Abstract
Numerous studies have found that depression is a strong independent risk factor for incident coronary heart disease (CHD), with increasing risk among those with higher levels of depressive symptoms. We examined the association between measures of inflammation (C-reactive protein, interleukin-6, soluble intracellular adhesion molecule-1), depressive symptoms, and CHD incidence among 1,794 participants of the population-based Canadian Nova Scotia Health Survey. There were 152 (8.5%) incident CHD events (141 nonfatal, 11 fatal) during the 15,514 person-years of observation (incidence rate=9.8 events/1000 person-years). Depression and inflammation were correlated at baseline and each significantly predicted CHD in separate models. When both risk factors were in the same model, each remained significant. The association between depressed group by the Center for Epidemiological Studies—Depression scale (score of 10+ vs. 0–9) and CHD incidence (HR=1.60, 95% CI = 1.12–2.27) was not reduced by the addition of inflammatory markers to the model (HR=1.59, 95% CI = 1.12–2.26). Findings were similar after adjustment for aspirin, lipid-lowering medication or anti-depressant use, and the association did not vary by sex, smoking status, age, obesity, cardiovascular medication use or antidepressant use. In conclusion, increased inflammation explains only a very small proportion of the association between depression and incident CHD.
Keywords: depression, inflammation, coronary artery disease, Center for Epidemiologic Studies Depression Scale (CES-D), C-reactive protein (CRP), Soluble Intercellular Adhesion Molecule-1 (sICAM-1)
Introduction
Three pieces of evidence from a number of studies have led researchers to hypothesize that inflammation is a biologically plausible mechanism to at least partially explain how depressive symptoms increase the risk of CHD incidence: 1) depressive symptoms independently predict incident CHD1–3; 2) inflammatory biomarkers predict incident CHD4–6 and; 3) cross-sectional studies indicate that there is a statistically significant correlation between depressive symptoms and inflammatory markers7, 8. Thus, it has been widely speculated that proinflammatory processes are the link between depressive symptoms and CHD8–12. The replication of all three of these findings in a single study is required to properly test if inflammation is implicated as a mechanism. Only one recent study13 has examined these findings within a single sample, but it was limited to women with suspected coronary ischemia. Therefore, we investigated whether inflammation accounts for some of the excess CHD risk conferred by depression in a large population-based sample of initially healthy Nova Scotians who were prospectively followed over 10 years for CHD occurrence.
Methods
The NSHS95 study14 is a population-based survey implemented by Heart Health Nova Scotia in partnership with the Nova Scotia Department of Health to estimate the distributions of selected health indicators and preventive practices of Nova Scotians. The sample (n=3,227) was drawn based on a probability sample designed by Statistics Canada, the national statistical agency and census bureau, to be representative of the Nova Scotian population by age, gender and geographic location. The targeted population consisted of all non-institutionalized Nova Scotians age 18 and above whose names were listed in the Medical Service Insurance register (MSI), the government-sponsored, universal health insurance plan. The overall recruitment percentage (72%) is comparable to other large health surveys, and weights applied from propensity analyses to test for response bias revealed no meaningful biases15. Pregnant women were excluded from the survey, as their CHD risk factor levels were not considered stable.
Exclusion criteria for the present analysis included participants who had a pre-existing medical diagnosis of cardiovascular disease or who did not attend the clinic session. Participants whose high sensitivity C-reactive protein (hs-CRP) levels were above 10 mg/L were also excluded as acute or chronic inflammatory infections (by proxy) might obfuscate the role of inflammatory markers in the association between depression and CHD16.
Details of data collection are presented in Rowan et al,17 where results of depression to 4-year CHD outcome are shown, but without any inflammatory marker information. Briefly, from March through November of 1995, participants were asked to attend a health care clinic for assessment of height and weight by a nurse (twice, average used to calculate body mass index [BMI]). Nurses also measured resting systolic (SBP) and diastolic (DBP) blood pressures four times using a manual random zero sphygmomanometer; we averaged two readings from home and two readings from the clinic session, which was usually one week later.
Participants were asked to fast for 12 hours before giving blood samples. Those who reported fasting for at least 8 hours were considered to have valid fasting blood draws. After centrifugation, one aliquot was immediately shipped on ice for centralized lipid determination. Total cholesterol and high-density cholesterol were assayed from plasma samples by the Lipid Research Laboratory, University of Toronto18. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula19. Remaining plasma samples were frozen and stored at −80 degrees Celsius, and subsequently assayed for inflammatory markers.
On the home visit, the nurse requested all prescription and non-prescription medications/supplements. The nurse recorded the generic drug name, the brand name, the number of pills in the bottle, the date of prescription, and the dosing instructions. The nurse then asked the participant to report which medications were taken regularly, and on what schedule. Drugs were coded using the World Health Organization Nordic Anatomical, Therapeutic and Chemical (ATC) code classification valid in 199520. Data on aspirin, antihypertensive, lipid-lowering, and antidepressant medication were employed.
Participants provided written consent, allowing linkage to their health care utilization data, and for the storage and future use of blood assays. IRB approval was originally obtained for the baseline protocol, and was subsequently obtained for inflammatory marker testing at both Dalhousie University in Canada and Columbia University.
Depressive symptoms were measured using the Centers for Epidemiological Studies-Depression (CES-D) scale21, a 20-item self-report instrument that was designed for use in epidemiological studies as a measure of current level of depressive symptoms. This scale was treated as a continuous measure, and the Cronbach alpha in this sample was .88.
Three inflammatory markers were measured for this analysis, high sensitivity hs-CRP, interleukin-6 (IL-6) and soluble intercellular adhesion molecule-1 (sICAM-1). Hs-CRP levels were measured in plasma samples using a latex-enhanced immunonephelometry assay (Cardiophase BN II; Dade Behring, DE). This assay has a sensitivity of less than 0.2 mg/L with an intra-assay precision (coefficient of variation; CV) of less than 4.4% and an inter-assay CV of less than 5.7%. Hs-CRP is reported to have long-term stability during storage at −80°C, a long half-life, negligible diurnal variation, and little covariation with sex.22 In one study, 13 years of storage slightly increased values (although the correlation from baseline to 13-year levels remained above .90).23 Recommendations for clinical use suggest that a single assessment is sufficient, except in persons with current acute infections, trauma, or acute hospitalizations, who may temporarily have hs-CRP levels of 10 mg/L or higher16. Therefore, participants with highly elevated hs-CRP levels (≥ 10 mg/L) should be excluded from analyses of cohort data16. IL-6 levels were assessed using a high-sensitivity enzyme-linked immunosorbent assay kit (Quantikine HS IL-6; R&D Systems, MN) with a sensitivity of 0.156 pg/mL and intra-assay and inter-assay CVs of 3% to 9%. sICAM-1 levels were measured using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, MN) with a sensitivity of 0.35 ng/mL and intra-assay and inter-assay CVs of 3% to 10%.
The diagnostic codes associated with medical care delivered to the survey participants provides an accurate measure for outcome assessment24 as Nova Scotia provides universal health care insurance, and events occurring outside Nova Scotia are reimbursed and therefore captured. Data on occurrences of hospital discharges with a diagnosis code for International Classification of Disease manual (ICD-9) codes for ischemic heart disease (410.×–414.×, or the equivalent ICD-10 codes), were gathered from the provincial health care database through March 31, 2005, the ten-year period following the initial enrollment of participants. To ensure that all events were incident and not recurrent, all participants were asked about prior CHD events in the survey and we reviewed discharge diagnoses for each participant for four years prior to the baseline survey administration; survey respondents with a prior documented/reported CVD event were excluded. Death status and cause of death, including those who died in-hospital, at home, or outside of the province, were extracted from the national vital statistics database for the same ten-year period. For CHD morbidity, a data quality committee from the Department of Health of Nova Scotia meets regularly with health records personnel to ensure accuracy and to adjudicate data entry irregularities. For CHD mortality, deaths are coded provincially and transferred to Statistics Canada, where a national process of determining the underlying cause of death is applied.
The primary aim was to test whether statistical adjustment for inflammatory markers reduces any of the excess CHD incidence risk due to depressive symptoms. The relationship between baseline depression, inflammation, demographics and cardiac risk factors were tested by simple and partial correlations. Linear regression was used to quantify the cross-sectional relationship between depressive symptoms and log-transformed hs-CRP, log-transformed IL-6, and sICAM-1 biomarker levels5.
The period of follow-up was defined for each participant as starting at the participant’s age at baseline and extending until either his/her age at first CHD event or age on March 31, 2005 for those who did not have an event during the follow-up period.
Cox proportional hazards models were used to estimate the adjusted hazard ratios of depression and inflammation for incident CHD. Models were estimated in which depressive symptoms alone, one inflammatory marker alone, or depressive symptoms and an inflammatory marker were the primary predictors. We tested the significance of quadratic terms to check for curvilinearity in the relationships of depressive symptoms and inflammatory measures with CHD incidence. With CRP and IL-6 log-transformed to reduce severe skewness, there was no evidence of curvilinearity.
All models were adjusted for sex and age at baseline, the latter to control for cohort and/or healthy survivor effects, as well as for Framingham risk score, to determine whether depression and/or inflammation predict CHD over and above traditional cardiovascular risk factors. We used the Framingham risk score that incorporates the combined effects of seven traditional cardiovascular risk factors – sex, age, total cholesterol, HDL cholesterol, blood pressure, history of diabetes, and cigarette smoking – into a single risk score25. Each factor is collapsed into categories and each category is assigned a score. The scores are then summed to create the overall risk score. The categorization and assigned scores are defined separately for males and females. Although the original Framingham Risk Score was not defined for participants age 75 or older, the scoring algorithm is linear with age for males and quadratic with age for females and so risk scores could be determined for older participants. For those few participants who were missing a small number of risk factors, a regression-based approach was used to impute the expected value of the total Framingham score conditioned on the available risk factors.
We tested the proportional hazards assumption using the Kolmogorov-type supremumtest26 based on 1,000 simulated replications; this assumption was met (all p>.20).
We conducted secondary analyses to address potential limitations and subgroup differences suggested in previous studies.7, 27 To examine whether inflammatory markers explain the relationship between depressive symptoms and CHD differently by subgroup, we tested the significance of multiplicative interaction terms for sex (male vs female), smoking status (current vs all others), age (≥65 years vs<65 years), obesity (BMI >30 vs all others), cardiovascular medication use (aspirin, antihypertensive, or lipid-lowering medication use vs none), and antidepressant use (yes vs no). All analyses were performed using SAS statistical software (version 9.1).
Results
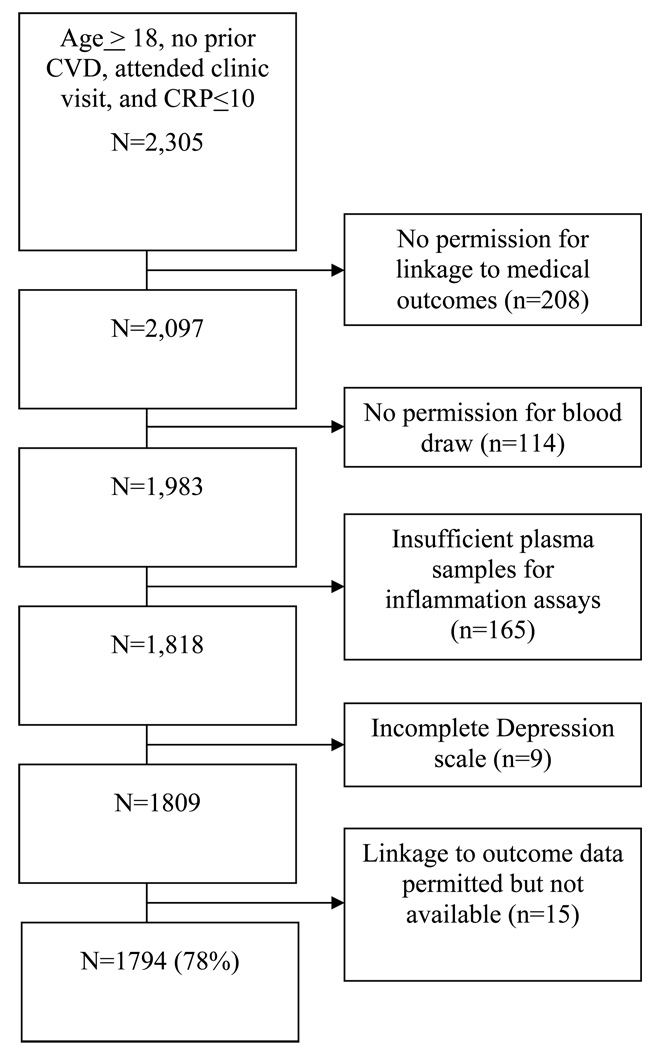
Of the 2,305 survey respondents who met the inclusion criteria for this study, data on 1,794 (78%) participants are included in the analysis (see Figure 1). Survey respondents were excluded due to refusal to permit linkage to medical outcome data, unwillingness or inability to provide sufficient blood, failure to complete the depression scale, or inability to link electronic medical outcome data to the study database. Those available for analysis reported significantly lower depressive symptom scores than did the excluded group (7.2 vs 8.2; p=0.02), but there were no other significant demographic differences.
Figure 1.
Participants Available for Analysis from the Nova Scotia Health Survey
Table 1 shows the baseline characteristics of the 1,794 adults included in our analysis. Table 2 shows the pair-wise correlations for the control variables and for baseline depression and inflammation, controlling for age, sex, and Framingham risk score. The regression of baseline log(hs-CRP) and log(IL-6) levels on baseline depressive symptoms, controlling for sex, age, and Framingham risk score, showed that each standard deviation increase in depressive symptoms was associated with an 8.3% higher level of hs-CRP and with a 4.7% higher level of IL-6.
Table 1.
Baseline Characteristics of Population-based, Coronary Heart Disease (CHD)-free, Men and Women Aged 18–98 Years: Continuous Variables reported as mean ± standard deviation and categorical variables reported as number (percent of sample)
| Variable | Mean (Standard Deviation) OR N (%) |
|---|---|
| Age, years | 46.3 ± 18.3 |
| Male | 895 (49.9%) |
| Active smoker* | 478 (26.6%) |
| Body mass index (kg/m2) | 26.7 ± 5.1 |
| Diabetes mellitus | 58 (3.2%) |
| Center for Epidemiologic Studies- Depression score | 7.2 ± 7.7 |
| Center for Epidemiologic Studies- Depression grouping (10+ vs. 0–9) |
477 (26.6%) |
| Total cholesterol (mmol/L) | 5.3 ± 1.1 |
| High density Lipoprotein (mmol/L) | 1.3 ± 0.3 |
| Low density Lipoprotein (mmol/L) | 3.3 ± 0.9 |
| Systolic blood pressure (mmHg) | 124.2 ± 16.6 |
| Diastolic blood pressure (mmHg) | 76.5 ± 9.4 |
| Framingham risk score | 1.4 ± 9.1 |
| High sensitivity C-reactive protein (mg/L)** | 0.17 ± 1.25 |
| Interleukin-6 (pg/mL)** | 0.16 ± 0.79 |
| High sensitivity C-reactive protein ≥ 3.0 (mg/L) | 465 (26.0%) |
| Soluble Intercellular Adhesion Molecule-1 (ng/mL) | 565.0 ± 329.7 |
| Use of | |
| Aspirin | 73 (4.1%) |
| Lipid-lowering medication | 23 (1.3%) |
| Antihypertensive medication | 182 (10.1%) |
| Antidepressants | 52 (2.9%) |
Total sample size=1794.
Defined as smoking more than 1 cigarette per day in the past year.
log transformed (natural log)
Table 2.
Simple and Partial Correlations Controlling for Age, Sex, and Framingham Risk Score, in Population-based, Coronary Heart Disease-free Adults Aged 18–98 Years
| Simple Correlations (p-value) with Control Variables |
Partial Correlations (p-value), Controlling Age, Sex, and Framingham Risk Score |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Age | Male | Framingham risk score |
Center for Epidemiologic Studies- Depression |
High sensitivity Creactive protein |
Interleukin- 6 |
Soluble Intercellular Adhesion Molecule |
| Age | 1 | ||||||
| Male vs Female* | −0.04 (p=0.06) |
1 | |||||
| Framingham risk score |
0.78 | 0.28 | 1 | ||||
| Center for Epidemiologic Studies- Depression score |
−0.14 | −0.09 | −0.12 | 1 | |||
| High sensitivity C-reactive protein |
0.27 | −0.12 | 0.22 | 0.07 | 1 | ||
| Interleukin-6 | 0.31 | 0.00 (p=0.96) |
0.25 | 0.06 | 0.32 | 1 | |
| Soluble Intercellular Adhesion Molecule-1 |
−0.55 | 0.04 (p=0.09) |
−0.46 | 0.01 (p=0.53) |
0.06 | 0.15 | 1 |
Total sample size ~1794 (varies slightly for different pairwise comparisons).
All p-values <0.01 unless otherwise noted
Point biserial correlations
There were 152 (8.5%) incident CHD events (141 nonfatal and 11 fatal events) during the 15,514 person-years (9.8 events/1000 person-years). The hazard rate (HR) estimates from separate Cox proportional hazards regression analyses (Table 3) show that depressive symptoms, log(hs-CRP) and sICAM-1 each significantly predict CHD risk. Adjusting for age, sex and Framingham risk score, each increase of one standard deviation in CES-D scores increased the rate of incident CHD by 28%. Results were also significant in models predicting CHD from inflammatory markers; however, IL-6 was not significant after covariate adjustment.
Table 3.
Bivariate Association of Each Covariate, Depressive Symptoms and Inflammatory Markers with 10-year Incident Coronary Heart Disease or Mortality
| Predictor | Category of Exposure | Bivariate Hazard Ratio (95% CI) |
Adjusted* Bivariate Hazard Ratio (95% CI) |
|---|---|---|---|
| Sex of Participant | Male (vs Female) | 1.58 (1.14–2.18) | - |
| Age | Per SD (1 SD = 18.2 yrs) | 6.30 (2.22–17.9) | - |
| Framingham risk score | Per SD (1 SD = 9.1 points) | 3.64 (2.42–5.49) | - |
| Center for Epidemiologic Studies- Depression scale |
Per SD (1 SD = 7.7 points) | 1.28 (1.11–1.48) | 1.28 (1.11–1.48) |
| Center for Epidemiologic Studies- Depression scale |
10+ vs. 0–9 | 1.55 (1.09–2.20) | 1.60 (1.12–2.28) |
| Log high sensitivity C- reactive protein |
Per SD (1 SD = 1.25) | 1.40 (1.14–1.71) | 1.28 (1.04–1.57) |
| Soluble Intercellular Adhesion Molecule-1 (natural log transformed) |
Per SD (1 SD = 330 ng/mL) | 1.33 (1.06–1.66) | 1.32 (1.05–1.67) |
| Log Interleukin-6 | Per SD (1 SD = 0.79) | 1.17 (1.00–1.36) | 1.07 (0.90–1.27) |
Total sample size =1794.
Adjusted for sex, age, and Framingham risk score
When both depressive symptoms and log(hs-CRP) levels were included in the same model, both remained significant and independent predictors, with only minor reduction in their HRs (Table 4, model 1). When sICAM-1 (model 2) or log(IL-6) (model 3) was added to a model with CES-D, no meaningful reduction in the estimate for depressive symptoms occurred, suggesting that inflammation explains little or none of the association between depression and CHD. In a model that included depressive symptoms, log(hs-CRP) and sICAM-1 (model 4), each exhibited a statistically significant independent effect on risk of incident CHD, but the effect of depressive symptoms was attenuated by only 6% after adjusting for the inflammatory measures. We also repeated model 4, using depressed grouping (score of 10+ vs. 0–9) rather than the continuous scale, and found the same results (see model 4b). Re-conducting these analyses with CHD individual risk factors included, rather than the summary Framingham score, resulted in identical findings—depression’s effect on CHD is not reduced (data not shown).
Table 4.
Hazard ratios and 95% confidence intervals per 1 standard deviation increase in baseline value of predictor for incident Coronary Heart Disease morbidity or mortality comparing a model with (Center for Epidemiological Studies- Depression), adjusted for age, sex, and Framingham risk score (model A) to a model additionally including inflammatory markers (model B)
| N | Model A | Model B | Depression Parameter Estimate Difference |
||||
|---|---|---|---|---|---|---|---|
| Center for Epidemiological Studies- Depression scale |
Center for Epidemiological Studies- Depression scale |
Log High sensitivity C-reactive protein |
Soluble Intracellular Adhesion molecule-1 |
Log Interleukin -6 |
|||
| 1. | 1790 | 1.27 (1.10–1.47) | 1.26 (1.09–1.45) | 1.25 (1.02–1.53) | 5.68% | ||
| 2. | 1792 | 1.28 (1.11–1.48) | 1.28 (1.11–1.48) | 1.32 (1.04–1.66) | −0.08% | ||
| 3. | 1751 | 1.28 (1.10–1.48) | 1.27(1.10–1.47) | 1.05 (0.88–1.25)° | 1.22% | ||
| 4. | 1788 | 1.28 (1.11–1.48) | 1.26 (1.09–1.46) | 1.24 (1.01–1.52) | 1.29 (1.02–1.63) | 5.62% | |
| 4b. Center for Epidemiological Studies- Depression ≥ 10 |
1788 | 1.60 (1.12–2.27) | 1.59 (1.12–2.26) | 1.26 (1.03–1.54) | 1.29 (1.03–1.63) | 0.88% | |
| 5. Men | 890 | 1.25 (1.02–1.52) | 1.22 (1.00–1.50) | 1.23 (0.94–1.61)° | 1.10 (0.80–1.51)° | 8.45% | |
| 6. Current smokers | 475 | 1.49 (1.21–1.83) | 1.55 (1.25–1.92) | 1.00 (0.68–1.46)° | 1.58 (1.04–2.40) | −10.77% | |
| 7. Age ≥65 years | 345 | 1.23 (0.94–1.61)° | 1.20 (0.92–1.58)° | 1.23 (0.90–1.66)° | 1.70 (1.17–2.48) | 11.10% | |
| 8. Body Mass Index ≥30 |
404 | 1.46 (1.15–1.86) | 1.48 (1.16–1.89) | 0.95 (0.66–1.36)° | 1.17 (0.71–1.92)° | −3.70% | |
| 9. Not prescribed cardiac** medications |
1563 | 1.19 (0.99–1.43)° | 1.16 (0.97–1.40)° | 1.33 (1.04–1.70) | 1.28 (0.97–1.67)° | 12.38% | |
| 10. Not prescribed anti- depressant medication |
1736 | 1.24 (1.06–1.46) | 1.22 (1.04–1.43) | 1.23 (1.00–1.53) | 1.29 (1.01–1.64) | −8.89% | |
not significant
aspirin, lipid-lowering medications and anti-hyerpertensive medications
1 standard deviation of depressive symptoms = 7.7 points;
1 standard deviation of log(high sensitivity C-reactive protein) = 1.25; 1 standard deviation of soluble intracellular adhesion molecule-1 = 330 ng/mL; 1 standard deviation of log(interleukin-6) = 0.79
Finally, we tested if inflammation might explain the risk for CHD incidence conferred by depression in subgroups defined by sex, smoking status, age group, obesity, cardiovascular medication use (aspirin, lipid-lowering, or anti-hypertensive), or antidepressant medication (models 5–10). We conducted tests of interaction terms to examine whether the association between depression or inflammation and CHD varied by subgroup and found that depressive symptoms predict CHD incidence more strongly in smokers (p<0.02) and hs-CRP predicts CHD incidence more strongly in the non-obese (p<0.02). No other interactions reached statistical significance.
Discussion
Recent population-based studies have concluded that depression is an independent risk factor for incident CHD mortality, controlling for established and emerging risk factors28. Thus, the search for the mechanisms by which depression confers its risk remains urgent, and inflammation continues to have strong appeal as the most viable candidate11. However, studies have rarely examined the inter-relations among depression, inflammation, and subsequent CHD incidence9, particularly in randomly-selected, population-based samples. This study indicates that depressive symptoms and inflammation are modestly correlated. Further, depressive symptoms are associated with an increased risk of incident CHD events, independent of both traditional risk factors and inflammatory biomarkers, particularly hs-CRP and sICAM-1 levels. Therefore, inflammatory biomarkers neither fully nor partially explain the association between depression and CHD incidence.
Our study does not support the long-held theory that inflammation mediates the increased risk of incident CHD events associated with depression. Our findings are similar to those reported in a retrospective, nested, case-control study7 of middle-aged European men, in which depressive symptoms were predictive of a combined endpoint of incident angina pectoris, nonfatal myocardial infarction, and coronary death, but the increased risk was not reduced by considering hs-CRP, IL-6, sICAM-1, or fibrinogen levels. Another population-based, prospective study of apparently healthy European men29 did show that depression status interacted synergistically with CRP to increase CHD risk, such that those with both depression and high hs-CRP levels were at substantially higher risk. In our population-based sample, there was no evidence of such an interaction among men or women. Vaccarino et al13 demonstrated that depression and inflammation (hs-CRP and IL-6) were independent predictors of nonfatal myocardial infarction, stroke, congestive heart failure, and cardiovascular death in women referred for coronary angiogram to evaluate symptoms of chest pain or suspected coronary ischemia (tests indicated that 16% of the enrolled participants had baseline obstructive CHD). Finally, recent findings from the Whitehall II study30 suggest that inflammation does not mediate the relation of negative affect and psychological distress to CHD incidence in a large cohort of British civil servants. However, this study did not examine depression specifically nor did it find a relation of negative affect and psychological distress with elevated levels of inflammation, as measured by hs-CRP, IL-6, and fibrinogen.
Many of these studies did not directly test the independent contributions of depression and inflammation in relation to the onset of incident CHD events in asymptomatic men and women from the general population. In contrast, this was a prospective study of a randomly selected, nationally representative, population-based sample of healthy men and women. The data collection process made it possible for us to accurately include only people with no diagnosis of CVD at baseline and to verify CHD status up to ten years after the survey date using centralized, computerized administrative databases with excellent and independent quality control of diagnostic codes. Additionally, the random sample collection and the low loss to follow-up help to minimize concerns of significant selection bias. Depressive symptoms were modestly, albeit highly significantly, comorbid with inflammation; the association is similar in size to that found in other large studies7,8. Thus, our findings provide strong evidence that inflammation does not explain the link between depression and CHD morbidity and mortality.
There are potential limitations to our study. First, we only have measures of depressive symptoms and inflammation at baseline, so we cannot determine whether the depression preceded the inflammation, the inflammation preceded the depression or the two developed simultaneously; nor can we explore mediation in the formal sense recently recommended by Kraemer and others.31 Future studies with repeated measures during follow-up may help to reveal the most biologically plausible process by which these risk factors interact. Also, data on medication use was only available at baseline. It seems likely that some participants began, stopped and/or switched some of their medications over the ten years of follow up, and we cannot assess how the duration and changes in medication use impact the associations under study here. Also, we only examined the use of aspirin, antihypertensive, lipid-lowering, and antidepressant medications so there may be other potentially relevant medications that were omitted from our analyses. There was such a low rate of baseline statin medication use (less than 1% in 1995) so we could not determine if our results would differ for those receiving statins.
There are many plausible biological mechanisms suggested to explain why depression is a risk marker for CHD incidence—dysregulated autonomic function, endothelial dysfunction, reduced omega-3 fatty acid levels, hyperintensities of white matter (vascular depression), upregulated platelet aggregation9,32,33—as well as behavioral mechanisms, such as nonadherence to medication use.34 Despite the evidence that inflammation underlies the development and progression of CHD, our study strongly suggests that inflammation does not appear to be a likely mechanism for explaining the link between depression and CHD incidence.
Acknowledgments
Funding: The study was supported by grants HL-088117, HL-076857, HL-084034, HL-072866, and HL-04458 from the National Heart, Lung, and Blood Institute, Bethesda, Md. This project was also supported by Grant UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The National Health & Welfare of Canada, the Nova Scotia Department of Health, and the Heart and Stroke foundation of Canada all supported the original data collection. The study sponsors had no role in the study design, collection, analysis, writing, or interpretation of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 2.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 3.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 7.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P, Group PS, Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P, Group PS. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 8.Panagiotakos DB, Pitsavos C, Chrysohoou C, Tsetsekou E, Papageorgiou C, Christodoulou G, Stefanadis C. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. Eur Heart J. 2004;25:492–499. doi: 10.1016/j.ehj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Shimbo D, Chaplin W, Crossman D, Haas D, Davidson KW. Role of depression and inflammation in incident coronary heart disease events. Am J Cardiol. 2005;96:1016–1021. doi: 10.1016/j.amjcard.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 10.Elovainio M, Keltikangas-Jarvinen L, Pulkki-Raback L, Kivimaki M, Puttonen S, Viikari L, Rasanen L, Mansikkaniemi K, Viikari J, Raitakari OT. Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychol Med. 2006;36:797–805. doi: 10.1017/S0033291706007574. [DOI] [PubMed] [Google Scholar]
- 11.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the third national health and nutrition examination survey. Arch Intern Med. 2004;164:1010–1114. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 12.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 13.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 14.MacLean D, Scott J, Beanlands H, Hood R, Cogdon A, LeBlanc B, Davidson KW, Mills T, Wolf HK, Elliott D, Kephart G, Farquhar JW. The 1995 Nova Scotia Health Survey. Halifax, Nova Scotia: Dalhousie University, Heart Health Nova Scotia; 1996. [Google Scholar]
- 15.Lawson BJ. Evaluation of non-response bias in the Nova Scotia Health Survey 1995. Halifax, Nova Scotia, Canada: Dalhousie University; 1999. [Google Scholar]
- 16.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F Centers for Disease C, Prevention, American Heart A. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 17.Rowan PJ, Haas D, Campbell JA, Maclean DR, Davidson KW. Depressive symptoms have an independent, gradient risk for coronary heart disease incidence in a random, population-based sample. Ann Epidemiol. 2005;15:316–320. doi: 10.1016/j.annepidem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Connelly PW, MacLean DR, Horlick L, O'Connor B, Petrasovits A, Little JA. Plasma lipids and lipoproteins and the prevalence of risk for coronary heart disease in Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ. 1992;146:1977–1987. [PMC free article] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS, Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) classification index. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 1995
- 21.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 22.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107:370–371. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa S, Kayaba K, Gotoh T, Nakamura Y, Kario K, Ito Y, Kajii E. Comparison of C-reactive protein levels between serum and plasma samples on long-term frozen storage after a 13.8 year interval: the JMS Cohort Study. J Epidemiol. 2007;17:120–124. doi: 10.2188/jea.17.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manga P, Broyles RW, Angus DE. The determinants of hospital utilization under a universal public insurance program in Canada. Med Care. 1987;25:658–670. doi: 10.1097/00005650-198707000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 26.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 27.Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W. Influence of depressive mood on the association of CRP and obesity in 3205 middle aged healthy men. Brain Behav Immun. 2003;17:268–275. doi: 10.1016/s0889-1591(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 28.Surtees PG, Wainwright NW, Luben RN, Wareham NJ, Bingham SA, Khaw KT. Depression and Ischemic Heart Disease Mortality: Evidence From the EPIC-Norfolk United Kingdom Prospective Cohort Study. Am J Psychiatry. 2008;165(4):515–523. doi: 10.1176/appi.ajp.2007.07061018. [DOI] [PubMed] [Google Scholar]
- 29.Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W. C-reactive protein, depressed mood, and the prediction of coronary heart disease in initially healthy men: results from the MONICA-KORA Augsburg Cohort Study 1984–1998. Eur Heart J. 2005;26:2537–2542. doi: 10.1093/eurheartj/ehi456. [DOI] [PubMed] [Google Scholar]
- 30.Nabi H, Singh-Manoux A, Shipley M, Gimeno D, Marmot MG, Kivimaki M. Do psychological factors affect inflammation and incident coronary heart disease: the Whitehall II Study. Arterioscler Thromb Vasc Biol. 2008;28:1398–1406. doi: 10.1161/ATVBAHA.108.167239. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 32.Lett HSMA, Blumenthal JAP, Babyak MAP, Sherwood AP, Strauman TP, Robins CP, Newman MFMD. Depression as a Risk Factor for Coronary Artery Disease: Evidence, Mechanisms, and Treatment. Psychosom Med. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 33.Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29:1110–1117. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 34.Rieckmann N, Gerin W, Kronish IM, Burg MM, Chaplin WF, Kong G, Lesperance F, Davidson KW. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol. 2006;48:2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]