Abstract
Drug delivery to the skin is limited by the strong barrier properties of skin’s outer layer of stratum corneum. Micron-scale needles have been developed to deliver drugs across this barrier layer and into the skin in a minimally invasive manner. One method of delivery involves coating these microneedles with a drug that rapidly dissolves off within the skin. As a variation on this approach, this study examines microneedles with holes cut through their shafts to form “pockets” that can be filled with drug formulations using a dip-coating method. Our results (i) demonstrated the filling of microneedle pockets having a variety of different sizes and shapes, (ii) quantified the amount of drug that can be filled into pockets and coated onto microneedle surfaces, (iii) developed composite microneedle structures that sequester one model drug within the microneedle pocket and coat another model drug on the microneedle surface and (iv) showed that pocketed microneedles can deliver a model drug to a targeted depth within the skin. We conclude that pocketed microneedles offer unique capabilities for controlled drug delivery to the skin.
Keywords: A. metals, D. microstructure, D. surface properties, D. transport properties
Introduction
Drug delivery to the skin is needed for a variety of medical scenarios. Most dermatological applications benefit from localized delivery to the skin at the site of action. In addition, systemic delivery via the skin using transdermal patches has also been highly successful [1]. Finally, there is growing interest to deliver vaccines to the skin for a more potent immune response [2]
Progress on these applications is severely limited by the strong barrier properties of the skin imposed primarily by the outer layer of stratum corneum, which measures just 10–20 μm in thickness, but permits transport only of low doses of small, lipophilic molecules [1]. Although a small number of drugs with suitable characteristics can be delivered across stratum corneum and into the skin at therapeutic levels, there has been intensive research to develop ways to increase skin permeability and to deliver larger quantities, especially of large, hydrophilic compounds like peptides and proteins.
One method to deliver drugs into the skin with a minimally invasive approach involves the use of micron-scale needles that are large enough to pierce across the stratum corneum, but small enough to avoid causing pain. These microneedles have been fabricated by adapting the tools of the microelectronics industry for inexpensive mass production. Employing a number of different delivery strategies, microneedles have been used to deliver small molecules, peptides, proteins, oligonucleotides, DNA, vaccines and other compounds into the skin in vitro and in animals in vivo [3]. Human clinical trials are underway.
A particularly attractive use of microneedles is to coat them with a drug that rapidly dissolves off the microneedles when inserted into the skin. Robust coating methods have been developed for this approach [4] and in vivo delivery of ovalbumin as a model vaccine has been studied in detail [5].
We have extended this approach by fabricating microneedles with one or more holes cut through the center of the microneedle [6]. These “pockets” can then be filled with a drug formulation. Drug delivery from microneedle pockets offers the opportunity to sequester drug on just a portion of the microneedle shaft and, in that way, target delivery to a specified depth within the skin. This approach also lends itself to isolating a drug within the pocket and then over-coating the microneedles with other materials that can serve as a protective layer, as a second drug coating, or other functions. Pockets can also be filled with liquid drug formulations held in place by surface tension. In this study, we examine pocketed microneedles in detail.
Experimental Methods
Fabrication of microneedles
Using methods described in detail previously [4], microneedles were cut into 75 μm thick stainless steel sheets (Trinity Brand Industries, SS 304; McMaster-Carr, Atlanta, GA, USA) using an infrared laser (Resonetics Maestro, Nashua, NH, USA). Holes through microneedle shafts to form “pockets” were similarly cut. Microneedles were then electropolished, washed and dried.
Coating solution formulations
Coating solution formulations (w/v % unless otherwise noted) were prepared using DI water (unless otherwise noted) according to the following recipes: Formulation I: 25% sucrose (Fisher Chemicals), 0.1% sulforhodamine (Molecular Probes, Eugene, OR, USA). Formulation II: 80% (v/v) glycerol, 20% (v/v) aqueous solution of 1% sodium fluorescein (Sigma, St. Louis, MO, USA). Formulation III: 20% sucrose, 1% polyvinyl alcohol (Sigma), 0.05% gWiz™ luciferase plasmid DNA (Aldevron, Fargo, ND, USA; made fluorescent with YOYO-1, tag). Formulation IV: Formulation 3 plus 0.2% (w/w) Tween 20 (Sigma). Formulation V: 1% carboxymethylcellulose sodium salt (CMC, low viscosity, USP grade, CarboMer, San Diego, CA, USA), 0.5% Lutrol F-68 NF (BASF, Mt. Olive, NJ, USA), 0.01% or 0.1% or 3% vitamin B2 (riboflavin-5′-phosphate sodium salt dihydrate, Fisher Chemicals). Formulation VI: 5% poly(lactic-co-glycolic acid) (PLGA, Absorbable Polymer International, Pelham, AL, USA) in acetonitrile solvent (Fisher Chemicals). Formulation VII: 1% CMC, 0.5% Lutrol F-68 NF, 0.2% sodium fluorescein (Sigma).
The various excipients used in the formulations act as either viscosity enhancers (sucrose, glycerol and CMC) or as surfactants (polyvinyl alcohol, Tween 20 and Lutrol F-68).
Micron-scale dip-coating
Single microneedles, and arrays of multiple microneedles, were coated using a micron-scale dip-coating process developed previously [4]. Coatings were formed by dipping the microneedle into a coating solution once, unless otherwise noted. The withdrawal speed of microneedles from the coating solution was manually maintained at approximately 2 mm/s to produce films and at approximately 0.35 mm/s to fill just the pockets. To assess coating uniformity, dipped microneedles were examined either by (i) fluorescence microscopy using an Olympus IX70 fluorescence microscope (Olympus America, Center Valley, PA, USA) with a CCD camera (RT Slider, Diagnostic Instruments, Sterling Heights, MI, USA), or (ii) brightfield microscopy using an Olympus SZX12 stereo microscope (Olympus America) with a CCD camera (Leica DC 300, Leica Microsystems, Bannockburn, IL, USA).
Selective coating of pockets
To selectively coat either just the pockets or both the pocket and the microneedle surface, different coating excipients were tested. Just the pockets were coated with a solid phase using formulation I (rectangular pocket, 400 μm × 50 μm, n=3) and formulation III (ten 10-μm diameter circular pockets, n=3), or with a liquid phase using formulation II (three 90-μm diameter circular pockets, n=3). By the addition of a surfactant to formulation III, both the microneedle flat surface and the pocket were coated using formulation IV (ten 10-μm diameter circular pockets, n=3).
Quantification of vitamin B2
In-plane microneedle rows each containing five microneedles with and without rectangular pockets (400 μm × 50 μm) were coated with formulation V containing either 0.01% (n=5), 0.1% (n=5) or 3% (n=5) vitamin B2. The mass of riboflavin in the coatings was determined by dissolving the coatings off the microneedles in DI water and then measuring riboflavin concentration by calibrated fluorescence spectroscopy (SpectraMax Gemini, Molecular Devices, Sunnyvale, CA, USA; excitation = 450 nm, emission = 534 nm).
Multi-layer coatings
To produce multi-layer coatings with protected pockets, microneedles (n=3) with a rectangular pocket (400 μm × 50 μm) were dipped sequentially into formulations I, VI and VII. Microneedles were air dried between sequential dips. Microneedles were then dipped into water for 1 min to dissolve off the topmost coating without removing the contents of the protected pocket.
Microneedle delivery into skin in vitro
Single, pocketed microneedles (one 90 μm diameter circular pocket) coated with formulation I (n=3) were manually inserted into abdominal porcine cadaver skin for 20 s and then removed. Microneedle insertion speed was manually maintained at 1 to 2 cm/s. After removing the microneedles, the skin surface was examined by brightfield microscopy for coating residue. The skin was then examined histologically to assess the extent of delivery of microneedle coatings into the skin. The use of porcine cadaver skin has been approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee (IACUC).
Results and Discussion
Controlled coating of pockets
Microneedles with pockets were fabricated by laser etching stainless steel into the desired microneedle geometries. Fig. 1A shows a representative microneedle in which a single rectangular pocket has been formed. This microneedle measures 700 μm in length, 160 μm in width and 50 μm in thickness, and tapers at a 55° angle to a sharp tip with less than 1 μm radius of curvature. The pocket measures 400 μm in length and 50 μm in width.
Figure 1.
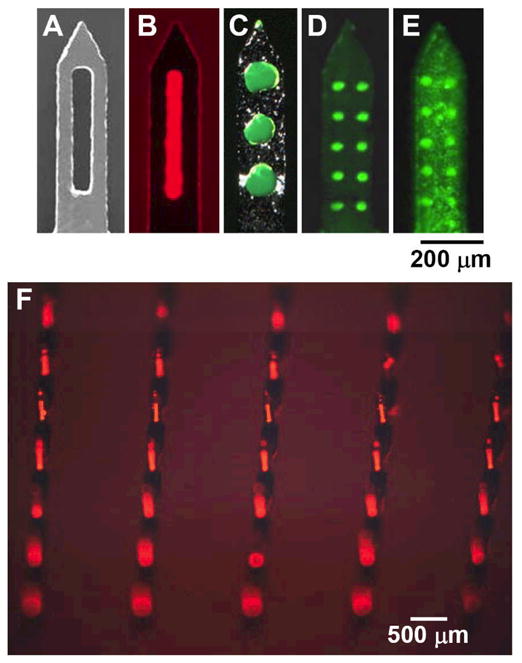
Pocketed microneedles. (A) Representative microneedle with a large central pocket. Microneedles with pockets of different sizes and shapes filled with (B) sulforhodamine, (C) fluorescein, and (D) plasmid DNA as model drugs. (E) A microneedle with plasmid DNA filling the pockets and coating the microneedle surface. (F) An array of pocketed microneedles filled with sulforhodamine.
Using a dip-coating process, microneedle pockets were selectively filled with fluorescent model drugs. When coating non-pocketed microneedles, the coating solution should be formulated to include a surfactant, to facilitate wetting of the microneedles surface with the coating solution, and a viscosity enhancer, to increase the thickness of the liquid film attached to the microneedle after dipping and to increase the residence time of this film on the microneedle while it dries [6]. In contrast, when selectively filling microneedle pockets, without coating the microneedle surface, the coating solution was formulated to include a viscosity enhancer, but not a surfactant. Thus, by keeping the surface tension high, the coating process could be controlled to prevent wetting and subsequent coating of the microneedle surface.
Selective filling of microneedle pockets in this way is shown in Figs. 1B–1D for pockets of different shapes and sizes. Alternatively, by adding a surfactant to the coating solution, both the microneedle pockets and the microneedle surface were coated, as shown in Fig. 1E. Fig. 1F shows that this approach can also be applied to filling pockets in two-dimensional arrays containing multiple microneedles.
Filling capacity of pockets
Because microneedles are small and the pocket dimensions are further constrained by the need to maintain mechanical integrity of the pocketed microneedles for insertion into the skin, the mass of drug that can be filled into pockets is limited. For example, for the miconeedles used in this study (700 μm long × 160 μm wide), a rectangular pocket (400 μm long × 50 μm wide) represents a pocket size near the upper size limit. Wider pockets (e.g., 60–80 μm wide) produced fragile microneedles, which bent while attempting insertion into the skin (data not shown).
Based on the rectangular pocket dimensions (with a pocket volume of 400 μm × 50 μm × 50 μm = 10−6 cm3) and assuming a density of 1 g/cm3 for the coatings, the theoretical maximum mass that can be completely filled into the pocket is 1 μg. Accounting for the solids content (on a dry basis) of coating solutions with 3% (w/v) Vitamin B2 (on a wet basis), pockets can be filled with a maximum of 0.6 μg drug, where the remaining mass is composed of coating excipients (e.g., surfactant, viscosity enhancer). To compare with this calculation, we experimentally filled just the pockets using a coating solution containing 1% or 3% Vitamin B2 based on formulation I, and found that they contained 0.02 or 0.09 μg of Vitamin B2, respectively [6], which is much less than the predicted maximum. This discrepancy is probably due to the void volume associated with evaporated liquid, which was not accounted for in the calculation. Guided by this analysis, microneedle arrays containing 100–1000 needles could therefore be used to deliver up to on the order of 100 μg of drug. This may be sufficient for a number of dermatological and vaccination applications.
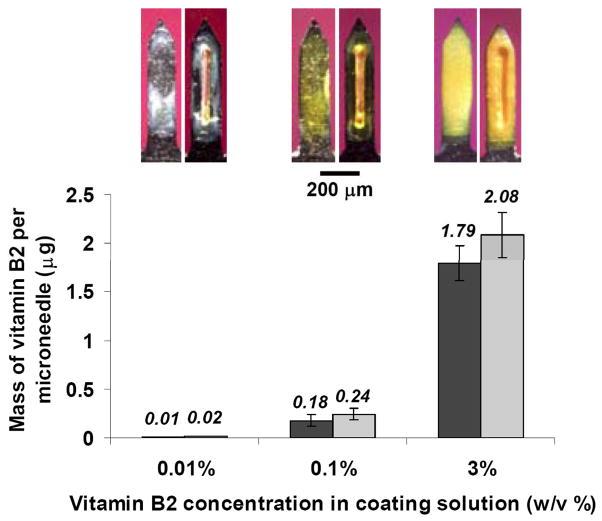
The amount of drug on a microneedle could be maximized by coating both the microneedle surface and filling pockets, as shown in Fig. 1E. The amount of drug on microneedles with only surface coatings was compared to microneedles with both surface coatings and filled pockets. This comparison is shown as a function of coating solution concentration in Fig. 2. However, differences between the amount of Vitamin B2 on the two needle designs were not statistically significant (ANOVA, p=0.53). This suggests that microneedle pockets may find greater use as a means to isolate or target small doses of drug than to increase drug loadings on microneedles.
Figure 2.
Drug loading into microneedle pockets and onto microneedle surfaces. The mass of Vitamin B2 per microneedle is shown as a function of Vitamin B2 concentration in the coating solution for non-pocketed microneedles with surface coatings and pocketed microneedles with drug coated onto the surface and filled within the pockets. Inset images show representative non-pocketed and pocketed microneedles at each concentration, used to generate data in the graph.
Multi-layered coatings
In addition to coating microneedles and filling pockets with the same drug using a one-step process, surface coatings and filled pockets were designed to contain different drugs using a multi-step process. As an example of this, Fig. 3A shows a microneedle pocket filled with red-fluorescent sulforhodamine. This needle was then completely coated with a thin layer of biodegradable polymer (PLGA), which was subsequently over-coated with green-fluorescent fluorescein. The resulting microneedle is shown in Fig. 3B. The polymer coating served to isolate the sulforhodamine in the pocket and thereby to prevent it from being removed during the surface coating with fluorescein.
Figure 3.
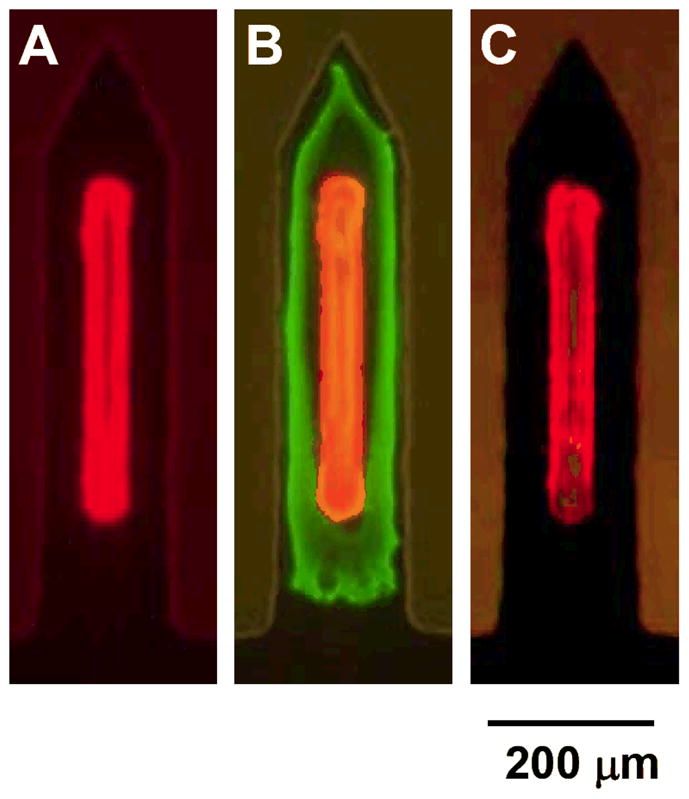
Microneedle with a composite coating that sequestered sulforhodamine within the microneedle pocket and coated fluorescein on the microneedle surface by a multi-step process. (A) Pocketed microneedle after the first step of filling the pocket with sulforhodamine. (B) Microneedle after the second and third steps of completely coating the needle with PLGA and then over-coating with fluorescein, respectively. (C) Microneedle after dipping into water for 1 min, which removed the over-coat of fluorescein, but did not affect the sulforhodamine sequestered in the pocket and covered with a film of PLGA.
Finally, the fully coated microneedle was dipped into water, which rapidly removed the surface coating of fluorescein, but left the sulforhodamine in the pocket intact (Fig. 3C). Leaving the microneedle in water (or skin) for a longer time should biodegrade the polymer coating and thereby release the sulforhodamine from the pocket. By selecting a suitable polymer, the release kinetics from the pocket can be controlled by polymer degradation or dissolution kinetics or, if desired, release could be eliminated by using a non-degradable polymer.
Targeted delivery to skin
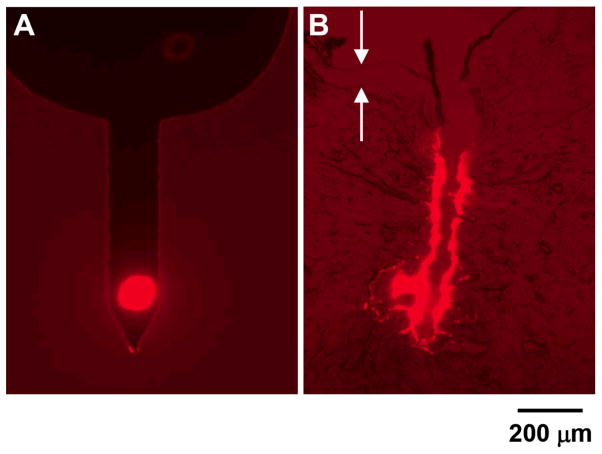
An attractive feature of pocketed microneedles is that they can target drug delivery to specific depths in the skin. For some dermatological applications, it might be desirable to deliver a drug selectively to the middle of the dermis. This would avoid the highly vascularized regions found in the upper and lower dermis and thereby reduce systemic absorption. Fig. 4A shows a microneedle with a pocket filled near the microneedle tip designed for this purpose. After insertion and removal from porcine cadaver skin, the histological section presented in Fig. 4B shows targeted deposition of the red-fluorescent model drug at a depth of approximately 600 μm into the skin, which is in the central region of the dermis between the highly vascularized regions. Some of the model drug was also deposited along the needle insertion track.
Figure 4.
Targeted delivery within the skin using a pocketed microneedle. (A) Microneedle with a pocket centered at 170 μm from the needle tip that is filled with sulforhodamine. (B) Histological section of porcine cadaver skin after insertion and removal of a microneedle like the one showed in (A). Arrows point to the upper and lower edges of the epidermis.
Conclusion
Microneedles can be fabricated with pockets of various sizes and shapes that can be selectively filled with model drug compounds. Microneedles can also be coated with drug, such that the same drug coats the microneedle surface and fills the microneedle pockets or different drugs are located on the surface and in the pocket. Microneedles can be inserted into the skin to release model drug from a pocket at a targeted depth within the skin. Altogether, this study shows that pocketed microneedles can be developed for controlled drug delivery to the skin.
Acknowledgments
We thank Dr. Mark Allen, Richard Shafer, Dr. Shawn Davis, and Ed Birdsell for help with laser operation. This work was supported in part by the National Institutes of Health.
References
- 1.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 2.Partidos CD, Beignon AS, Mawas F, Belliard G, Briand JP, Muller S. Immunity under the skin: potential application for topical delivery of vaccines. Vaccine. 2003;21(7–8):776–780. doi: 10.1016/s0264-410x(02)00597-2. [DOI] [PubMed] [Google Scholar]
- 3.Prausnitz MR, Gill HS, Park J-H. In: Modified Release Drug Delivery. Rathbone, editor. Informa Healthcare; In press. [Google Scholar]
- 4.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 6.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]