Abstract
Alterations in circadian rhythm generation may be related to the development of mood disorders. Although it has been reported that the most popular antidepressant, selective serotonin reuptake inhibitors (SSRIs) affect circadian phase, no data are available that describe the effects of SSRIs on other circadian parameters (period, amplitude and damping rate) in dissociated cells. In the present study we used real-time monitoring of bioluminescence in rat-1 fibroblasts expressing the Period1-luciferase transgene, and that in Period1-luciferase transgenic mouse suprachiasmatic nucleus (SCN) explants, in order to characterize the effects of SSRI on circadian oscillator function in vitro. We found that mRNA of the serotonin transporter (SERT), a target of SSRIs, was expressed in rat-1 fibroblasts. Sertraline, fluoxetine, fluvoxamine, citalopram and paroxetine all significantly shortened the period of Period1-bioluminescence rhythms in rat-1 fibroblasts. The amplitude was reduced by sertraline, and the damping rate was decreased by sertraline, fluoxetine, flvoxamine and paroxetine. The effect of sertraline was dose-dependent, and it also shortened the circadian period in the SCN. SERT is associated with lipid microdomains, which are required for efficient SERT activity. Indeed, cholesterol chelating reagent methyl-β-cyclodextrin significantly reduced the period and the amplitude in rat-1 fibroblasts. Furthermore, lipid binding reagent xylazine significantly reduced the period. In summary our data present evidence that SSRIs affect circadian rhythmicity. The action of SSRIs is likely mediated by suppression of SERT activity. A better understanding of the relationship between mental illness and biological timing may yield new insight into disease etiology and avenues for treatment.
Keywords: SSRI, SERT, Lipid raft, Methyl-β-cyclodextrin, Xylazine, Real-time circadian bioluminescence, monitoring, Fibroblasts, Mouse suprachiasmatic nucleus
Introduction
Circadian rhythms are cyclic variations in biological activity which are generated by internal (circadian) clocks in most living organisms on earth (Dunlap et al., 2003). The molecular circadian clock in mammals has been elucidated over the past decade (Ko and Takahashi, 2006). In mammals, the central circadian clock is localized to the suprachiasmatic nucleus (SCN) of the hypothalamus, which synchronizes circadian functions within the body and is necessary for behavioral and hormonal rhythms. Peripheral circadian oscillators have also been identified in many types of cells and tissues (Balsalobre et al.,1998; reviewed in Fukuhara and Tosini, 2003; Brown et al., 2005; Yamazaki et al., 2000; Yoo et al., 2004; Welsh et al., 2004). The expression patterns of circadian clock genes and proteins are similar between SCN neurons and peripheral tissues (Ko and Takahashi, 2006), suggesting that the circadian machinery is similar between the central (SCN) and peripheral cells.
Light is the most effective naturally occurring time cue to entrain the central circadian clocks to the exact 24-hour cycles. In addition, many chemicals affect circadian rhythm generation by shifting clocks and/or affecting period length. For example, lithium lengthens period of SCN neuronal rhythms and behavioral rhythms in many organisms (Klemfuss, 1992). In a previous study, we have shown that SP600125, an inhibitor of one of the mitogen-activated protein kinases (MAPKs) c-Jun N-terminal kinase (JNK), similarly extends period in fibroblasts and the mouse SCN (Chansard et al., 2007). In addition, valproic acid shortens the period in rat-1 fibroblasts (Chansard et al., 2007). These results further suggest that peripheral cell models have a potential to allow us to study the effects of chemicals on circadian rhythm generation. Indeed, the fibroblast model has been used to record circadian rhythms in rodents and even humans (Balsalobre et al., 1998; Brown et al., 2005; Chansard et al., 2007). This model also enables us to reduce the number of animals to be used.
It has been suggested that alterations in circadian rhythm generation may underlie the development of mood disorders; for example, affected phases have been reported to correlate with depression and bipolar disorders (Ehlers et al.,1988; Grandin et al., 2006; Lader, 2007; Malkoff-Schwartz et al., 2000; McClung, 2007). Incidentally, some therapeutic treatments, such as some antidepressants, total sleep deprivation, and bright light therapy have been shown to affect circadian rhythm generation. One of the most popular antidepressant, selective serotonin reuptake inhibitors (SSRIs) have been reported to affect circadian phase in vivo and in vitro (Gannon and Millan, 2007; Prosser et al., 2006; Sprouse et al., 2006). However, no data are available that describe the effects of these agents on circadian parameters (period, amplitude and damping rate) in dissociated cellular oscillator organizations in periphery.
In the present study, we used real-time monitoring of bioluminescence in rat-1 fibroblasts, stably transfected with Period1-luciferase (Period1 promoter; one of circadian clock genes) reporter gene (Chansard et al., 2007) as well as Period1-luciferase transgenic mouse SCN explants to test any effects that SSRIs might have on the circadian rhythm generation. Since SSRI inhibits serotonin reuptake by inhibiting the activity of serotonin transporter (SERT), we first confirmed that SERT mRNA is expressed in rat-1 fibroblasts. Results of real-time-bioluminescence recording suggest that SSRI significantly shortened the period in rat-1 fibroblasts and the mouse SCN. SERT is associated with lipid microdomains, which are required for efficient serotonin transport activity (Magnani et al., 2004). Therefore, to further support our findings, SERT activity was blocked using a cholesterol chelating reagent methyl-β-cyclodextrin (β-CD) and lipid binding reagent xylazine. Both chemicals significantly shortened the period in rat-1 fibroblasts.
Materials and methods
Animals
Transgenic mice carrying a Period1-luciferase transgene as a reporter of activity were generously provided by Dr. Hajime Tei (Mitsubishi Kagaku Institute of Life Sciences, Tokyo, Japan). Period1-luciferase mice were maintained at Morehouse School of Medicine. All animals were housed under a 12-hour light:12-hour dark cycle (light on, 0700–1900) in a temperature-controlled environment and had access to food and water ad libitum. Zeitgeber time (ZT0) was defined as light onset and ZT12 as light offset. Wild type siblings were used for RT-PCR experiment. Both heterozygous and homozygous mice were used in our bioluminescence recording experiments. All experiments were run under the oversight of IACUC of the Atlanta University Center and NIH guidelines.
Drugs
Sertraline hydrochloride, fluvoxamine maleate, paroxetine hydrochloride, methyl-β-cyclodextrin, and xylazine were purchased from Sigma-Aldrich, St Louis, MO, USA. Fluoxetine hydrochloride and citalopram hydrobromide were purchased from TOCRIS Bioscience, Ellisville, MO, USA.
Amplification of serotonin transporter (SERT) mRNA expression
An adult male wild type mouse was sacrificed by decapitation under CO2 anesthesia. A whole brain was removed from the animal and the brain was kept at −80 °C until RNA extraction. Detailed procedures for RNA extraction and RT-PCR methods were described in our previous study (Fukuhara et al., 2002). PCR was carried out using following primers: Sert, sense: 5′-ATCATAGCCTGGGCGCTCTAC-3′, antisense: 5′-CATGTAGCCAAGCACCGTGAA-3′ ([Ushijima et al., 2005], GenBank accession no. AF013604) and Gapdh, sense: 5′- AGACAGCCGCATCTTCTTGT-3′, antisense: 5′-TGATGGCAACAATGTCCACT-3′ (GenBank accession no. X02231). Thirty seven cycles of amplification were carried out following 30 s of denaturation at 94 °C. Once the temperature reached 94 °C, it was decreased to 57 °C for 10 s, and raised to 72 °C for 40 s The PCR products were separated by electrophoresis through a 3% agarose gel.
Rat-1 fibroblasts culture
Detailed procedures are described in the previous study (Chansard et al., 2007). In brief, 3 days before starting experiments, 106 rat-1 fibroblasts, stably transfected with Period1-luciferase reporter gene ([Izumo et al., 2003], generous gift from Carl Johnson, Vanderbilt University, Nashville, TN, USA), were seeded in a 35 mm Petri dish. Cells were cultured at 36 °C under 5% CO2–95% air atmosphere in 2mL of Dulbecco's Eagle Modified Medium (Gibco, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum,100 units/mL penicillin and 100 µg/mL streptomycin. For all experiments, circadian rhythms were synchronized among the cells by replacing the culture medium with 2 mL of assay medium (Hirota et al., 2002), constituted of serum-free DMEM, 10 mM HEPES, 350 mg/mL sodium bicarbonate, 2% B27 supplement, 25 units/mL penicillin, and 25 µg/mL streptomycin. For bioluminescence analysis, 0.1 mM luciferin was added to the assay medium. Sertraline, fluvoxamine, paroxetine, fluoxetine, citalopram, and xylazine were added when medium was replaced with the assay medium. 30 µM of each chemical was used to test the effects on circadian parameters, because the µM range of SSRIs has been successfully used in the previous circadian studies (Prosser et al., 2006; Sprouse et al., 2006). Cells were pre-incubated with methyl-β-cyclodextrin (β-CD) for 24 h before medium was replaced with the assay medium. β-CD or xylazine was continuously supplied in the assay medium while cells were placed in the Lumicycle apparatus.
SCN tissue culture
Detailed procedures are described in the previous study (Chansard et al., 2007). In brief, the Period1-luciferase mice were sacrificed by decapitation under CO2 anesthesia at ZT3. A brain was removed from animal and dissected at 125-micrometer thickness using a vibratome (World Precision Instruments, Sarasota, FL, USA). The brain section containing the SCN was further dissected as 1 mm bottom × 1 mm height triangle. Each piece of SCN was cultured individually on Millicell culture membranes (Millipore, Billerica, MA, USA) placed in 35 mm culture dishes. The SCN was cultured for 3 days before monitoring bioluminescence levels at 36 °C under 5% CO2–95% air atmosphere. At ZT7 the medium was changed to fresh assay medium, and dishes were placed in the Lumicycle.
Bioluminescence monitoring and data analysis
As shown in our previous study (Chansard et al., 2007), each chemical or their solvent as their vehicle was added in the recording medium, and dishes were sealed using cover slips and grease. The samples were placed in a real-time-bioluminescence monitoring system (Lumicycle, Actimetrics, Wilmette, IL, USA) placed in a 36 °C air incubator. Photons emitted from cells in each dish were monitored by photomultipliers for 60 s at 10-minute intervals. The data were acquired using a PCI-6601 card and a NI-DAQ 7.1 acquisition software (Lumicycle Software, Actimetrics).
Data analyses were performed using the Lumicycle Data Analysis Software (version 2.10, Actimetrics) and OriginPro (version 7, Origin Lab, Northampton, MA, USA). First, a chi-square periodogram analysis were performed on each baseline-subtracted data to establish whether or not there was a significant circadian rhythm (P<0.05; Sokolove and Bushell, 1978). Using Lumicycle software the raw data were subjected to baseline correction, performed by μ a polynomial curve to the data. The baseline fit curve obtained was subtracted from the raw data and the resulting baseline-subtracted data was used to determine the period and amplitude. The damping rate was defined as the number of days necessary for the amplitude of the rhythm to decrease to 1/e (≈ 36.79%) of its initial value. Using OriginPro, the raw data were 2 h smoothed. For the period calculation, the second peak and fourth peak were chosen and split by 3 days.
The baseline level of bioluminescence, which corresponds to the background level, was evaluated at 28.79 ± 7.66 counts/s.
Statistics
Data are expressed as means ± SEM, and to obtain statistical significance each value was subjected to parametric and non-parametric statistics.
Results
Sert mRNA expression
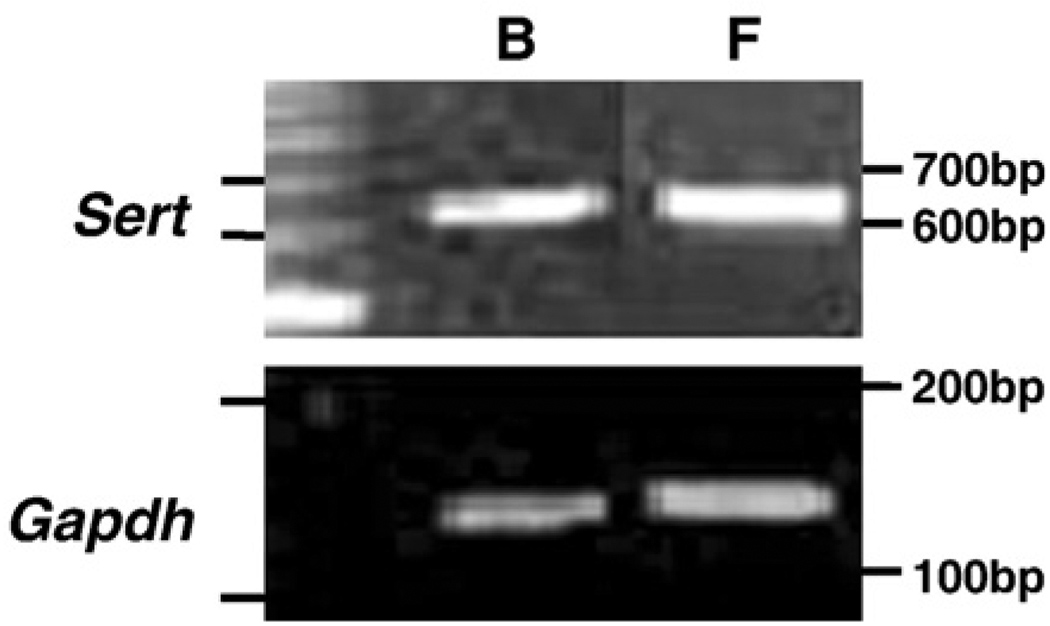
RT-PCR was first performed to detect the mRNA expression of Sert in rat-1 fibroblasts. As a positive control, mouse whole brain was used to amplify Sert mRNA expression (Ushijima et al., 2005). In both rat-1 fibroblasts and whole brain, expected sizes of Sert and Gapdh DNA fragments were amplified as single bands (Fig. 1).
Fig. 1.
Amplification of serotonin transporter (SERT) in rat-1 fibroblasts and mouse whole brain. Total RNA isolated from rat-1 fibroblasts (F) and mouse whole brain (B) were subjected to RT-PCR to amplify Sert and Gapdh. Ethidium bromide-stained agarose gel indicates amplicons for Sert (614 bp) and Gapdh (142 bp) in both samples.
The effects of SSRIs in rat-1 fibroblasts
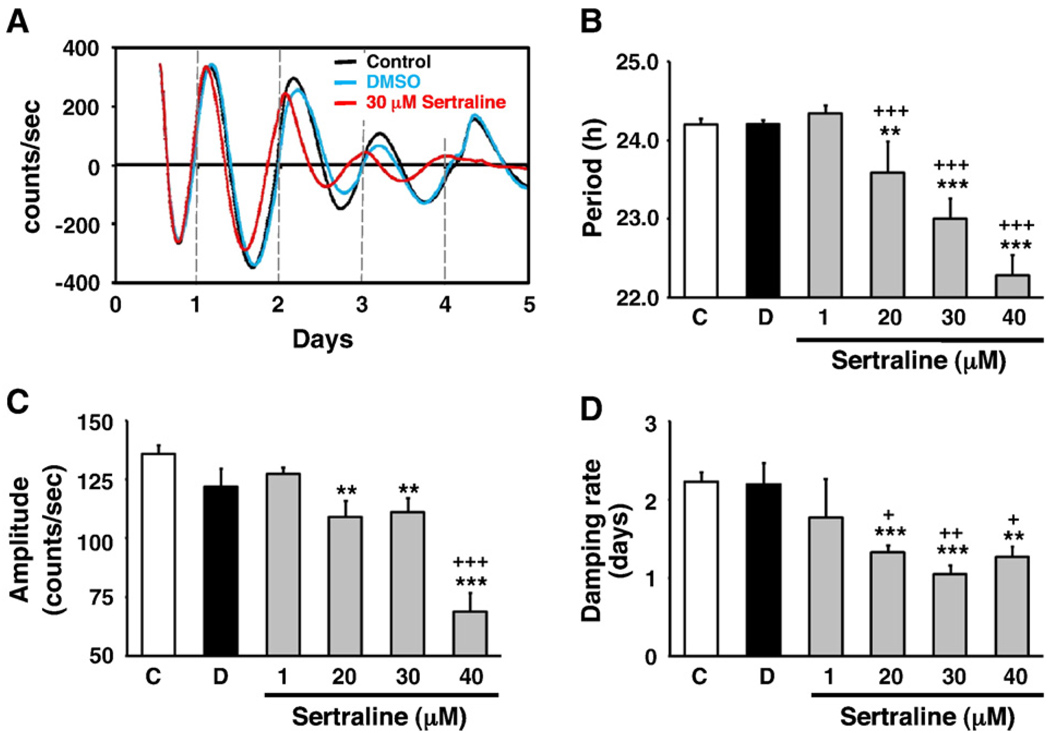
Since Sert mRNA was expressed in rat-1 fibroblasts, we then examined the effects of SSRIs on circadian parameters; period, amplitude, and damping rate, in rat-1 fibroblasts. As shown in Table 1, all SSRI treatments shortened the period compared with control and their vehicle-treated samples. The amplitudes were affected by sertraline, but not other SSRIs. Damping rate was affected by sertraline, fluoxetine, fluvoxamine and paroxetine treatment (Table 1). Since all SSRIs shortened the period, we further examined the effects of the most popular SSRI, sertraline. Fig. 2A showed the representative record of real-time-bioluminescence rhythms, affected by sertraline. Sertraline dose-dependently shortened the period (Fig. 2B). Although 1 µM sertraline did not significantly shorten the period, 20 µM and 40 µM sertraline shortened the period to 23.59±0.13 h (P<0.005 vs control, P<0.0005 vs DMSO) and 22.28±0.05 h (P<0.0005 vs control, P<0.0005 vs DMSO), respectively. Additionally, sertraline affected the amplitude. 20 µM and 40 µM sertraline reduced the amplitude to 109.14±6.76 h (P<0.005 vs control) and 68.77±8.02 counts/s (P<0.0005 vs control, P<0.0005 vs DMSO), respectively. Damping rate also was affected; 20 µM and 40 µM sertraline reduced the damping rate to 1.33±0.09 h (P<0.0005 vs control, P<0.05 vs DMSO) and 1.27±0.13 counts/s (P<0.005 vs control, P<0.05 vs DMSO), respectively.
Table 1.
The effects of selective serotonin reuptake inhibitors on circadian parameters in rat-1 fibroblasts
| Chemical | Tested concentrations |
Period (h) |
Amplitude (counts/s) |
Damping rate (days) |
|---|---|---|---|---|
| Control | – | 24.20±0.08 | 135.84±3.72 | 2.23±0.12 |
| DMSO | 0.1% | 24.21±0.05 ns | 121.86±7.82 ns | 2.20±0.27 ns |
| Citalopram | 30 µM | 23.29±0.17**,+++ | 139.42±23.06 ns, ns | 1.91±0.34 ns, ns |
| Fluoxetine | 30 µM | 23.33±0.10***,+++ | 134.52±5.30 ns, ns | 0.99±0.02***,++ |
| Fluvoxamine | 30 µM | 23.54±0.23*,++ | 120.71±16.46 ns, ns | 1.27±0.08***,++ |
| Paroxetine | 30 µM | 22.61±0.11***,+++ | 130.46±16.39 ns, ns | 1.38±0.17**,++ |
| Sertraline | 30 µM | 23.00±0.26**,+++ | 112.89±5.38**, ns | 1.05±0.11***,++ |
Student τ was performed. ns, not significant;
P<0.05,
P<0.005,
P<0.0005 (Control vs);
P<0.005,
P<0.0005 (DMSO vs);
ns, not significant.
Fig. 2.
The effects of sertraline in rat-1 fibroblasts. (A) Synchronized Period1-driven bioluminescence rhythms were monitored in the presence of sertraline in rat-1 fibroblasts. Representative base-line normalized records for control (black), vehicle (DMSO, blue), and sertraline (red) are shown. 30 µM sertraline significantly affected the period, amplitude and damping rate. (B–D) The dose-dependent effects of sertraline (1–40 µM) on the circadian period (B), amplitude (C) and damping rate (D). C, Control; D, DMSO. Values are means±SEM (N = 4, from two independent experiments).**, P<0.005; ***, P<0.0005 (Control vs); +, P<0.05; ++, P<0.005; +++, P<0.0005 (DMSO vs). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The effects of sertraline in the mouse SCN
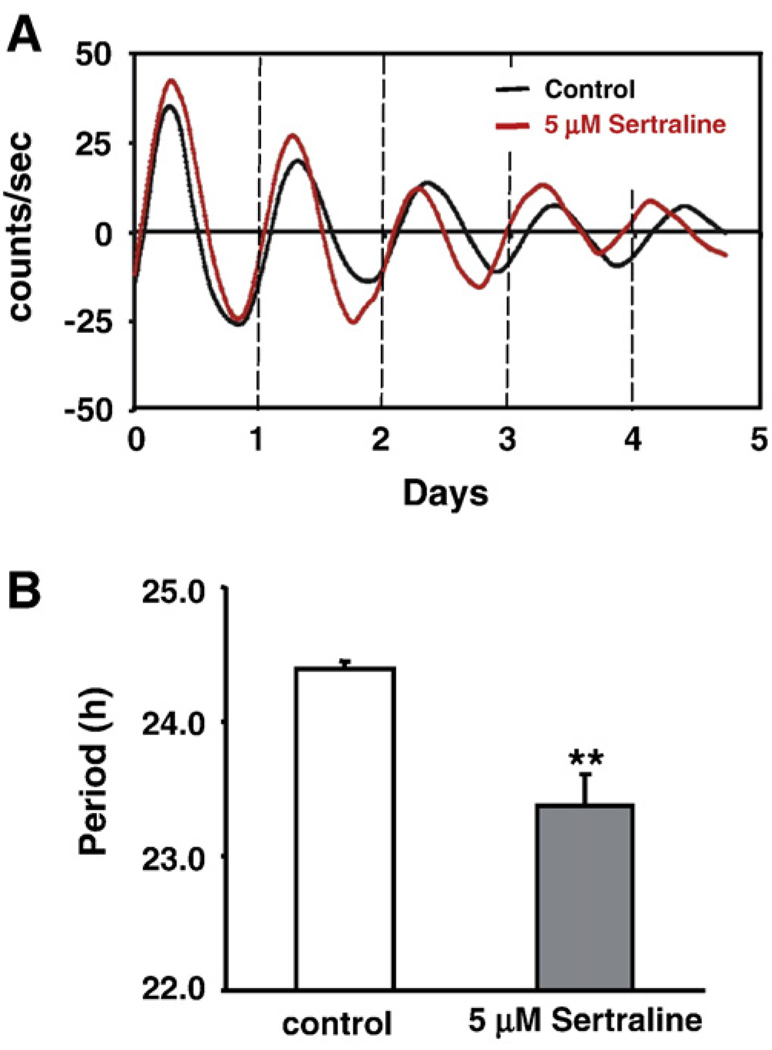
To test whether sertraline similarly affected the circadian parameters in the central clock, the effects of sertraline in the mouse SCN were tested (Fig. 3). Control SCN explants showed period (24.39±0.06 h), amplitude (43.39±10.34 counts/s), and damping rate (2.78±0.43 days) that are similar to those previously reported (Chansard et al., 2007), whereas 5 µM sertraline significantly shortened the period (23.38± 0.23 h, P<0.005, Fig. 3B) without affecting amplitude (33.72± 9.86 counts/s, P>0.05) and damping rate (3.09±0.24 days, P>0.05).
Fig. 3.
The effects of sertraline in the Period1-luciferase transgenic mouse SCN explants. (A) SCN slices from Period1-Luciferase transgenic mice showed the shortened period in the presence of 5 µM sertraline. A representative patterns for control (black) and sertraline-treated (red) samples are shown. (B) Quantitative data showing that sertraline significantly shortened the period in the SCN. Values are means±SEM (N = 4, from three independent experiments). Comparisons with the control condition; **, P<0.005. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The effects of lipid raft inhibition in rat-1 fibroblasts
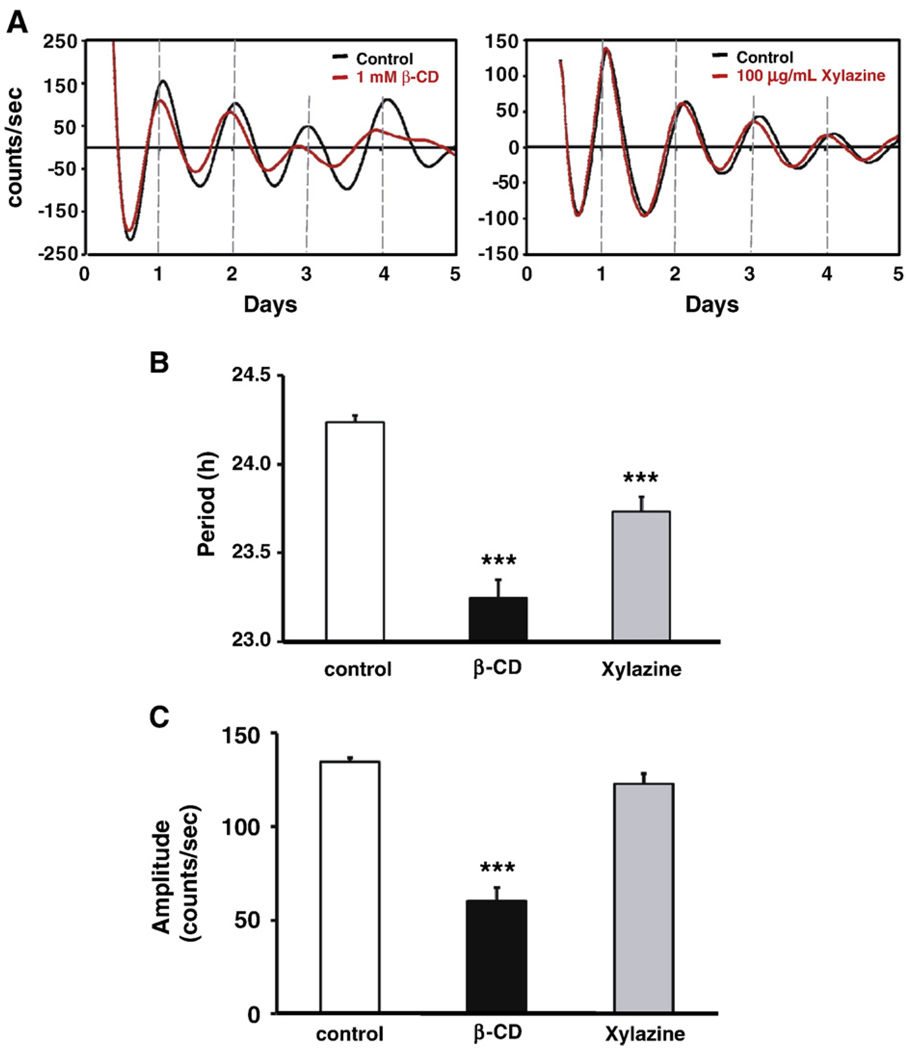
Cholesterol is a major component of lipid rafts. As the association of SERT with lipid rafts may represent a mechanism for regulating the SERT activity (Scanlon et al., 2001; Magnani et al., 2004), we further used a cholesterol chelating reagent β-CD to analyze the effects of cholesterol depletion, consequently the inhibition of SERT activity, on circadian rhythms. Our preliminary results showed that 0.5, 0.8, and 1 mM β-CD shortened the period, but cells were not able to survive in the presence of 2 mM β-CD. Therefore, we used 1 mM β-CD for further experiments. As shown in Fig. 4 β-CD significantly shortened the period to 22.49±0.21 h (P<0.0005), and reduced the amplitude to 60.46±6.77 counts/s (P<0.0005), without affecting damping rates.
Fig. 4.
The effects of lipid raft inhibitions on the Period1 promoter-driven bioluminescence rhythms in rat-1 fibroblasts. (A) A cholesterol chelating reagent β-CD and lipid binding reagent xylazine significantly shortened the period. Normalized data for control (black), β-CD (red in the left panel), and xylazine (red in the right panel) are shown. Amplitude was also affected by β-CD treatment. (B) Quantitative data showing the effects of β-CD and xylazine on the period. (C). Quantitative data indicate that β-CD, but not xylazine affected amplitude. Values are means±SEM (N = 5, from two independent experiments. Comparisons with the control condition; ***, P<0.005. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We also examined the effects of a non-specific lipid binding reagent xylazine, which can inhibit the cell proliferation at the concentration of 100 µg/mL in cultured cells, to further support the β-CD results (Huo et al., 2003). Xylazine (100 µg/mL) treatment significantly shortened the period to 23.47±0.16 h (P<0.005, Fig. 4),whereas 50 µg/mL xylazine did not affect the period (data not shown). Amplitude and damping rate were not affected by 100 µg/mL xylazine treatment (in both cases, P>0.5).
Discussion
In the present study, we found that SSRIs significantly shorten the period, and accelerate damping rate in fibroblasts (Table 1, Figs. 2 and 3). A common target of all the SSRIs is the SERT (Harten,1993). In mouse, we found that Sert mRNA is expressed ubiquitously, such as in fibroblasts and whole brain (Fig. 1) as well as the SCN, heart, lung, liver and kidney (data not shown), thus it suggests that SSRI could affect SERT functions in many tissues, organs and cells, but not only in the CNS. Furthermore, since serotonin synthesis has been suggested in mammalian skin, including fibroblasts (Slominski et al., 2003), fibroblasts could represent an appropriate model to test the effects of SSRI. The fact that sertraline similarly shortens the period in the SCN and fibroblasts (Figs. 2A, B and 3). It suggests that fibroblasts can be used to evaluate the effects of SSRIs on circadian oscillator functions.
Although the degree of shortened period length was not significant among the SSRIs, there is a tendency that paroxetine has stronger impact than other SSRIs tested, and fluvoxamine showed the least effects (Paroxetine>fluvoxamine). It is interesting to mention that SSRIs binding affinity to the SERT and the degree of shortened period likely well correlate (Hirano et al., 2005), thus it suggests that the effects of SSRIs on the period are unlikely of a non-specific nature.
We found that damping of Period1 promoter-driven bioluminescence rhythms in fibroblasts are accelerated by SSRI treatments, except for citalopram (Table 1). Further studies will provide evidence whether such damping is caused by desynchronization of circadian rhythm generation among cells, or damped rhythms within a cell. Since citalopram does not affect the damping rate, it would be of interest to further find if citalopram has any clinical relevance superior to other SSRIs.
Since SSRIs inhibit SERT activity, it is plausible to speculate that SSRIs shorten the period by inhibiting SERT activity. To further support this speculation, the effects of SERT activity suppression by lipid raft and cholesterol binding reagents were further examined (Fig. 4). SERT is an N-glycosylated integral membrane protein with 12 transmembrane regions, and has been shown to associate with lipid raft. The association with lipid raft on the cell membrane is required for an efficient 5-HT transporter activity (Magnani et al., 2004). The loss of interaction with lipid microdomains is detrimental to SERT function, possibly due to conformational changes that mainly affect the translocation of the substrate across the plasma membrane. The conformational changes of SERT by cholesterol depletion using β-CD result in the reduction of SERT activity (Scanlon et al., 2001). A nonspecific lipid binding reagent xylazine is another type of chemical that can interrupt proper lipid raft functions (Huo et al., 2003). We found that both chemicals shorten the period (Fig. 4A and B). However, it is worth to note that only β-CD affected the amplitude (Fig. 4C) that is probably due to different mechanisms by which two chemicals affect membrane properties. The observation, taken together with the SSRIs data, suggests that the SSRI likely shortens the period by inhibiting the SERT functions. However, a possibility that other membrane associated molecule, affected by β-CD and xylazine, contributes to the regulation of circadian rhythm generation, cannot be excluded.
The mechanisms how SSRIs shorten the period downstream from the SERT are not yet fully elucidated. One of the potential molecules that mediate the effects of SSRI is glycogen synthase kinase-3 (GSK3). Indeed, fluoxetine has been reported to affect the level of phosphorylated form of GSK3β in the mouse brain (Li et al., 2007). GSK3 has been recognized as an important enzyme that modulates many aspects of neuronal functions, including circadian rhythm generation and mood (Phiel and Klein, 2001; Gould et al., 2006; Yin et al., 2006; Kaladchibachi et al., 2007). Another potential intracellular target by which SSRI affects the period is MAPKs. Chou et al. (2007) have shown in their paper that paroxetine phosphorylates three MAPKs: extracellular- regulated kinase (ERK), JNK, and p38. Previous studies, including our paper have reported that inhibition of JNK or p38 affects the period in mammals, chick, and Xenopus (Hayashi et al., 2003; Hasegawa and Cahill, 2004; Chansard et al., 2007). Future studies will provide crucial answers to find if these kinases are recruited to regulate the period upon SSRI treatment.
It has been suggested that affected circadian rhythm generation (sleep–wake cycles) and the development of certain mental illnesses, such as depression, bipolar disorders, and seasonal affective disorders are somehow correlated (Ehlers et al., 1988; Malkoff-Schwartz et al., 2000; Grandin et al., 2006; Lader, 2007; McClung, 2007). A recent study has shown that when total sleep deprivation and light therapy advance phase of activity–rest rhythms in bipolar depression patients, the degree of depression is significantly reduced/improved (Benedetti et al., 2007). Phase advancing effects of these two therapies support the significant relations between circadian rhythm generations and regulation of mood. In addition to the circadian hypothesis, functional deficiencies in the regulation of 5-HT levels have been implicated in the pathophysiology of depressive syndromes, and restoring the normal functions of 5-HT associated signaling pathways has been one of the targets of antidepressants (Heninger et al., 1996). Indeed, the second-generation antidepressants SSRIs have been a major choice in clinical treatment. Our data, presenting evidence that SSRIs affect circadian rhythmicity by suppressing SERT activity, may help better understand the relationship between mental illness and biological timing to yield new insight into disease etiology and avenues for effective therapeutic treatments.
Acknowledgments
The authors would like to thank Dr. Hajime Tei, Akiko Hida-Fukada and Shin Yamazaki for providing us Period1-luciferase transgenic mice, and Dr. Carl H. Johnson for providing rat-1 fibroblasts stably transfected with a Period1-luciferase reporter gene. This study was supported by NS034194 from NINDS, IBN-9876754 from NSF Center for Behavioral Neuroscience and the Keck Foundation.
References
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93(6):929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Barbini B, Colombo C, Smeraldi E. Phase advance is an actmetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiology International. 2007;24(5):921–937. doi: 10.1080/07420520701649455. [DOI] [PubMed] [Google Scholar]
- Brown SA, Fleury-Olela F, Nagoshi F, Hauser C, Juge C, Meier CA, Chicheportiche R, Dayer JM, Albrecht U, Schibler U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biology. 2005;3(10):1813–1818. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansard M, Molyneux P, Nomura K, Harrington ME, Fukuhara C. c-Jun N-terminal kinase inhibitor SP00125 modulates the period of mammalian circadian rhythms. Neuroscience. 2007;145(3):812–823. doi: 10.1016/j.neuroscience.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CT, He S, Jan CR. Paroxetine-induced apoptosis in human osteosarcoma cells: activation of p38 MAP kinase and caspase-3 pathways without involvement of [Ca2+]i elevation. Toxicology and Applied Pharmacology. 2007;218(3):265–273. doi: 10.1016/j.taap.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ, DeCoursey PJ. Chronobiology: Biological Timekeeping. first ed. Sunderland, Massachusetts: Sinauer Associates, Inc.; 2003. [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Archives of General Psychiatry. 1988;45(10):948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Dirden JC, Tosini G. Regulation of period 1 expression in cultured rat pineal. Neurosignals. 2002;11(2):103–114. doi: 10.1159/000058547. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Tosini G. Peripheral circadian oscillators and their rhythmic regulation. Frontiers in Bioscience. 2003;8:642–651. doi: 10.2741/1042. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Millan MJ. Evaluation of serotonin, noradrenaline and dopamine reuptake inhibitors on light-induced phase advances in hamster circadian activity rhythms. Psychopharmacology. 2007;195(3):325–332. doi: 10.1007/s00213-007-0903-z. [DOI] [PubMed] [Google Scholar]
- Gould TD, Picchini AM, Einat H, Manji HK. Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Current Drug Targets. 2006;7(11):1399–1409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clinical Psychology Review. 2006;26(6):679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Harten JV. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clinical Pharmacokinetics. 1993;24(3):203–220. doi: 10.2165/00003088-199324030-00003. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. Regulation of the circadian oscillator in Xenopus retinal photoreceptors by protein kinases sensitive to the stress-activated protein kinase inhibitor, SB 203580. The Journal of Biological Chemistry. 2004;279(21):22738–22746. doi: 10.1074/jbc.M401389200. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Sanada K, Hirota T, Shimizu F, Fukada Y. p38 mitogen-activated protein kinase regulates oscillation of chick pineal circadian clock. The Journal of Biological Chemistry. 2003;278(27):25166–25171. doi: 10.1074/jbc.M212726200. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29(1):2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, Hashimoto H, Yamada S. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. British Journal of Pharmacology. 2005;144(5):695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukuda Y. Glucose down-regulates per1 and per2 mRNA levels and induces circadian gene expression in cultured rat-1 fibroblasts. The Journal of Biological Chemistry. 2002;277(46):44244–44251. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. The Journal of Biological Chemistry. 2003;278(13):11561–11569. doi: 10.1074/jbc.M211785200. [DOI] [PubMed] [Google Scholar]
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladchibachi SA, Doble B, Anthopoulos N, Woodgett JR, Manoukian AS. Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. Journal of Circadian Rhythms. 2007;12(5):3. doi: 10.1186/1740-3391-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacology & Therapeutics. 1992;56(1):53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Human Molecular Genetics. 2006;15(2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Lader M. Limitations of current medical treatments for depression: disturbed circadian rhythms as a possible therapeutic target. European neuropsychopharmacology. 2007;17(12):743–755. doi: 10.1016/j.euroneuro.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. The International Journal of Neuropsychopharmacology. 2007;10(1):7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. The Journal of Biological Chemistry. 2004;279(37):38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, Houck PR, Kupfer DJ. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychological Medicine. 2000;30(5):1005–1016. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorder. Pharmacology & Therapeutics. 2007;114(2):222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Klein PS. Molecular targets of lithium action. Annual Review of Pharmacology and Toxicology. 2001:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Lee HM, Wehner A. Serotonergic pre-treatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neuroscience. 2006;142(2):547–555. doi: 10.1016/j.neuroscience.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Scanlon MS, Williams CD, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. Journal of Cellular Physiology. 2003;196(1):144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. Journal of Theoretical Biology. 1978;72(1):131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Braselton J, Reynolds L. Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biological Psychiatry. 2006;60(8):896–899. doi: 10.1016/j.biopsych.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Ushijima K, Sakaguchi H, Sato Y, To H, Koyanagi S, Higuchi S, Ohdo S. Chronopharmacological study of antidepressants in forced swimming test of mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;315(2):764–770. doi: 10.1124/jpet.105.088849. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Current Biology. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311(5763):1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey LP, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]