Abstract
We describe the staff time required by the Prevention Care Manager tailored telephone support intervention, which significantly increased breast, cervical and colorectal cancer screening rates among female patients of Community Health Centers in New York City. For a sample of 38 women whose intervention was timed, Prevention Care Managers spent an average of 99 minutes per woman on the phone and on related follow-up tasks over 18 months, or 248 minutes for each additional cancer screening test. Potential modifications to decrease the time required include automation of common tasks and the use of administrative data to further tailor outreach calls.
Keywords: Cancer screening, patient care management, telephone counseling
Every year in New York City, over 700 women die of colorectal cancer, 1100 die of breast cancer, and 150 die of cervical cancer.1 The cancer death rate in New York City is 1.3 times higher in low-income neighborhoods than in high-income neighborhoods.2 Lower cancer screening rates among low-income and minority women may contribute to their higher rates of cancer morbidity and mortality.3, 4 Low screening rates affect the health of individuals, families, and populations, and also negatively affect the HEDIS quality ratings compiled annually for Medicaid Managed Care Organizations (MMCOs), which include an assessment of rates of mammography and Pap testing.5
The Prevention Care Manager (PCM) intervention was designed to test the ability of telephone support to increase cancer screening rates among 50-69 year old female patients of Community /Migrant Health Centers (C/MHCs) in New York City, many of whom are low-income and/or members of ethnic minorities.6 Dartmouth Medical School and Clinical Directors Network (CDN), a practice-based research network (PBRN) in New York City (www.CDNetwork.org),7 collaborated on the project. Trained Prevention Care Managers (PCMs) provided telephone support to women who were overdue for mammography, Pap testing, and/or colorectal cancer screening. As reported elsewhere, this intervention was found to increase screening rates for all 3 cancers.8 Among PCM women, breast cancer screening rates increased by 12%, Pap test rates increased by 7%, colorectal cancer screening rates by 13%, and the number of women up-to-date on all 3 cancer screenings increased by 14%, compared with Usual Care.
To establish the business case needed for health care planners and policy makers to advocate for and adopt such telephone support for cancer screening, the costs associated with implementing the intervention need to be understood. These costs are primarily driven by the amount of staff time spent, both per woman and per additional test received. In order to track the amount of PCM time spent per woman, all PCM contact with women and actions on their behalf over the 18 month intervention were timed for a subset of women. In this paper we report on the results from this timing subset, and suggest how the intervention could be streamlined for more widespread dissemination.
Methods
Subjects
Women were eligible for this study if they were 50 to 69 years of age, had been receiving care at one of the 11 participating C/MHCs for at least 6 months prior to enrollment in the PCM project, and spoke English or Spanish. CDN-based research assistants approached women in C/MHC waiting rooms, described the study, and obtained written informed consent from women who were willing to participate. Each consenting woman's medical record was reviewed to confirm eligibility. Women who were found to be up-to-date on all 3 cancer screenings were excluded, as were women who were under active cancer treatment or acutely ill at the time of recruitment. In addition, women whose chart documented an unresolved urgent abnormal result on any cancer screening were excluded from the study, and their primary care clinicians were notified of the need for follow-up care. Enrollment for the study ran from November 2001 through October 2002. Women were grouped by age and C/MHC, and randomly assigned to receive either the PCM intervention or Usual Care. The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College, the Institutional Review Board at CDN, and by all relevant bodies responsible for human subjects review and protection at participating C/MHCs.
Timing Subset
In order to accurately assess the staff time required for an experienced PCM to provide tailored cancer screening telephone support, we delayed the collection of timing data until well into the study (December 2002), when we expected that PCMs would have achieved the peak of the learning curve. By this time, all recruitment was complete, and telephone calls to women had become routine. Every day for two weeks, each PCM assigned the first Intervention woman successfully contacted for an initial follow-up call to the timing subset.
Design
The intervention is described in greater detail elsewhere.8 In brief, women in the intervention group received a series of telephone calls and mailings from a trained PCM, who provided educational information as well as motivational and scheduling support toward becoming up-to-date for all three cancer screenings. All PCMs were female, most were college graduates, and prior to the intervention, each received 7 hours of training from the Principal Investigator (AJD) and Project Director (AC), in 2 group sessions. These research-supported PCMs were comparable in education and experience to Medical Assistants or Health Educators found in the FQHCs. Telephone calls and mailings were conducted in the woman's primary language (English or Spanish). During these calls, the PCM asked women about barriers to screening,9 and then responded to each woman's specific barriers using a structured script. Commonly reported barriers included the absence of a clinician's recommendation for a screening, chronic illness and other competing priorities, difficulty scheduling appointments, and worry about the test. PCMs continued to call women until they were up-to-date for all targeted cancer screenings or the intervention period was over. While telephone calls were guided by a script, the content and length of conversations, as well as the frequency of calls, were tailored to the needs of each subject. Some women received a single call, while others presented substantial and persistent barriers, and in some cases received up to 20 calls.
In addition to telephone support, PCMs also carried out various administrative tasks on behalf of women: PCMs prepared and mailed women Provider Recommendation Letters and Patient Activation Cards listing overdue screenings, to bring to their next primary care appointment; they also scheduled appointments, and informed and reminded patients by phone and by mail about these appointments. Language appropriate educational material on needed cancer screening tests was mailed to women, and PCMs conducted research to respond to individual patient inquiries.
Data collection
For the timing subset, time (in minutes) was recorded manually by each PCM as she conducted phone calls with women or carried out follow-up tasks. Certain tasks, such as discussing barriers to mammography, were screening specific, while others, such as scheduling an appointment with a primary care provider, were not assigned to any particular screening. In a small number of cases where timing data were not recorded for an individual call, the average time spent on timed calls of that type (e.g. initial follow-up call, subsequent follow-up call) was used as a proxy. All times reported are over the duration of the 18 month intervention. Primary language was recorded at enrollment; all other characteristics were collected during the post-intervention review of medical records.
Analysis
In our outcome analysis, women who had received Pap testing or mammography within 18 months were considered up-to date. While the United States Preventive Services Task Force (USPSTF) recommends that women receive Pap tests at least every 3 years after a series of normal tests,10 and the HEDIS quality measure follows suit,5 our study population included many women with cervical cancer risk factors whose clinicians had recommended shorter screening intervals. Eighteen months is the midpoint of the USPSTF mammography recommendation (every 1-2 years),11 and is more aggressive than the HEDIS mammography quality measure interval (2 years).5 For colorectal cancer, a woman was considered up-to-date if she had received a home Fecal Occult Blood Test (hFOBT) within 18 months, a double-contrast barium enema or sigmoidoscopy within 5 years, or a colonoscopy within 10 years. This 18 month period for hFOBT provides a 6-month grace period to the USPSTF 12 month recommendation,12 used as part of the HEDIS colorectal cancer screening quality measure with commercial insurers.5
Data from medical record reviews, PCM telephone logs and timing forms were recorded separately, and merged for analysis. For continuous variables, we report mean, standard deviation, range, and interquartile range with comparisons performed using Student's T-test. For categorical variables, we report percentages in each group with comparisons performed by estimating the odds ratio using logistic regression. Multivariate linear regression and multivariate logistic regression were used for covariate adjustment of continuous and categorical variables respectively. 95% confidence intervals are reported with a p-value less than 0.05 taken to indicate statistical significance without adjustment for multiple comparisons.
Results
In the full study, a total of 1413 women were randomized to receive either the Prevention Care Manager Intervention or Usual Care (Figure 1). Of these, 23 whose medical records could not be located for the follow-up record review were excluded. Because the timing subset did not include women who spoke Haitian Creole, we excluded the 4 Creole speaking women from this analysis. In addition, we excluded women whose follow-up record review revealed that they were actually up-to-date on all 3 cancer screening tests at baseline (n=280), and women with a history of breast, cervical, or colorectal cancer (n=40). Our analysis sample included 531 women assigned to receive Usual Care, and 535 women assigned to receive the PCM intervention, out of which 38 (7%) were included in the timing subset.
Figure 1.
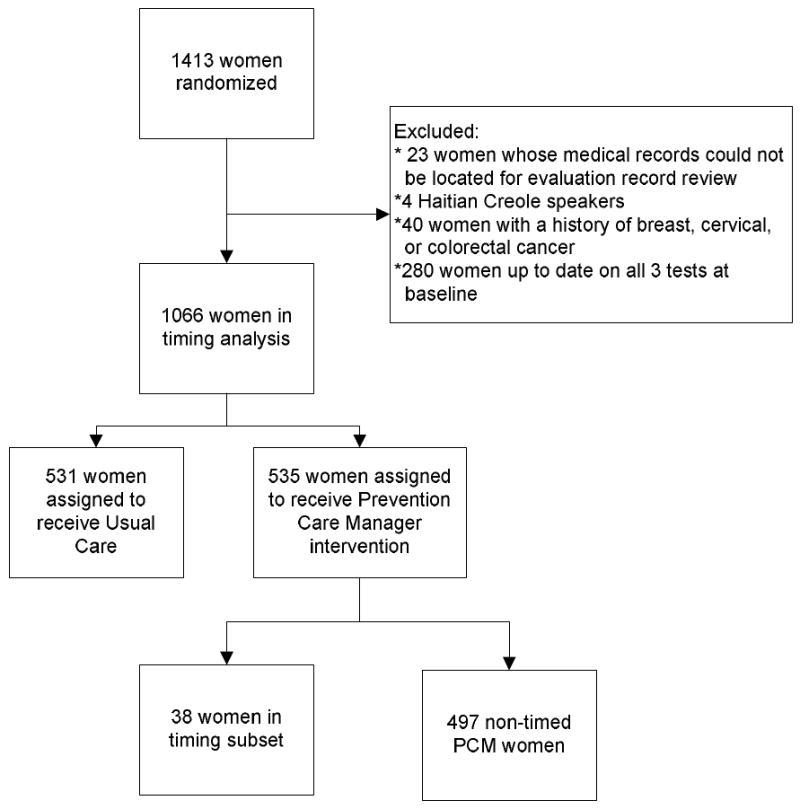
Consort diagram
As noted above, women in the timing subset were identified through a systematic approach. A comparison of the timing subset with non-timed PCM women and women assigned to receive Usual Care indicates that the three groups are demographically similar (Tables 1a and 1b). Most women were single, divorced, or widowed, were publically insured through Medicaid and/or Medicare, and had been receiving care at their C/MHC for at least three years. A larger proportion of women in the timing subset spoke English as their primary language than in the Intervention or Usual Care groups, because bilingual PCMs and those who only spoke English each selected one woman into the timing subset per day, rather than at a rate proportional to the primary language of the study population (63% Spanish, 37% English).
Table 1. Table 1a. Baseline characteristics of Intervention subjects: Timing subset and Non-timed.
| Characteristic | Timing Subset | Non-timed Intervention | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| # | % | # | % | ||||
| Mean age at consent (S.D.) | 58.6 yrs (6.0) | 58.0 yrs (5.2) | 0.6 | (-1.4,2.6) | 0.54 | ||
| Primary language | |||||||
| English | 21 | 55.3% | 175 | 35.2% | |||
| Spanish | 17 | 44.7% | 322 | 64.8% | 0.44* | (0.21,0.90) | 0.013 |
| Marital Status | |||||||
| Married/Cohabiting | 8 | 21.6% | 125 | 28.6% | 0.69 | (0.26,1.60) | 0.36 |
| Single/Divorced/Widowed | 29 | 78.4% | 312 | 71.4% | |||
| Insurance | |||||||
| Medicaid | 29 | 76.3% | 395 | 79.5% | 0.83 | (0.37,2.06) | 0.64 |
| Medicare | 7 | 18.4% | 98 | 19.7% | 0.92 | (0.33,2.21) | 0.85 |
| Employer / Other | 6 | 15.8% | 43 | 8.7% | 1.98 | (0.64, 5.16) | 0.14 |
| Uninsured | 4 | 10.5% | 27 | 5.4% | 2.05 | (0.49,6.37) | 0.2 |
| Unknown | 0 | 0.0% | 7 | 1.4% | 0 | (0.00,7.25) | 0.46 |
| Years at Health Center prior to consent | |||||||
| Less than 3 years | 9 | 23.7% | 147 | 30.8% | |||
| 3 or more years | 29 | 76.3% | 331 | 69.2% | 1.43 | (0.64,3.52) | 0.36 |
| Table 1b. Baseline characteristics of the Intervention Timing subset and Usual Care subjects | |||||||
| Characteristic | Timing Subset | Usual Care / Control | OR | 95% CI | P value | ||
| # | % | # | % | ||||
| Mean age at consent (S.D.) | 58.6 yrs (6.0) | 57.8 yrs (5.2) | 0.8 | (-1.3,2.8) | 0.45 | ||
| Primary language | |||||||
| English | 21 | 55.3% | 207 | 39.0% | |||
| Spanish | 17 | 44.7% | 324 | 61.0% | 0.52* | (0.25,1.06) | 0.048 |
| Marital Status | |||||||
| Married/Cohabiting | 8 | 21.6% | 142 | 29.3% | 0.66 | (0.26,1.54) | 0.32 |
| Single/Divorced/Widowed | 29 | 78.4% | 342 | 70.7% | |||
| Insurance | |||||||
| Medicaid | 29 | 76.3% | 415 | 78.2% | 0.9 | (0.40,2.23) | 0.79 |
| Medicare | 7 | 18.4% | 92 | 17.3% | 1.08 | (0.39,2.59) | 0.86 |
| Employer / Other | 6 | 15.8% | 52 | 9.8% | 1.73 | (0.56,4.45) | 0.24 |
| Uninsured | 4 | 10.5% | 29 | 5.5% | 2.04 | (0.49,6.29) | 0.2 |
| Unknown | 0 | 0.0% | 6 | 1.1% | 0 | (0.00,9.07) | 0.51 |
| Years at Health Center prior to consent | |||||||
| Less than 3 years | 9 | 23.7% | 152 | 29.6% | 1.35 | (0.61,3.33) | 0.44 |
| 3 or more years | 29 | 76.3% | 362 | 70.4% | |||
OR: Odds Ratio
CI: Confidence Interval
S.D.: Standard Deviation
NOTE: Percentages reflect denominators for non-missing data.
p < 0.05
While baseline and follow-up cancer screening rates varied slightly between the timing subset and non-timed intervention women (Table 2a), no differences were significant. Women in both groups were up-to-date for an average of two tests at follow-up, representing an increase of 0.7 tests among the timing subset and 0.6 tests among the non-timed intervention patients. A comparison between the timing subset and women assigned to receive Usual Care (Table 2b) also shows no significant differences in either baseline or follow-up screening rates for individual tests. Women in the timing subset had increased their number of up-to-date tests by 0.7 between baseline and follow-up, compared with an increase of 0.3 tests among Usual Care. The 0.4 difference in the average increase in up-to-date tests between these two groups is statistically significant (p=.015).
Table 2. Table 2a: Comparison of Baseline and Follow-up Up to date rates among Intervention Subjects: Timing subset and Non-Timed.
| Timing Subset | Non-Timed Intervention | OR | 95% CI | P value | |||
|---|---|---|---|---|---|---|---|
| Up-to-date at baseline | # | % | # | % | |||
| Pap test | 13 | 40.6% | 199 | 53.2% | 0.6 | (0.27,1.33) | 0.17 |
| Mammography | 16 | 42.1% | 235 | 47.3% | 0.81 | (0.39,1.66) | 0.54 |
| Colon cancer screening | 4 | 12.5% | 50 | 11.4% | 1.11 | (0.27,3.36) | 0.85 |
| Up-to-date at follow-up | |||||||
| Pap test | 20 | 62.5% | 251 | 67.1% | 0.82 | (0.37,1.90) | 0.6 |
| Mammography | 24 | 63.2% | 325 | 65.4% | 0.91 | (0.44,1.95) | 0.78 |
| Colon cancer screening | 16 | 50.0% | 221 | 50.5% | 0.98 | (0.45,2.16) | 0.96 |
| # | # | Difference | 95% CI | P value | |||
| Mean number of tests Up-to-date at follow-up | 1.9 (sd 0.9) | 2 (sd 1.0) | 0.1 | (-0.4,0.2) | 0.64 | ||
| Mean increase in number of Up-to-date tests between baseline and follow-up | 0.7 (sd 0.9) | 0.6 (sd 1.1) | 0.1 | (-0.2,0.4) | 0.61 | ||
| Table 2b: Comparison of Baseline and Follow-Up Up to date rates among the Intervention Timing Subset and Usual Care | |||||||
| Timing Subset | Usual Care / Control | OR | 95% CI | P value | |||
| Up-to-date at baseline | # | % | # | % | |||
| Pap test | 13 | 40.6% | 184 | 48.0% | 0.74 | (0.33,1.63) | 0.42 |
| Mammography | 16 | 42.1% | 261 | 49.2% | 0.75 | (0.36,1.54) | 0.4 |
| Colon cancer screening | 4 | 12.5% | 55 | 11.6% | 1.09 | (0.27,3.29) | 0.88 |
| Up-to-date at follow-up | |||||||
| Pap test | 20 | 62.5% | 204 | 53.3% | 1.46 | (0.66,3.38) | 0.31 |
| Mammography | 24 | 63.2% | 294 | 55.4% | 1.38 | 0.67,2.96) | 0.35 |
| Colon cancer screening | 16 | 50.0% | 168 | 35.4% | 1.83 | (0.83,4.01) | 0.096 |
| # | # | Difference | 95% CI | P value | |||
| Mean number of tests Up-to-date at follow-up | 1.9 (sd 0.9) | 1.6 (sd 1.0) | 0.3 | (-0.1, 0.6) | 0.11 | ||
| Mean increase in number of Up-to-date tests between baseline and follow-up | 0.7 (sd 0.9) | 0.3 (sd 1.1) | 0.4 | (0.1,0.7) | 0.015 | ||
NOTE: Percentages reflect denominators for non-missing data.
Table 3 reports on the amount of PCM time spent with timing subjects both on the phone and between calls. PCMs spent an average of 64 minutes in telephone contact with subjects, with initial calls for these 38 women averaging 16 minutes and subsequent calls averaging 14 minutes. PCMs spent an average of 35 minutes per subject providing follow-up support between calls, spending almost 18 minutes compiling and mailing material, 8 minutes scheduling appointments and reminding women about these appointments, and 9 minutes providing other support such as developing responses to questions from subjects or contacting providers. Combining contact and follow-up time, PCMs spent an average of 99 minutes with each timing subject over the 18 month intervention period. As shown in Table 2b, women in the timing subset received an average of 0.4 more tests by follow-up than did their usual care counterparts. An estimated duration of 99 minutes total time over 18 months, including 64 minutes of contact time, averages out to 248 minutes of PCM time, including 161 minutes of contact time, for each additional test received.
Table 3. PCM time spent per subject (n=38).
| Total time (in minutes) per woman | |||
|---|---|---|---|
| Category of time spent | Mean (SD) | Range | Interquartile Range |
| PCM contact with woman | 64.4 (38.2) | 8-140.7 | 32-100 |
| PCM Follow-up | 34.7 (29.7) | 0-108 | 14-40 |
| Compile and send material to women | 17.5 (17.7) | 0-82 | 6.2-24 |
| Schedule appointments, send appointment reminder letters | 8.0 (14.6) | 0-48 | 0-7 |
| Other legwork tasks (research, mail “no contact letter”, etc.) | 9.3 (17.4) | 0-86 | 0-13 |
| Total time spent by PCM | 99.2 (54.1) | 8-243 | 53-132.8 |
Time spent on the strictly research aspects of this study is not included in the time estimates reported above. This time included the enrollment interview, during which consent was obtained (mean of 9.5 minutes), and checks of medical records to confirm or correct information reported by subjects (mean of 23 minutes). In order to ensure treatment fidelity, the New York City-based Project Director (AC) also spent an average of 17 minutes per timing subject supervising the PCMs, over an average of 4 sessions per subject.
As shown in Table 4, PCMs spent an average of 17 minutes in conversation and on follow-up tasks related to both mammography and Pap testing, and 28 minutes for colorectal cancer screening. The remaining non-test specific time was spent developing and maintaining rapport and providing general screening support. For all tests, PCMs spent more than twice as much test-specific time on average with women who failed to become up-to-date on that test by follow-up than they did with women who were up-to-date at follow-up. In addition, nearly half of the women (16 of 38) in our timing subset reported on the initial call that they had never been screened for colon cancer; by follow-up, 8 of these 16 women were up-to-date on colon cancer screening.
Table 4. Test specific time in contact with woman and carrying out follow-up tasks, and screening status at follow-up.
| Timing subset | Up-to-date at Follow-up | Overdue at Follow-up | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Screening test | n | Mean time (minutes) | S.D. | n | Mean time (minutes) | S.D. | n | Mean time (minutes) | S.D. | |
| Mammography | 38 | 17.1 | 18.4 | 24 | 11.8 | 17.0 | 14 | 26 | 17.9 | 0.02 |
| Pap test | 32 | 17.3 | 17.8 | 20 | 10.1 | 12.9 | 12 | 29.4 | 18.9 | 0.00 |
| Colorectal Cancer screening | 32 | 28.1 | 25.3 | 16 | 18.4 | 15.5 | 16 | 37.9 | 29.6 | 0.03 |
Note: statistical comparison is between time spent with subjects up-to-date for a given test and that spent with subjects who were overdue for that same test.
We also carried out an exploratory analysis using multivariate linear regression to determine if any of the characteristics described in Tables 1a and 1b, either alone or together, predicted time spent. No variable alone or in combination was predictive given the limited number of patients with timing data.
Discussion
An effective telephone counseling intervention was provided in approximately 99 minutes per woman over an 18 month period, or about 5 minutes per woman per month. Approximately an hour of this time was spent on the phone with each woman; the rest was spent on follow-up tasks to help women overcome barriers to screening.
While other telephone counseling interventions have increased cancer screening rates with substantially less than an hour of telephone time,13-17 this intervention was unique in packaging all 3 cancer screenings recommended for this population – breast, cervical, and colorectal – into one multiple-call intervention, delivered to a primarily low-income and minority population. Champion et al. found increased mammography rates following 30 to 45 minutes of in-person or telephone counseling provided by a practice-based graduate nursing assistant, but suggested that spending this amount of time for one test may amount to an unaffordable luxury to many health care practices.18 Integrating 2 additional cancer screenings into the counseling protocol is one clear step toward greater efficiency19; PCMs worked with women on up to 3 cancer screenings, spending an average of 64 minutes of phone contact time, considerably less than 3 times the phone time reported by Champion. While other telephone interventions which have devoted less time or fewer calls to each woman failed to increase screening rates among previously non-adherent women,20, 21 half of our timing subjects who had never been screened for colon cancer screening became up-to-date during the intervention period. Once screened for the first time, these women may be more likely to be screened in the future, with these future tests representing additional rewards for the PCM time invested. As women shared their barriers and then worked with a PCM to overcome them, they experienced an interpersonal connection that may result in more positive long-term impacts on screening rates than less-intensive interventions or more generic approaches such as mass mailings.16, 22
We also found that PCMs spent significantly more time providing test-specific support to women who were still overdue at the end of the study on cancer screenings than they did to women who were up-to-date at follow-up. This is partly a function of the intervention protocol, in which PCMs continued calling women and providing test-specific support for willing subjects until a woman was up-to-date. Women who quickly became up-to-date required fewer calls and less PCM time than those with more persistent barriers who remained overdue for the entire intervention period. It also indicates that some women may be unreceptive to the PCM approach no matter how much PCM time is spent; these women represent a potential source of inflation of the timing figures reported in this study. A less intensive protocol, with fewer calls to resistant women, might have achieved comparable increases in screenings at a lower cost of PCM time. This also suggests that further PCM intervention with resistant patients is unlikely to result in further cancer screening, and that other types of intervention should be evaluated for use with these patients. Future research is needed to determine the appropriate threshold of time or number of calls beyond which further telephone contact with women is ineffective.
Additional research is also needed to explore whether individual characteristics can be used to identify women who will be more or less receptive to this particular type of intervention. Saywell, Stockdale and Crane have recommended using patient cancer screening history to tailor cancer screening interventions.13, 21, 23 This screening data can be collected efficiently through a simple query of health plan or health center administrative electronic data. A two-tiered intervention, in which previously adherent women receive a less intensive intervention than those who have never been screened or are many years overdue, could help direct PCM resources where they are most needed. For example, of the timing subjects who became up-to-date on colon cancer screening, PCMs spent an average of 27 minutes with those who had never been screened, and only 10 minutes with those who were previously adherent.The less time-intensive intervention for recently adherent women could involve fewer and/or shorter telephone calls, reminding them that it is time to be screened rather than exploring and addressing barriers. An automated reminder system could be used to deliver these reminder calls. Time spent on follow-up tasks for all subjects could also be substantially reduced by batching and automating PCM mailings, using data lists and personalized letter templates downloaded from electronic health records where available, or electronic billing and scheduling records.
Finally, it is important to note that the most PCM time was spent providing test-specific support for colorectal screening as compared to either mammography or Pap testing, which reflects the more recent addition of colorectal cancer screening to the routine screening regimen and lower baseline colorectal screening rates.
Some limitations should be noted. Timing data were recorded by the PCMs, rather than directly observed, and may be subject to under or over-reporting. An electronic timing device could reduce error and reporting bias. The timing subset, although representative of the entire study population, was small, which limited our ability to conduct meaningful outcome analyses on further subsets. We did not explicitly calculate the actual costs of the intervention, but used staff time as a proxy for cost in this analysis, and did not attempt to factor in costs for supplies or overhead.
A number of recent studies have assessed the actual dollar costs and cost-effectiveness of colon cancer screening interventions. While not directly comparable to our estimates of personnel time required, the cost per additional individual screened in these studies varied widely, from $43 to $319 for customized mailings to patients,24, 25 $106 to $978 for physician-directed interventions,26, 27 and up to nearly $6000 for a patient-directed mail intervention which included a telephone reminder call.25
“Patient navigators” have also been found to be effective at increasing colon cancer screening rates. 28-33 These navigators closely resemble the PCMs described in this study, except that they often begin their work after a patient has received a colon cancer screening referral from a provider, while much PCM time was spent working toward the provision of just such a referral. A cost effectiveness analysis of one Colonoscopy Patient Navigator program in New York City reported costs at three participating hospitals ranging from $199 to $708 for each additional colonoscopy completed.34 Factoring in the increased hospital revenue provided by these additional colonoscopies, two of the three hospitals achieved a net revenue benefit from their Colonoscopy Navigation Programs.34
A streamlined version of the PCM intervention has been subsequently tested in a National Cancer Institute- funded dissemination pilot study conducted through Affinity Health Plan, a Medicaid Managed Care organization (MMCO) serving primarily low-income and minority women in and around New York City (that provides reimbursements to the participating FQHCs).35 Affinity's electronic administrative and claims data were used to select eligible women and for outcome analyses, and the PCM intervention was made more efficient by reducing the number of calls, shortening the total length of the intervention, and batching mailings. Further testing of a streamlined PCM intervention among female patients of a larger sample of MMCOs in New York City is currently underway.
Our findings highlight the potential of utilizing an individualized, multi-dimensional, multiple call approach to address the array of barriers faced by underserved and minority populations in obtaining cancer early detection services. As Yarnall et al. have pointed out, there is simply not enough time in the day for primary care physicians to provide all recommended preventive services to their patients.36 Focussed help from non-clinicians, such as PCMs, may increase the chance of these preventive services being delivered in a timely manner. While the PCM intervention was effective at increasing access to cancer early detection in a multi-lingual, low-income population, the cost in PCM time is potentially high. Additional less-staff intensive strategies should be considered, including automated telephone reminder systems, personalized form letters, and group visits, as MMCOs and other organizations seek to improve patient outcomes and screening rates in this and other clinical areas. We are currently evaluating a similar PCM intervention set in three NYC-based MMCOs, and will be able to explore differences in time requirements and implementation in future publications.
Acknowledgments
The authors thank the following Community Health Centers for participating in the study or pilot study: Joseph P. Addabbo Family Health Center, Bedford Stuyvesant Family Health Center, Betances Health Center, Brownsville Multi-Service Family Health Center, East Harlem / Boriken Community Health Center, Family Physician Health Center, Montefiore Medical Group and Comprehensive Family Care Center, Morris Heights Community Health Center, Park Ridge Health Center, Ryan-NENA Community Health Center, Sunset Park Family Health Center Network, Urban Health Plan, and William F. Ryan Community Health Center. We also acknowledge the 12 staff members of Clinical Directors Network who served as Prevention Care Managers during this study.
Support: This project was supported by National Cancer Institute grants RO1CA087776 and RO1CA119014.
Abbreviations
- PCM
Prevention Care Manager
- HEDIS
Health Plan Employer Data and Information Set
- C/MHC
Community/Migrant Health Center
- FQHC
Federally Qualified Community Health Centers
- MMCO
Medicaid Managed Care Organization
- CDN
Clinical Directors Network
- SD
Standard Deviation
- USPSTF
United States Preventive Services Task Force
- PBRN
Practice-based Research Network
- hFOBT
home Fecal Occult Blood Test
Footnotes
Elements of this paper were presented at the second meeting of the International Primary Care and Cancer Research Group, April 23-24, 2009, Copenhagen, Denmark
Conflict of Interest Statement: None of the authors have any potential conflicts of interest.
Contributor Information
Christina M. Robinson, Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH.
Michael L. Beach, Norris Cotton Cancer Center and Department of Anesthesiology, Dartmouth Medical School, Hanover, NH.
Mary Ann Greene, Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH.
Andrea Cassells, Clinical Directors Network, New York, NY.
Jonathan N. Tobin, Clinical Directors Network and Department of Epidemiology and Population Health, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY.
Allen Dietrich, Norris Cotton Cancer Center and Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH.
References
- 1.Summary of Vital Statistics 2007. New York City, NY: New York City Department of Health and Mental Hygiene; 2008. [Google Scholar]
- 2.Karpati A, Kerker B, Mostashari F, et al. Health Disparities in New York City 2004. New York City, NY: New York City Department of Health and Mental Hygiene; 2004. [Google Scholar]
- 3.Jacobellis J, Cutter G. Mammography screening and differences in stage of disease by race/ethnicity. Am J Public Health. 2002;92(7):1144–50. doi: 10.2105/ajph.92.7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Malley AS, Forrest CB, Feng S, et al. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. 2005;165(18):2129–35. doi: 10.1001/archinte.165.18.2129. [DOI] [PubMed] [Google Scholar]
- 5.HEDIS 2009 Summary Table of Measures, Product Lines and Changes. National Committee for Quality Assurance. [10/7/2009];2009 at http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2009/2009_Measures.pdf.
- 6.Davis K. A need to transform the U.S. Health Care System: Improving Access, Quality, and Efficiency. National Association of Community Health Centers Plenary Address. 2006 March 27, 2006. [Google Scholar]
- 7.Sardell A. Clinical networks and clinician retention: the case of CDN. J Community Health. 1996;21(6):437–51. doi: 10.1007/BF01702604. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144(8):563–71. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogedegbe G, Cassells AN, Robinson CM, et al. Perceptions of barriers and facilitators of cancer early detection among low-income minority women in community health centers. J Natl Med Assoc. 2005;97(2):162–70. [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force. Screening for Cervical Cancer. [2/3/2010];2003 at http://www.ahrq.gov/clinic/uspstf/uspscerv.htm.
- 11.U.S. Preventive Services Task Force. Screening for Breast Cancer. [10/7/09];2002 at http://www.ahrq.gov/clinic/uspstf/uspsbrca.htm.
- 12.U.S. Preventive Services Task Force. Screening for Colorectal Cancer. [10/7/09];2002 doi: 10.7326/0003-4819-137-2-200207160-00014. at http://www.ahrq.gov/clinic/uspstf/uspscolo.htm. [DOI] [PubMed]
- 13.Crane LA, Leakey TA, Ehrsam G, et al. Effectiveness and cost-effectiveness of multiple outcalls to promote mammography among low-income women. Cancer Epidemiol Biomarkers Prev. 2000;9(9):923–31. [PubMed] [Google Scholar]
- 14.Lauver DR, Settersten L, Kane JH, et al. Tailored messages, external barriers, and women's utilization of professional breast cancer screening over time. Cancer. 2003;97(11):2724–35. doi: 10.1002/cncr.11397. [DOI] [PubMed] [Google Scholar]
- 15.Lipkus IM, Rimer BK, Halabi S, et al. Can tailored interventions increase mammography use among HMO women? Am J Prev Med. 2000;18(1):1–10. doi: 10.1016/s0749-3797(99)00106-3. [DOI] [PubMed] [Google Scholar]
- 16.Rimer BK, Conaway M, Lyna P, et al. The impact of tailored interventions on a community health center population. Patient Educ Couns. 1999;37(2):125–40. doi: 10.1016/s0738-3991(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 17.Costanza ME, Stoddard AM, Luckmann R, et al. Promoting mammography: results of a randomized trial of telephone counseling and a medical practice intervention. Am J Prev Med. 2000;19(1):39–46. doi: 10.1016/s0749-3797(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 18.Champion V, Maraj M, Hui S, et al. Comparison of tailored interventions to increase mammography screening in nonadherent older women. Prev Med. 2003;36(2):150–8. doi: 10.1016/s0091-7435(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 19.Chen TH, Chiu YH, Luh DL, et al. Community-based multiple screening model: design, implementation, and analysis of 42,387 participants. Cancer. 2004;100(8):1734–43. doi: 10.1002/cncr.20171. [DOI] [PubMed] [Google Scholar]
- 20.Stoddard AM, Fox SA, Costanza ME, et al. Effectiveness of telephone counseling for mammography: results from five randomized trials. Prev Med. 2002;34(1):90–9. doi: 10.1006/pmed.2001.0960. [DOI] [PubMed] [Google Scholar]
- 21.Saywell RM, Jr, Champion VL, Skinner CS, et al. A cost-effectiveness comparison of three tailored interventions to increase mammography screening. J Womens Health (Larchmt) 2004;13(8):909–18. doi: 10.1089/jwh.2004.13.909. [DOI] [PubMed] [Google Scholar]
- 22.Saywell RM, Jr, Champion VL, Zollinger TW, et al. The cost effectiveness of 5 interventions to increase mammography adherence in a managed care population. Am J Manag Care. 2003;9(1):33–44. [PubMed] [Google Scholar]
- 23.Stockdale SE, Keeler E, Duan N, et al. Costs and cost-effectiveness of a church-based intervention to promote mammography screening. Health Serv Res. 2000;35(5 Pt 1):1037–57. [PMC free article] [PubMed] [Google Scholar]
- 24.Shankaran V, McKoy JM, Dandade N, et al. Costs and cost-effectiveness of a low-intensity patient-directed intervention to promote colorectal cancer screening. J Clin Oncol. 2007;25(33):5248–53. doi: 10.1200/JCO.2007.13.4098. [DOI] [PubMed] [Google Scholar]
- 25.Lairson DR, DiCarlo M, Myers RE, et al. Cost-effectiveness of targeted and tailored interventions on colorectal cancer screening use. Cancer. 2008;112(4):779–88. doi: 10.1002/cncr.23232. [DOI] [PubMed] [Google Scholar]
- 26.Khankari K, Eder M, Osborn CY, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007;22(10):1410–4. doi: 10.1007/s11606-007-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf MS, Fitzner KA, Powell EF, et al. Costs and cost effectiveness of a health care provider-directed intervention to promote colorectal cancer screening among Veterans. J Clin Oncol. 2005;23(34):8877–83. doi: 10.1200/JCO.2005.02.6278. [DOI] [PubMed] [Google Scholar]
- 28.Jandorf L, Gutierrez Y, Lopez J, et al. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82(2):216–24. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christie J, Itzkowitz S, Lihau-Nkanza I, et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100(3):278–84. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 30.Nash D, Azeez S, Vlahov D, et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83(2):231–43. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6(4):443–50. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24(2):211–7. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers RE, Hyslop T, Sifri R, et al. Tailored navigation in colorectal cancer screening. Med Care. 2008;46(9 Suppl 1):S123–31. doi: 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]
- 34.Elkin E, Snow J, Zauber AG. Assessing the Economic Impact of Colonoscopy Patient Navigator Programs. The Sixth Annual NYC Colon Cancer Prevention and Control (C5) Summit (powerpoint presentation).2009. [Google Scholar]
- 35.Dietrich AJ, Tobin JN, Cassells A, et al. Translation of an efficacious cancer-screening intervention to women enrolled in a medicaid managed care organization. Ann Fam Med. 2007;5(4):320–7. doi: 10.1370/afm.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]


