [Google Scholar]
- 22.Chang B, et al. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int J Cancer. 2005;113(5):719–729. doi: 10.1002/ijc.20636. [DOI] [PubMed] [Google Scholar]
- 23.Furuno T, et al. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem Pharmacol. 2001;62(8):1037–1046. doi: 10.1016/s0006-2952(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 24.Oyanagi E, et al. L-Carnitine suppresses oleic acid-induced membrane permeability transition of mitochondria. Cell Biochem Funct. 2008;26(7):778–786. doi: 10.1002/cbf.1506. [DOI] [PubMed] [Google Scholar]
- 25.Luo X, et al. L-carnitine attenuates doxorubicin-induced lipid peroxidation in rats. Free Radic Biol Med. 1999;26(9–10):1158–1165. doi: 10.1016/s0891-5849(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, et al. Acetyl-L-carnitine suppresses apoptosis of thioredoxin 2-deficient DT40 cells. Arch Biochem Biophys. 2008;478(2):154–160. doi: 10.1016/j.abb.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Kuwajima M, et al. Cardiomegaly in the juvenile visceral steatosis (JVS) mouse is reduced with acute elevation of heart short-chain acyl-carnitine level after L-carnitine injection. FEBS Lett. 1999;443(3):261–266. doi: 10.1016/s0014-5793(98)01732-3. [DOI] [PubMed] [Google Scholar]
- 28.Sparagna GC, et al. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2000;279(5):H2124–2132. doi: 10.1152/ajpheart.2000.279.5.H2124. [DOI] [PubMed] [Google Scholar]
- 29.Chang B, et al. L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys. 2002;405(1):55–64. doi: 10.1016/s0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 30.Pillich RT, et al. Reduction of apoptosis through the mitochondrial pathway by the administration of acetyl-L-carnitine to mouse fibroblasts in culture. Exp Cell Res. 2005;306(1):1–8. doi: 10.1016/j.yexcr.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Miguel-Carrasco JL, et al. The role of inflammatory markers in the cardioprotective effect of L-carnitine in L-NAME-induced hypertension. Am J Hypertens. 2008;21(11):1231–1237. doi: 10.1038/ajh.2008.271. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari R, et al. Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review. Ann N Y Acad Sci. 2004;1033:79–91. doi: 10.1196/annals.1320.007. [DOI] [PubMed] [Google Scholar]
- 33.Bueno R, et al. L-carnitine and propionyl-L-carnitine improve endothelial dysfunction in spontaneously hypertensive rats: different participation of NO and COX-products. Life Sci. 2005;77(17):2082–2097. doi: 10.1016/j.lfs.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 34.Smith WA, et al. Effect of glycine propionyl-L-carnitine on aerobic and anaerobic exercise performance. Int J Sport Nutr Exerc Metab. 2008;18(1):19–36. doi: 10.1123/ijsnem.18.1.19. [DOI] [PubMed] [Google Scholar]
- 35.Pepine CJ. The therapeutic potential of carnitine in cardiovascular disorders. Clin Ther. 1991;13(1):2–21. discussion 21. [PubMed] [Google Scholar]
- 36.Goa KL, Brogden RN. l-Carnitine. A preliminary review of its pharmacokinetics, and its therapeutic use in ischaemic cardiac disease and primary and secondary carnitine deficiencies in relationship to its role in fatty acid metabolism. Drugs. 1987;34(1):1–24. doi: 10.2165/00003495-198734010-00001. [DOI] [PubMed] [Google Scholar]
- 37.Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54(1):101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 38.Stanley WC, et al. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33(2):243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 39.Iliceto S, et al. Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) Trial. J Am Coll Cardiol. 1995;26(2):380–387. doi: 10.1016/0735-1097(95)80010-e. [DOI] [PubMed] [Google Scholar]
- 40.Digiesi V, et al. L-carnitine adjuvant therapy in essential hypertension. Clin Ter. 1994;144(5):391–395. [PubMed] [Google Scholar]
- 41.Horvath GA, et al. Newborn screening for MCAD deficiency: experience of the first three years in British Columbia, Canada. Can J Public Health. 2008;99(4):276–280. doi: 10.1007/BF03403754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurabayashi T, et al. L-carnitine supplementation decreases the left ventricular mass in patients undergoing hemodialysis. Circ J. 2008;72(6):926–931. doi: 10.1253/circj.72.926. [DOI] [PubMed] [Google Scholar]
- 43.Iacobazzi V, et al. Response to therapy in carnitine/acylcarnitine translocase (CACT) deficiency due to a novel missense mutation. Am J Med Genet A. 2004;126A(2):150–155. doi: 10.1002/ajmg.a.20573. [DOI] [PubMed] [Google Scholar]
- 44.Cox GF, et al. Reversal of severe hypertrophic cardiomyopathy and excellent neuropsychologic outcome in very-long-chain acyl-coenzyme A dehydrogenase deficiency. J Pediatr. 1998;133(2):247–253. doi: 10.1016/s0022-3476(98)70228-8. [DOI] [PubMed] [Google Scholar]
- 45.Winter SC, et al. Plasma carnitine deficiency. Clinical observations in 51 pediatric patients. Am J Dis Child. 1987;141(6):660–665. doi: 10.1001/archpedi.1987.04460060076039. [DOI] [PubMed] [Google Scholar]
- 46.Bernard A, et al. L-carnitine supplementation and physical exercise restore age-associated decline in some mitochondrial functions in the rat. J Gerontol A Biol Sci Med Sci. 2008;63(10):1027–1033. doi: 10.1093/gerona/63.10.1027. [DOI] [PubMed] [Google Scholar]
- 47.Rajasekar P, Anuradha CV. Effect of L-carnitine on skeletal muscle lipids and oxidative stress in rats fed high-fructose diet. Exp Diabetes Res. 2007;2007:72741. doi: 10.1155/2007/72741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller DM, et al. Effects of oral L-carnitine supplementation on in vivo long-chain fatty acid oxidation in healthy adults. Metabolism. 2002;51(11):1389–1391. doi: 10.1053/meta.2002.35181. [DOI] [PubMed] [Google Scholar]
- 49.Wutzke KD, Lorenz H. The effect of l-carnitine on fat oxidation, protein turnover, and body composition in slightly overweight subjects. Metabolism. 2004;53(8):1002–1006. doi: 10.1016/j.metabol.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Nyman LR, et al. Homozygous carnitine palmitoyltransferase 1a (liver isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2005;86(1–2):179–187. doi: 10.1016/j.ymgme.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Cox KB, et al. Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum Mol Genet. 2001;10(19):2069–2077. doi: 10.1093/hmg/10.19.2069. [DOI] [PubMed] [Google Scholar]
- 52.Exil VJ, et al. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ Res. 2003;93(5):448–455. doi: 10.1161/01.RES.0000088786.19197.E4. [DOI] [PubMed] [Google Scholar]
- 53.Kurtz DM, et al. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci U S A. 1998;95(26):15592–15597. doi: 10.1073/pnas.95.26.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tolwani RJ, et al. Medium-chain acyl-CoA dehydrogenase deficiency in gene-targeted mice. PLoS Genet. 2005;1(2):e23. doi: 10.1371/journal.pgen.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong DL, et al. Pathologic characterization of short-chain acyl-CoA dehydrogenase deficiency in BALB/cByJ mice. Am J Med Genet. 1993;47(6):884–892. doi: 10.1002/ajmg.1320470616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibdah JA, et al. Lack of mitochondrial trifunctional protein in mice causes neonatal hypoglycemia and sudden death. J Clin Invest. 2001;107(11):1403–1409. doi: 10.1172/JCI12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokogawa K, et al. Loss of wild-type carrier-mediated L-carnitine transport activity in hepatocytes of juvenile visceral steatosis mice. Hepatology. 1999;30(4):997–1001. doi: 10.1002/hep.510300423. [DOI] [PubMed] [Google Scholar]