Abstract
The NR4A orphan nuclear receptor subfamily is comprised of the highly homologous receptors Nur77 (NR4A1), Nurr1 (NR4A2), and NOR1 (NR4A3). These evolutionarily conserved and ancient receptors function as ligand-independent transcription factors that regulate the expression of overlapping target genes. As early response genes, the basal expression level of these receptors is low but rapidly induced as a result of changes in environmental cues. The transcriptional activity of these receptors is primarily regulated by gene induction and posttranslational modifications of the receptor including phosphorylation. NR4A receptors were initially identified in the brain and early functional studies suggested a role for these receptors in signal- and cell-specific stimulation of both apoptosis and proliferation. More recent studies have revealed much broader functions of these orphan receptors including the regulation of genes involved in cancer, metabolism, energy balance, atherosclerosis, and vascular remodeling. In this review, we will discuss our current understanding of the molecular biology of NR4A receptors and summarize recent studies suggesting an important role of these orphan receptors in vascular biology.
1. Introduction
An emerging consensus underscores the importance of inflammatory and proliferative responses for the development of atherosclerosis and its complications[1]. A decade of intensive investigation has revealed that endothelial dysfunction and increased expression of adhesion molecules leading to monocyte recruitment into the arterial wall are the key initial events for atherosclerotic lesion formation[1]. This recruitment of monocytes, their differentiation into macrophages, and uptake of LDL-derived cholesterol promote early fatty streak formation[1,2]. The observation that hypercholesterolemic mice become resistant to atherosclerosis if they are bred to macrophage-deficient mice represents one of many lines of evidence that the net actions of macrophages promote lesion initiation and progression[3]. Continued intracellular cholesterol accumulation results in the generation of endogenous inducers of inflammatory gene expression and a broad range of cellular and humoral responses contributing to lesion progression. The resulting chronic inflammatory state and the enrichment of lipid-laden macrophages ultimately lead to the formation of a complex atherosclerotic lesion. In this process, the proliferation of smooth muscle cells (SMC) within the developing neointima may promote lesion development through the production of pro-inflammatory mediators and the synthesis of extracellular matrix molecules, which is required for the retention of lipoproteins and often constitutes the majority of the volume of the advanced lesion responsible for luminal obstruction[1,2].
In an era marked by the increasing prevalence of obesity and diabetes as key risk factors for cardiovascular disease, members of the nuclear hormone receptor (NR) superfamily have emerged as key transcription factors in metabolism and cardiovascular disease[4,5]. In addition to their function to act as molecular sensors of lipid and carbohydrate homeostasis, many nuclear receptors also exert pleiotropic effects to control inflammatory and proliferative responses in the vasculature. For example, this ability to integrate metabolic and vascular signaling networks has been well described for the ligand-activated peroxisome proliferator-activated receptors (PPAR) and liver X receptors (LXR)[5,6]. However, the nuclear receptor superfamily comprises a large number of receptors, for which ligands have not been identified and these remain classified as orphan nuclear receptors. For many of these orphan receptors, the physiological functions and their regulated target genes remain unknown, yet the high degree of conservation during evolution points to an important role in the transcriptional control of gene expression[7]. Among these orphan receptors is the NR4A subfamily of nuclear receptors, which consists of Nur77 (NR4A1, also referred to as NGFI-B or TR3), Nur-related factor-1 (Nurr1, NR4A2) and the Neuron-Derived Orphan Receptor-1 (NOR1, NR4A3)[8,9]. These NR4A receptors have recently been identified as key transcription factors regulating gene expression in metabolism and vascular disease. This review will summarize emerging evidence to support an important role for NR4A receptors in the development of atherosclerosis and its complications.
2. Molecular Biology of NR4A Orphan Nuclear Receptors
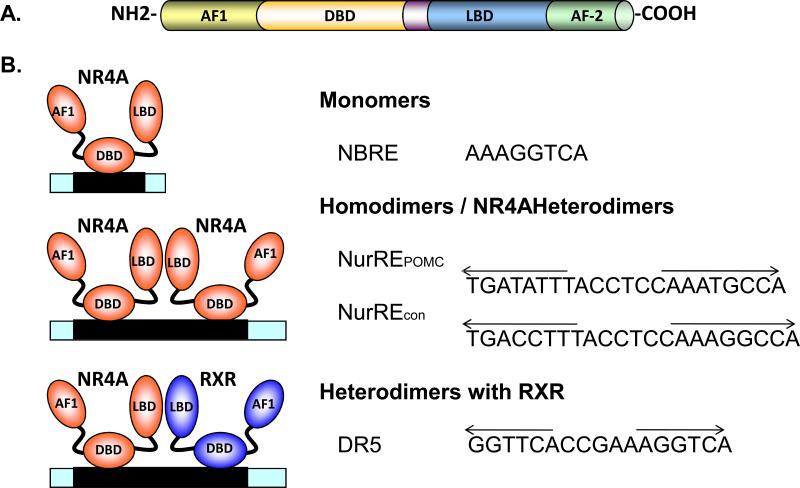
All members of the nuclear hormone receptor superfamily share a highly conserved molecular structure[7] (Fig. 1A). The N-terminal domain is variable in length and in amino acid composition. This domain contains the activation function 1 (AF-1), which mediates the ligand-independent transactivation of nuclear receptors. The central DNA binding domain (DBD) is the most conserved region among the different nuclear receptors. It is responsible for the specific response element (RE) recognition and DNA binding to the promoter of target gene. The ligand binding domain (LBD) and the ligand-dependent activation function 2 (AF-2) are located at the C-terminus. The ligand binding pocket (LBP) directs the interaction with ligands, and its particular size and amino acid sequence determine ligand specificity. The consequence of ligand binding is an allosteric switch and subsequent nuclear receptor activation[7-9].
Figure 1. Molecular biology of NR4A orphan nuclear receptors.
NR4A receptors share the conserved molecular structure consisting of a N-terminal AF-1 domain, the central DNA-binding domain (DBD) and C-terminal ligand binding domain (LBD) and the AF-2 domain (Panel A). NR4A receptors induce gene expression by binding as monomers to the NBRE site and as homodimers or heterodimers to the NurRE site in the promoter of their regulated target genes. NurREPOMC represents the binding sequence demonstrated in the pro-opiomelanocortin (POMC) promoter, while NurRECON represents the NurRE site comprising consensus NBRE sites. Nur77 and Nurr1, but not NOR1, heterodimerize with RXR and bind to the direct repeats of nuclear receptor binding motif separated by five nucleotides (DR5) (Panel B).
The three different NR4A receptors Nur77, Nurr1 and NOR1 share a highly conserved DNA binding domain (degree of conservation >90%) and a less conserved LBD (degree of conservation ∼60 %)[8,9]. However, the amino acid sequences of the N-terminal activation function domain, which mediates the ligand-independent activation of gene expression and dictates transactivation and co-factor recruitment among the NR4A receptors are very divergent (degree of conservation 20 − 30%)[10]. Crystallography of Nurr1 has demonstrated that its LBP is occupied by hydrophobic residues[11]. These hydrophobic residues are conserved throughout the NR4A subfamily, and their occupancy of the LBP suggested that all three receptors function as ligand-independent and constitutively active transcription factors[11]. However, a recent report has challenged this concept and suggested that cytosporone B (Csn-B) may serve as a potential agonist for Nur77 by binding to the Tyr-453 site, which is conserved in many nuclear receptor LBP[12]. Interestingly, the binding of Csn-B to Nur77 was demonstrated to enhance Nur77 expression and transcriptional activity. Since the Nur77 promoter contains a canonical binding site for NR4A receptors, these studies have pointed to a positive autoregulation of Nur77 as has been described for many other NR[12].
NR4A receptors are considered constitutively active receptors, and as early response genes the expression level of the receptor largely determines its transcriptional activity. The observation that a variety of extracellular stimuli, including mitogens, inflammatory stimuli, cytokines, peptide hormones, and cellular stress, rapidly induces the expression of NR4A receptors and influences their posttranslational modifications has resulted in the characterization of NR4A receptors as early response genes[13-18]. In addition, Nur77 is phosphorylated by several kinases, including Akt, ERK2, pp90rsk and c-Jun N-terminal kinase, which can affect its transcriptional activity[19-21]. NR4A nuclear receptors induce gene expression by binding as monomers to the nerve growth factor-induced clone B (NBFI-B) response element (NBRE, AAAGGTCA) and as homodimers or heterodimers to the Nur response element (NurRE) in the promoter of their regulated target genes[22] (Fig. 1B). Nur77 and Nurr1, but not NOR1, heterodimerize with RXR, which suggests a crosstalk with retinoic acid signaling pathway[23]. In addition to posttranslational modification by phosphorylation several factors have been identified to affect NR4A receptor-mediated transactivation of target genes in vitro. Prostaglandin A2 was recently identified to enhance NOR1-dependent transactivation, an effect that requires the presence of a functional LBD[24]. In addition, 6-mercaptopurine activates NR4A-mediated transactivation of target genes in muscle cells[25,26]. Interestingly, these studies demonstrated that the N-terminal AF-1 domain of NOR1 mediated this activation by 6-mercaptopurine and not the C-terminal LBD[25].
3. Regulation and Function of the NR4A Receptors in Vascular Cells
Macrophages
The macrophage constitutes an essential component for the development of atherosclerosis, having critical functions in cholesterol uptake and both innate and adaptive immunity[1]. All three members of the NR4A receptor subfamily have been demonstrated to be highly expressed in macrophages of human atherosclerotic lesions[14,15]. In vitro, NR4A receptors are rapidly induced by a variety of pro-inflammatory stimuli, including lipopolysaccharide (LPS), TNFα, IFNγ, IL-1β, oxidized LDL, and oxysterols[14,15]. The signaling pathways governing this rapid induction of NR4A receptors in macrophages involve an NF-κB-dependent transcriptional gene activation[27]. NF-κB has been widely recognized as a key transcription factor mediating the activation of a large library of inflammatory genes in macrophages[28]. Consistent with the pivotal role of this signaling pathway in inflammation, NR4A receptors are bona fide NF-κB target genes in macrophages.
Using murine macrophages, Pei et al. recently demonstrated that overexpression of Nur77 leads to a transcriptional activation of genes involved in inflammation, apoptosis, and cell cycle control[27]. Interestingly, retroviral-mediated overexpression of Nur77 increased the expression of IKKi and NIK, both of which are NF-κB activating kinases[27]. The identification of a canonical NBRE site in the murine IKKi promoter has confirmed IKKi as a bona fide target gene for Nur77[27]. These studies provided the first evidence that NR4A receptors function in a proinflammatory fashion in murine macrophages by amplifying NF-κB signaling pathways. In contrast to these findings, Bonta et al. have recently reported that overexpression of Nur77, Nurr1, and NOR1 reduces the expression levels of several cytokines and chemokines in the human THP-1 macrophage cell line, although the molecular mechanisms underlying this observation remain unknown[15]. In addition, in these studies it was observed that NR4A receptors reduce oxidized LDL uptake and decrease the expression of SR-A and CD36[15]. Considering this data, the role of NR4A receptors in the control of macrophage inflammation remains controversial. Therefore, it will be of particular importance to confirm potential differences between murine and human macrophages in future studies and to investigate the transcriptional mechanism governing NR4A-mediated regulation of inflammatory gene expression in both species.
Endothelial Cells
The activation of endothelial cells at atherosclerotic lesion-prone sites in the arterial tree results in the up-regulation of cell adhesion molecules and chemokines, which mediate the recruitment of circulating monocytes as one of the earliest events during atherosclerotic lesion formation[1,2]. All three NR4A receptors are transiently induced in response to various stimuli, including growth factors, inflammatory mediators, and hypoxia[29-34]. For example, Nur77 has been reported to be induced in response to inflammatory cytokines, including TNFα, LPS and IL-1β[29]. Using an Affymetrix oligonucleotide array system, NOR1 has been identified as the most potently induced gene in endothelial cells treated with VEGF[32]. The signal transduction mediating VEGF-induced NOR1 expression involves multiple pathways, including PKC activation, calcium mobilization, and calcineurin signaling[33]. These pathways ultimately converge in the phosphorylation of cAMP response element binding (CREB) protein and the subsequent binding of Ser133-phosphorylated CREB to the NOR1 promoter[33].
At present, little is known about the functional role of NR4A receptors in endothelial cells. The only direct NR4A target gene identified in endothelial cells is plasminogen activator inhibitor-1 (PAI-1)[29]. In response to TNFα stimulation, Nur77 transactivates the PAI-1 promoter by binding to a canonical NBRE consensus site[29]. However, whether NR4A receptors play a functional role in the regulation of fibrinolysis in vivo remains to be investigated. Furthermore, Rius et al. demonstrated that NOR1 expression in endothelial induces cell proliferation by promoting cell cycle progression and DNA synthesis[33]. In contrast, experiments by Arkenbout et al. suggested that overexpression of Nur77 exerts the opposite effect and induces cell cycle arrest[31]. The first in vivo evidence to confirm a functional role for Nur77 in endothelial cells was provided by Zeng et al., who observed decreased angiogenesis in Nur77-deficient mice[34]. In these studies, overexpression of Nur77 in HUVEC increased proliferation, cell survival and tube formation. Conversely, silencing of Nur77 decreased tube formation and VEGF-induced expression of cell cycle regulators cyclin D1, cyclin A and E2F[34]. In concert, these data establish a role for NR4A receptors in endothelial cell proliferation, survival and angiogenesis and point to potentially differential functions of NOR1 and Nur77, although further studies are required to identify the regulated target genes mediating these responses.
Smooth Muscle Cells
In addition to inflammation and endothelial dysfunction, proliferation of SMC is considered to play a pivotal role in the pathogenesis of atherosclerosis and the failure of interventional approaches used to treat related occlusive vascular complications[35]. While basal expression levels in the vasculature are low, all members of the NR4A receptor family are expressed in SMC of atherosclerotic lesions and in the neointima following vascular injury[13,16,36-38]. In vitro, NR4A receptor expression in SMC is induced by a variety of stimuli, including various mitogens, oxidized LDL, and conditioned media of activated macrophages[13,16,17,36,38-40]. For example, we have previously demonstrated that NOR1 mRNA expression is induced by >600-fold upon mitogenic stimulation of murine SMC[16]. In addition to mitogens, Nur77 transcript levels are also induced by cyclic stretch in both arterial and venous SMC[37]. In concert, these studies demonstrate a consistent induction of NR4A receptors in stimulated SMC and point to a key role of these receptors in the transcriptional control of SMC proliferation during vascular remodeling.
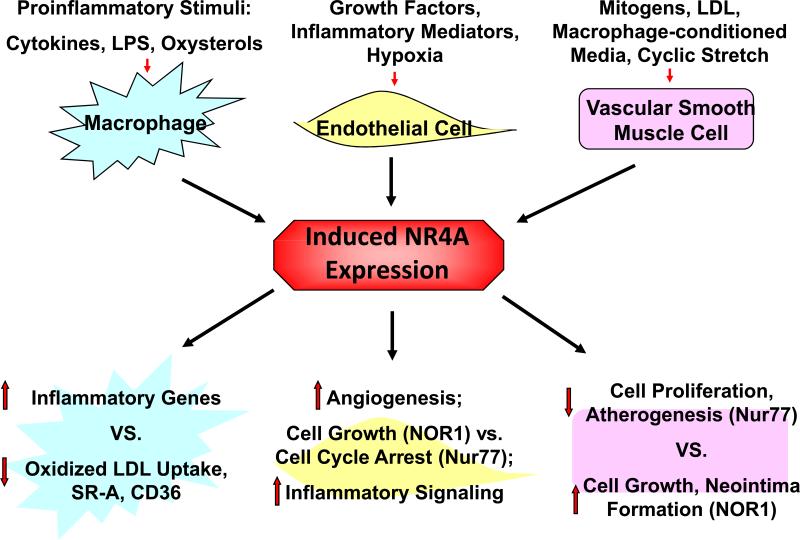
The signaling networks governing the regulation of NR4A receptors in SMC involve multiple pathways, including PKC activation, G-protein receptor activation, and calcium mobilization[13,40]. The transcriptional induction of NOR1 during growth factor stimulation of SMC is primarily mediated through the RAS-MEK-ERK1/2 signaling pathway[16]. Activation of ERK1/2 MAP kinase converges in CREB phosphorylation at the Ser-133 site and ultimately binding of phosphorylated CREB to three highly-conserved CRE sites between −79 and −46 in the NOR1 promoter[16] (Fig. 2). In contrast to NOR1, little is known about the transcriptional mechanisms underlying the expression of Nur77 and Nurr1 in SMC.
Figure 2. Regulation and function of NR4A orphan nuclear receptors in macrophages, endothelial cells and smooth muscle cells.
NR4A nuclear receptors have been demonstrated to be highly induced by a variety of stimuli, including both inflammatory and proliferative factors. Functional studies point to a critical role of NR4A nuclear receptors in the control of vascular gene expression programs (see text for details).
The first pioneering studies to investigate the functional role of NR4A receptors in SMC biology were performed by Arkenbout et al.[36]. Using adenovirus-mediated expression of a dominant-negative Nur77 mutant the authors provided evidence that Nur77 exerts an antiproliferative activity by reducing DNA synthesis and increasing p27Kip1 protein expression in SMC. Consistent with these observations transgenic overexpression of Nur77 under control of a SMC-specific promoter limits neotintima formation during vascular remodeling[36]. Follow-up studies by the same group confirmed the antiproliferative activity of Nur77 in vitro and demonstrated that this receptor prevents cyclic stretch-induced proliferation of SMC[37]. As detailed earlier 6-mercaptopurine augments Nur77-mediated transactivation of target genes[26]. Interestingly, 6-mercaptopurine has been reported to prevent neointima formation in wildtype mice but not in animals overexpressing a dominant-negative Nur77 mutant[37]. These studies have suggested that the inhibition of SMC proliferation by 6-mercaptopurine depends on Nur77 activity and may be mediated by augmentation of its ability to transactivate downstream target genes[26,37]. However, to date the transcriptional target genes that are activated by Nur77 in SMC and mediate this antiproliferative activity of the receptor remain unknown.
While these studies clearly indicate that Nur77 prevents SMC proliferation, NOR1 has been reported to act mitogenic suggesting a function that is distinct from that of Nur77. Martinez-Gonzalez et al. first employed antisense oligonucleotides to demonstrate that knock-down of NOR1 limits SMC proliferation and DNA synthesis[13]. Consistent with these initial observations, studies from our group revealed decreased proliferation of SMC isolated from NOR1-deficient mice[16,38] (Fig. 2). In vivo, the proliferative response and neointima formation following endovascular femoral artery guide wire injury is decreased in NOR1-deficient mice[38]. In vitro, NOR1-deficient SMC exhibit an arrest in the G1→S phase of the cell cycle and increased apoptosis in response to serum deprivation. Using a series of molecular approaches, we identified a canonical NBRE site in the cyclin D1 promoter, to which NOR1 is recruited in response to mitogenic stimulation of SMC. The subsequent transactivation of the cyclin D1 promoter by NOR1 results in increased cyclin D1 protein expression and the phosphorylation of the retinoblastoma protein[16,38]. These experiments identified cyclin D1 as a bona fide NOR1 target gene in SMC and provide a molecular mechanism underlying the mitogenic activity of NOR1 in SMC. In concert, these in vitro and in vivo studies establish an important role for NR4A receptors in the control of SMC proliferation and vascular remodeling. However, additional studies are required to define the regulated target genes, and it will be particularly important to determine the molecular basis underlying the differential function of NOR1 and Nur77 in SMC biology.
4. Conclusion
In conclusion, NR4A receptors are immediate/early response genes that are highly expressed in response to injury of the arterial wall. All cell types participating in vascular remodeling rapidly induce the expression of NR4A receptors in response to environmental cues. Recent studies have provided initial evidence to suggest fundamental roles of these orphan receptors in regulating genes involved in the control of inflammation, proliferation, apoptosis, thrombosis, and angiogenesis. Albeit this progress, little is known about the downstream target genes that mediate these responses. Considering for example that all three members of the NR4A receptor subfamily bind to the same NBRE site, it remains unclear whether there is functional redundancy and if so whether this is cell specific. In addition, an obvious question for future investigation is how to reconcile the distinct biological effects of NOR1 and Nur77 in SMC. Nurr77, unlike NOR1, can heterodimerize with RXR and mediate efficient transactivation in response to RXR-specific agonists[23]. Since ligand-induced RXR activation has been well established to inhibit mitogen-induced SMC proliferation[41], some of the antiproliferative activity of Nur77 may be mediated through RXR. Therefore, an emphasis of future research will have to be the definition of target genes and the analysis of the detailed transcriptional networks by which NR4A receptors control vascular gene expression programs.
5. Acknowledgements
Dennis Bruemmer is supported by the National Institutes of Health (RO1 HL084611).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Smith JD, et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92(18):8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla A, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 5.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116(3):607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefebvre P, et al. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germain P, et al. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58(4):685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65(3):609–618. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Pols TW, et al. NR4A nuclear orphan receptors: protective in vascular disease? Curr Opin Lipidol. 2007;18(5):515–520. doi: 10.1097/MOL.0b013e3282ef77d1. [DOI] [PubMed] [Google Scholar]
- 10.Wansa KD, et al. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277(36):33001–33011. doi: 10.1074/jbc.M203572200. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423(6939):555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 12.Zhan Y, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol. 2008;4(9):548–556. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Gonzalez J, et al. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ Res. 2003;92(1):96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- 14.Pei L, et al. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280(32):29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 15.Bonta PI, et al. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26(10):2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 16.Nomiyama T, et al. The NR4A orphan nuclear receptor NOR1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J Biol Chem. 2006;281(44):33467–33476. doi: 10.1074/jbc.M603436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, et al. Induction of nuclear orphan receptor NGFI-B gene and apoptosis in rat vascular smooth muscle cells treated with pyrrolidinedithiocarbamate. Arterioscler Thromb Vasc Biol. 2001;21(11):1738–1744. doi: 10.1161/hq1101.098550. [DOI] [PubMed] [Google Scholar]
- 18.Pirih FQ, et al. Parathyroid hormone induces the NR4A family of nuclear orphan receptors in vivo. Biochem Biophys Res Commun. 2005;332(2):494–503. doi: 10.1016/j.bbrc.2005.04.132. [DOI] [PubMed] [Google Scholar]
- 19.Davis IJ, et al. Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol. 1993;7(8):953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs CM, et al. ERK2 prohibits apoptosis-induced subcellular translocation of orphan nuclear receptor NGFI-B/TR3. J Biol Chem. 2004;279(48):50097–50101. doi: 10.1074/jbc.M409145200. [DOI] [PubMed] [Google Scholar]
- 21.Han YH, et al. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25(21):2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- 22.Maira M, et al. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19(11):7549–7557. doi: 10.1128/mcb.19.11.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zetterstrom RH, et al. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996;10(12):1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- 24.Kagaya S, et al. Prostaglandin A2 acts as a transactivator for NOR1 (NR4A3) within the nuclear receptor superfamily. Biol Pharm Bull. 2005;28(9):1603–1607. doi: 10.1248/bpb.28.1603. [DOI] [PubMed] [Google Scholar]
- 25.Wansa KD, et al. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem. 2003;278(27):24776–24790. doi: 10.1074/jbc.M300088200. [DOI] [PubMed] [Google Scholar]
- 26.Wansa KD, Muscat GE. TRAP220 is modulated by the antineoplastic agent 6-Mercaptopurine, and mediates the activation of the NR4A subgroup of nuclear receptors. J Mol Endocrinol. 2005;34(3):835–848. doi: 10.1677/jme.1.01739. [DOI] [PubMed] [Google Scholar]
- 27.Pei L, et al. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol. 2006;20(4):786–794. doi: 10.1210/me.2005-0331. [DOI] [PubMed] [Google Scholar]
- 28.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber F, et al. Direct binding of Nur77/NAK-1 to the plasminogen activator inhibitor 1 (PAI-1) promoter regulates TNF alpha -induced PAI-1 expression. Blood. 2003;101(8):3042–3048. doi: 10.1182/blood-2002-07-2331. [DOI] [PubMed] [Google Scholar]
- 30.McEvoy AN, et al. Corticotropin-releasing hormone signaling in synovial tissue vascular endothelium is mediated through the cAMP/CREB pathway. Ann N Y Acad Sci. 2002;966:119–130. doi: 10.1111/j.1749-6632.2002.tb04209.x. [DOI] [PubMed] [Google Scholar]
- 31.Arkenbout EK, et al. TR3 orphan receptor is expressed in vascular endothelial cells and mediates cell cycle arrest. Arterioscler Thromb Vasc Biol. 2003;23(9):1535–1540. doi: 10.1161/01.ATV.0000084639.16462.7A. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, et al. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler Thromb Vasc Biol. 2003;23(11):2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 33.Rius J, et al. NOR-1 is involved in VEGF-induced endothelial cell growth. Atherosclerosis. 2006;184(2):276–282. doi: 10.1016/j.atherosclerosis.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Zeng H, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203(3):719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor AM, McNamara CA. Regulation of vascular smooth muscle cell growth: targeting the final common pathway. Arterioscler Thromb Vasc Biol. 2003;23(10):1717–1720. doi: 10.1161/01.ATV.0000094396.24766.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arkenbout EK, et al. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106(12):1530–1535. doi: 10.1161/01.cir.0000028811.03056.bf. [DOI] [PubMed] [Google Scholar]
- 37.de Waard V, et al. TR3 nuclear orphan receptor prevents cyclic stretch-induced proliferation of venous smooth muscle cells. Am J Pathol. 2006;168(6):2027–2035. doi: 10.2353/ajpath.2006.050932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomiyama T, et al. Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. circulation. 2009;119(4):577–586. doi: 10.1161/CIRCULATIONAHA.108.822056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vries CJ, et al. Differential display identification of 40 genes with altered expression in activated human smooth muscle cells. Local expression in atherosclerotic lesions of smags, smooth muscle activation-specific genes. J Biol Chem. 2000;275(31):23939–23947. doi: 10.1074/jbc.M910099199. [DOI] [PubMed] [Google Scholar]
- 40.Rius J, et al. Involvement of neuron-derived orphan receptor-1 (NOR-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: role of CREB. Arterioscler Thromb Vasc Biol. 2004;24(4):697–702. doi: 10.1161/01.ATV.0000121570.00515.dc. [DOI] [PubMed] [Google Scholar]
- 41.Wakino S, et al. Retinoids inhibit proliferation of human coronary smooth muscle cells by modulating cell cycle regulators. Arterioscler Thromb Vasc Biol. 2001;21(5):746–751. doi: 10.1161/01.atv.21.5.746. [DOI] [PubMed] [Google Scholar]