Abstract
Three decades of structural analysis have produced the view that the kinetochore in vertebrate cells is a disk-shaped structure composed of three distinct structural domains. The most prominent of these consists of a conspicuous electron opaque outer plate that is separated by a light-staining electron-translucent middle plate from an inner plate associated with the surface of the pericentric heterochromatin. Spindle microtubules terminate in the outer plate and, in their absence, a conspicuous corona of fine filaments radiates from the cytoplasmic surface of this plate. Here we report for the first time the ultrastructure of kinetochores in untreated and Colcemid-treated vertebrate somatic (PtK1) cells prepared for optimal structural preservation using high-pressure freezing and freeze substitution. In serial thin sections, and electron tomographic reconstructions, the kinetochore appears as a 50–75 nm thick mat of light-staining fibrous material that is directly connected with the more electron-opaque surface of the centromeric heterochromatin. This mat corresponds to the outer plate in conventional preparations, and is surrounded on its cytoplasmic surface by a conspicuous 100–150 nm wide zone that excludes ribosomes and other cytoplasmic components. High magnification views of this zone reveal that it contains a loose network of light-staining, thin (<9 nm diameter) fibers that are analogous to the corona fibers in conventional preparations. Unlike the chromosome arms, which appear uniformly electron opaque, the chromatin in the primary constriction appears mottled. Since the middle plate is not visible in these kinetochore preparations this feature is likely an artifact produced by extraction and coagulation during conventional fixation and/or dehydration procedures.
Introduction
Each replicated chromosome possesses two discrete sister kinetochores that are positioned on the opposite sides of its primary constriction. During mitosis, sister kinetochores firmly attach their associated chromosome to the spindle by capturing the plus ends of dynamically unstable microtubules (Mts) growing from the poles. Individual kinetochores then use these kinetochore fiber (K-Fiber) Mts as a scaffold for producing much of the force for chromosome poleward motion, and during this motion hold onto the ends of associated Mts as they grow and shrink by Mt subunit addition/dissociation within their confines. In addition to roles in chromosome attachment and force production, kinetochores also control the timing of anaphase onset by producing an inhibitor of this event until the kinetochore is properly attached to the spindle (reviewed in Hyman and Mitchison 1992; Inoue and Salmon 1995; Rudner and Murray 1996; Nicklas 1997; Rieder and Salmon 1998).
The mechanisms by which the kinetochore accomplishes its various functions are not apparent from its structure. In sections from conventionally fixed and stained vertebrate somatic cells unattached kinetochores are seen to consist of a circular 35–40 nm thick electron-opaque plate-like structure that consists of a dense meshwork of 10–20 nm thick fibers (reviewed in Jokelainen 1967; Rieder 1982; McEwen et al. 1993; Cooke et al. 1997; Yao et al. 1997). This plate is separated from the underlying centromeric heterochromatin by a 15–30 nm thick electron-lucent zone relatively devoid of structure. A conspicuous fine fibrillar corona material radiates 100 nm or more from the cytoplasmic surface of the plate, which can vary significantly in diameter between the chromosomes of a genome (but is only weakly correlated with chromosome size; see McEwen et al. 1998). As this kinetochore plate attaches to Mt ends the corona becomes less distinct (reviewed in Rieder 1982; Cassimeris et al. 1990), its diameter decreases significantly (see Jokelainen 1967; reviewed in Rieder 1982), and a dense staining inner plate appears in association with the surface of the chromatin underlying the Mt-binding outer plate (see Rieder 1979, 1982).
A number of kinetochore proteins have been identified, some of which have been ascribed putative functions (reviewed by Pluta et al. 1995). For example, the proteins CENP-C and CENP-G are located in the inner plate, while CENP-B, and probably CENP-A, are located in the centromeric DNA beneath the inner plate. These constitutively bound proteins are thought to play a role in forming and maintaining kinetochore structure, although CENP-B is probably redundant (Tomkiel et al. 1994; Warburton et al. 1997; He et al. 1998). Other proteins bind transiently during the cell cycle and appear to be involved in kinetochore function. Thus, although the location of Mad2 and Bubl within the kinetochore remain to be determined, these components are clearly involved in producing the wait anaphase signal until the kinetochore is attached to Mts (Chen et al. 1996; Taylor and McKeon 1997). 3F3/2 epitopes are located within the middle, electron-lucent kinetochore layer and have also been implicated in the checkpoint that controls anaphase onset (Campbell and Gorbsky 1995). The attachment and proper positioning of chromosomes on the spindle likely involves cytoplasmic dynein (Echeverri et al. 1996) and CENP-E (1997; Schaar et al. 1997; Wood et al. 1997), both of which are located in the outer plate and corona. The possible functions of other kinetochore components, including ZW10 (e.g., Starr et al. 1997), CENP-F (also known as mitosin; Liao et al. 1995) and MCAK (Wordeman and Mitchison 1995), remain to be determined. The composition of facultative proteins on the kinetochore changes during the course of mitosis. Thus antibody staining of phosphorylated 3F3/2 epitopes, Mad and Bub proteins is lost or diminished after the kinetochore acquires Mts. Several other components, including ZW10, cytoplasmic dynein, and CENP-E, fully or partially redistribute into the K-Fiber during late metaphase and early anaphase (Echeverri et al. 1996; Cooke et al. 1997; Starr et al. 1997; Yao et al. 1997).
A number of models have been proposed to explain kinetochore function, especially the way this organelle remains attached to growing and shortening Mts as it moves toward and away from its pole, while producing most of the force for chromosome poleward motion (reviewed in Hyman and Mitchison 1992; Inoue and Salmon 1995; Rieder and Salmon 1998). Many of these schemes ascribe different functional roles to each of the three structural domains that form the kinetochore. However, our concept of kinetochore structure is based entirely on fixation procedures developed more than 30 years ago (e.g., see Brinkley and Stubblefield 1966; Jokelainen 1967) that are known to extract the cytoplasmic components and induce subtle and even not so subtle structural changes. Indeed, although largely ignored when interpreting kinetochore structure, chromosomes in cells fixed by conventional buffered glutaraldehyde/osmium protocols shrink up to 9% in width and length during the dehydration process (Jensen and Bajer 1969). The situation is worse for pre-embedment labeling immuno-electron microscopy (EM) studies, which are based exclusively on detergent extraction and fixatives that maintain antigenicity but not high resolution structure.
A method based on rapid freezing followed by freeze substitution (FS) is now available that allows thicker specimens, including vertebrate cells in mitosis, to be processed for EM with minimal structural alterations (reviewed in Moor 1987; Dahl and Staehelin 1989; McDonald and Morphew 1993; Studer et al. 1995; McDonald 1998). This procedure relies on using a high-pressure freezing (HPF) apparatus to prevent ice crystal formation during freezing (i.e., vitreous freezing). Vitreous ice is then replaced with fixative dissolved in a dehydrant at −90°C, (i.e., FS). This HFP/FS approach has many advantages over conventional fixation protocols. Not only does it minimize the displacement, extraction, and coagulation of molecules that occur as a front of buffered chemical fixative sweeps through a cell, but it also minimizes the structural distortions produced in the specimen during dehydration due to changes in surface tension (Hippe-Sanwald 1993).
In this paper we document our initial observations on the structure of the vertebrate kinetochore in sections cut from cells fixed by HPF followed by FS and embedding. These images reveal that the electron-translucent middle layer of the kinetochore is greatly exaggerated in conventionally prepared specimens. The data further reveal that the kinetochore consists of fine fibers that radiate directly from the surface of the chromosome and connect with tangential fibers to form an extended mat that becomes condensed into a prominent outer plate in response to conventional fixation and dehydration protocols.
Materials and methods
For HPF, PtK1 cells were trypsinized from stock cultures and seeded onto sterilized 1-mm diameter disks cut from thin sheets of Mylar (a kind gift from Dr. Kent McDonald, Univ. California, Berkeley, Calif.). These disk cultures were grown in Ham’s F12 medium supplemented with 10% fetal bovine serum within 5 cm Petri dishes, 50 disks per dish, for 2–3 days at 37°C. In some experiments cells were treated in the dishes with 1.0 µM Colcemid (Sigma, St. Louis, Mo.) in medium for 4–7 h prior to fixation.
Just before HPF the center of each disk was scanned by phase-contrast light microscopy for the presence of one or more mitotic cells. Individual disks containing suitably positioned mitotic cells were then placed into a gold or brass “top-hat” specimen holder, designed for use with a Balzers HPC-010 HPF apparatus, which was subsequently filled with medium plus 10% (w/w) Ficoll (mol.wt. 70,000, Sigma, St. Louis, Mo.; see McDonald and Morphew 1993). Working quickly, the specimens were then exposed to a 0.5 s burst of liquid N2 pressurized to 2100 atmospheres. Under this condition vitreous freezing occurs within the sample in <12 ms. The frozen specimen was transferred to liquid N2 within 1 s from the completion of HPF. In total, approximately 2 min elapsed between the time each specimen was transferred to medium plus Ficoll and the time it was vitreously frozen.
After freezing, samples were placed in liquid N2, the top hats were opened and the frozen specimen transferred to the FS solution. We used an unpublished FS protocol developed by Mary Morphew for mitotic vertebrate cells in monolayers (University of Colorado, Boulder, Colo.). Specimens were incubated for 2 days in 0.5% glutaraldehyde plus 0.1% tannic acid in acetone at −90°C, followed by three acetone rinses (15 min each) at −90°C, and a 2 day incubation in 1.0% OsO4 plus 0.1% uranyl acetate in acetone at −90°C. All cold incubations were performed in a Balzers FSU 010 FS apparatus. Specimens were then warmed to room temperature over 4 h, rinsed three times with acetone (15 min each), and transferred to 50:50 Epon:acetone. After an overnight incubation in this Epon:acetone mixture the disks were placed in 100% Epon that lacked catalyst. Each disk was then scanned within the Epon for the presence of mitotic cells. Typically 30%–80% of the disks no longer had mitotic cells after FS, presumably because they were lost from the substrate during HPF or during solution changes after warming. Treating the surface of the disks with polylysine before seeding with cells did not substantially improve the recovery rate.
Disks on which the mitotic cells had been retained up to the Epon infiltration process were placed cell side up on a no.1.5 glass coverslip and covered with fresh Epon with catalyst. After incubating for 1–2 h at room temperature these preparations were placed in a 60°C oven for 2 days. After curing, the glass coverslip was etched away using HF acid (Rieder 1981), and the Mylar growth substrate was then peeled from the embedment with tweezers. As a result of this procedure the embedded cells were now found at the surface of the Epon wafer (just as in conventional flat-embedded preparations). The specimen area was then cut from the wafer and remounted for ultramicrotomy (Rieder 1981).
PtK1 cells that were to be fixed by conventional fixation protocols were grown on coverslips, with or without Colcemid, and then fixed as described previously (e.g., Roos 1973; McEwen et al. 1997). Briefly, the cultures were fixed for 30 min with 2.5% glutaraldehyde in phosphate buffer, pH 7.2, postfixed for 60 min with 2% OsO4 in deionized distilled water, washed, treated for 1 min with 0.15% tannic acid, and stained en bloc for 1–3 h with 1% uranyl acetate in dH20. The specimens were then dehydrated in a graded ethanol series, cleared with propylene oxide, and flat embedded in Epon. In some cases cells were transferred to medium plus 10% Ficoll for 5 min before glutaraldehyde fixation.
Selected cells were then either serially thin-sectioned (70–90 nm) and imaged at 80 kV on a Zeiss 910 EM (LEO, Thornwood, N.Y.), or serially thick (150–250 nm) sectioned for electron tomography using a JEOL JEM-4000FX (JEOL USA, Peabody, Mass.) intermediate voltage EM. All sections were stained with lead citrate and uranyl acetate prior to viewing at 80 or 200 kV (Rieder et al. 1985). For tomography, 20 nm colloidal gold was added to one surface of the sections. Tomographic double-tilt series were recorded in 2° intervals with a binned pixel size of 2.9 nm and three-dimensional reconstructions computed as described by McEwen and Marko (1998). Thin-section electron micrographs, and in a few cases tilt series images that had been recorded on film, were digitally scanned with a Dage model 81 camera (Michigan City, Ind.). The majority of tomographic tilt series were digitally recorded using a fiber-optically coupled CCD camera (Tietz Video and Image, Gauting Germany).
All figures were prepared using Adobe Photoshop (Adobe Systems, San Jose, Calif.). In some cases (i.e., Figs. 1B, 3, 4B, 6A, B) thin section images of high-pressure frozen specimens were enhanced for greater contrast of the kinetochore mat relative to the background and the chromatin.
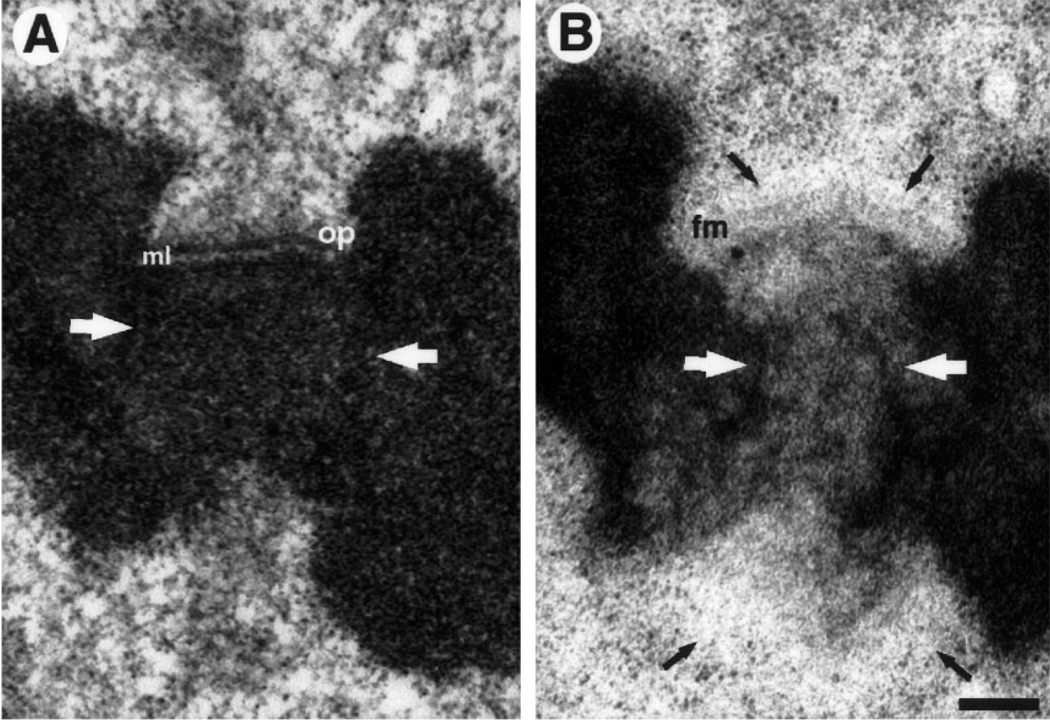
Fig. 1.
A, B. Comparison between high-pressure freezing/freeze substitution (HPF/FS) and conventional specimen preparations for Colcemid treated PtK1 cells. A Kinetochore prepared via conventional protocol. The outer plate (op) is a heavily stained, compact, structure that is separated from the underlying heterochromatin by a translucent middle layer (ml). A prominent fibrous corona radiates from the outer plate’s distal surface. Note the general extracted appearance of the surrounding cytoplasm, and the uniform staining of the centromeric chromatin (white arrows). B Sister kinetochores prepared via HPF/FS. In the top kinetochore, the fibrous mat structure (fm) is lightly stained and much more open than the outer plate in A. The corona appears as a cytoplasmic exclusion zone (black arrows) lacking in discernable substructure. In contrast to conventional preparations, the heterochromatin has a mottled appearance (white arrows) and the surrounding cytoplasm is smooth, uniform, and unextracted. The lower kinetochore is sectioned at an oblique angle. As a result the mat structure is not evident, and was not found in the neighboring serial sections. However, the exclusion zone is visible (arrows). Bar represents 250 nm
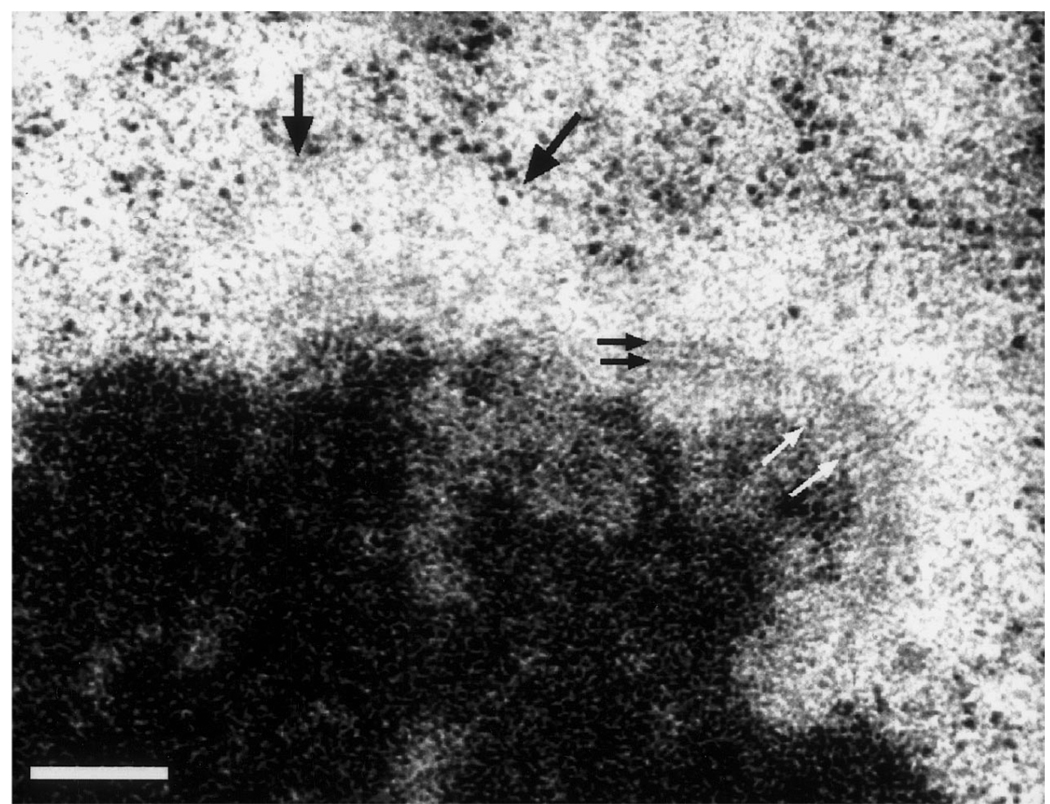
Fig. 3.
Higher magnification view of a Colcemid-treated HPF/FS kinetochore. This chromosome is oriented with a slight rotation into the section plane. The mat structure on the right-hand kinetochore appears to be constructed from fine fibers radiating out from the underlying chromatin (white arrows) and crossing fibers lying parallel to the chromatin surface (black arrows). The mat is not visible in the left-hand kinetochore in this section, but the kinetochore can be detected by the prominent exclusion zone with ribosomes piled up along the boundary (large black arrows). Bar represents 200 nm
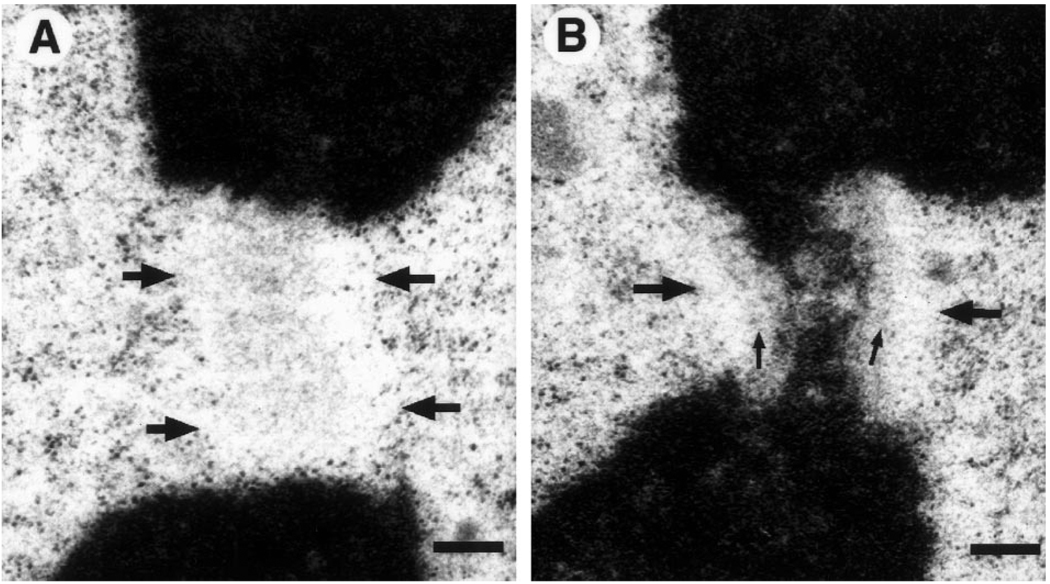
Fig. 4.
A, B. The exclusion zone in an obliquely cut section. A A roughly axial view of the exclusion zone (boundaries marked by large arrows), detected primarily by exclusion of ribosomes and other cytoplasmic material. A fine flocculent material, possibly of fibrous nature, is seen in this area. Presumably this material consists of corona fibers and/or an en face view of the mat structure B Serial section to A, showing the obliquely sectioned kinetochore. Both the mat structure (small arrows) and exclusion zone (large arrows) can be discerned. Bar represents 250 nm
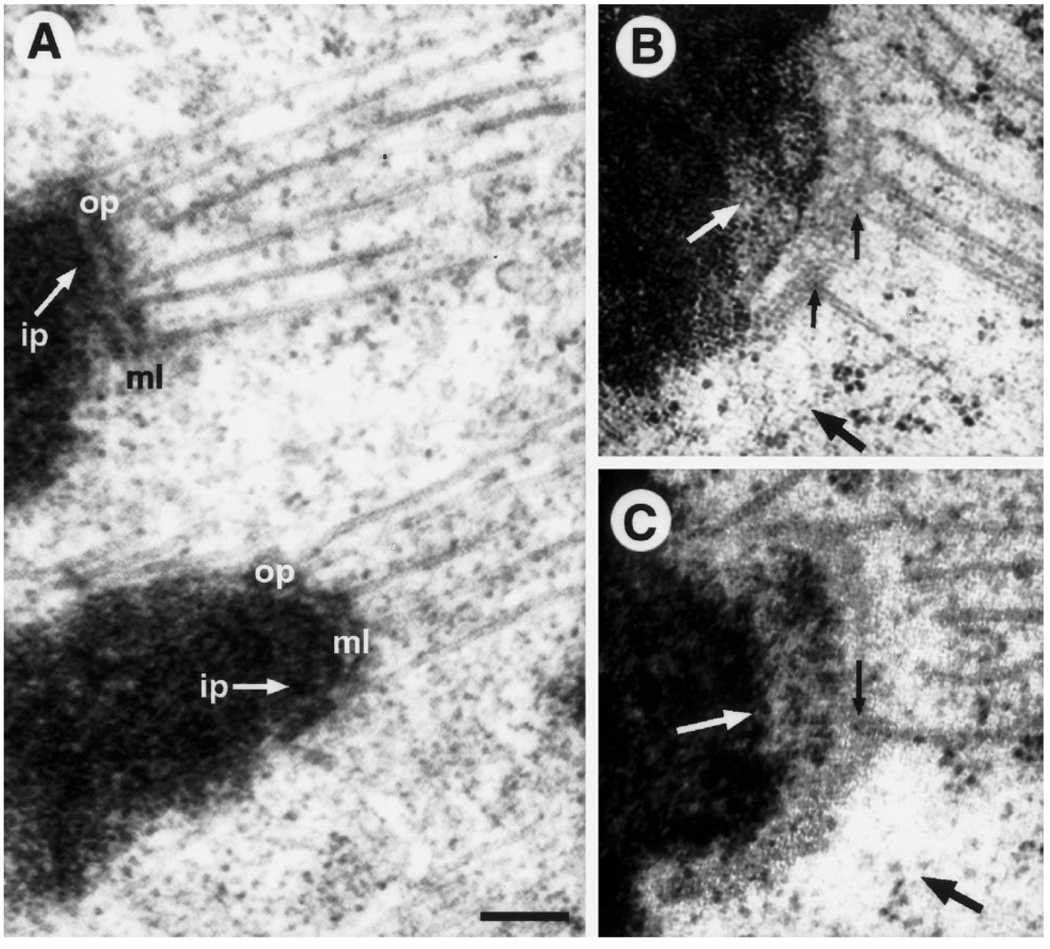
Fig. 6.
A–C. HPF/FS vs conventional specimen preparations for untreated PtK1 cells. A Adjacent kinetochores from a conventionally prepared metaphase cell. Note the prominent outer plate (op) structure that stains as heavily as chromatin, and is separated from the underlying inner plate (ip) by a well-defined, translucent, middle layer (ml).Microtubule (Mt) plus ends can not be clearly traced within the highly condensed outer plate. B, C Two different kinetochores from an anaphase cell prepared via HPF/FS. Note the absence of a prominent outer plate. Instead there is a lightly staining mat structure that sits above the chromatin without an intervening translucent layer. Mt plus ends can be traced (small black arrows) and they appear to flay apart and be continuous with the fibrous mat (in agreement with Mastronarde et al. 1997). The cytoplasmic exclusion zone can still be detected in some areas where there are no Mts (large black arrows). The mottled appearance of the centromeric heterochromatin is also evident (white arrows). Bar represents 200 nm
Results
The structure of the kinetochore in sections cut from conventionally fixed and dehydrated Colcemid-treated PtK1 cells has been previously detailed (e.g., see Rieder 1979; McEwen et al. 1993). In general the kinetochore consists of a conspicuous, roughly circular 35–40 nm thick electron-opaque outer plate that is composed of a dense meshwork of 10–20 nm thick fibers (Fig. 1A). This outer plate varies widely in diameter, depending on the chromosome, and is connected to the underlying pericentromeric heterochromatin by a 20–30 nm thick electronlucent zone which is seen in electron tomographic studies to contain 10–20 nm thick fibers (McEwen et al. 1993). A homogenous staining corona of fibrillar material radiates ~100–120 nm away from the cytoplasmic surface of the outer disk. For this study we were able to recognize a distinct plate structure in 60 out of 66 kinetochores examined in 70–80 nm thick serial sections from three conventionally fixed cells (for PtK1 cells, the most frequent chromosome number is 11, hence 22 kinetochores per cell). Thus the kinetochore plate in Colcemid-treated cells is sufficiently compact that it is still recognizable over a wide range of sectioning angles. The unattached kinetochores in these cells are similar in all respects to the unattached kinetochores seen during early prometaphase in non-Colcemid-treated vertebrate cells (see Rieder 1982).
We examined six different blocks of Colcemid-treated PtK1 cells prepared by HPF/FS. During cryofixation optimal structural preservation requires that the specimen be frozen so rapidly that cellular water forms vitreous (i.e., glass-like) rather than crystalline ice (Dahl and Staehelin 1989; Studer et al. 1989). The latter causes an extensive and unmistakable disruption of structure that is worse than the effects of conventional fixation protocols. The criteria we used to identified vitreously frozen and well-substituted mitotic cells were as follows. After staining, the sections cut from such cells had to show a uniform staining and ribosome distribution in the cytoplasm, the organelle profiles had to be smooth and rounded, and there could be no evidence of reticulation, especially in the chromatin – which is generally considered to be the cellular component most sensitive to ice damage (see McDonald 1994, for examples of good freezing vs mild ice damage).
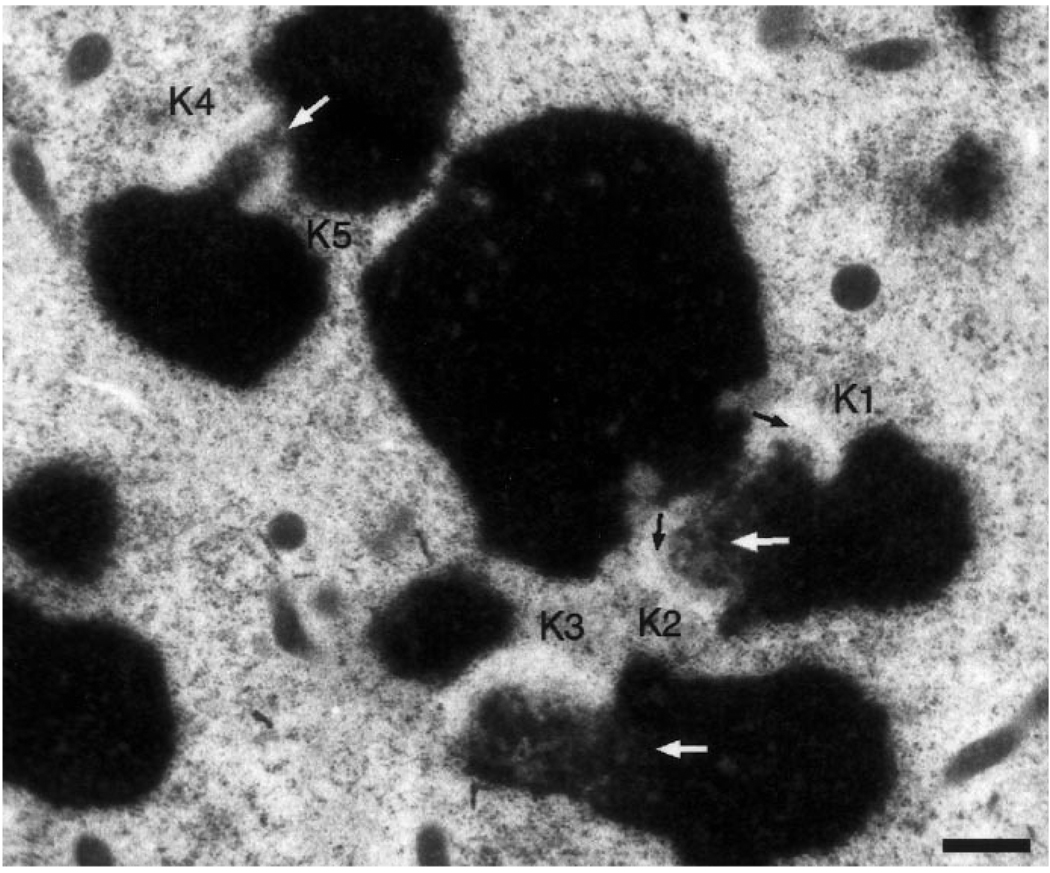
Kinetochores in HPF/FS Colcemid-treated cells have a strikingly different appearance than those in conventionally fixed cells (cf Fig. 1A,B). Instead of a densely staining, compact outer plate, cryofixed kinetochores consist of a 25–70 nm thick matted meshwork of fine, lightly staining fibers (Figs. 1B, 2, 3). This material is directly linked with the surface of the underlying pericentromeric heterochromatin. Unlike conventionally fixed material, kinetochores fixed by HPF/FS do not possess an electron lucent-middle layer. The mat of material associated with the surface of the heterochromatin consists of fibers, 10–15 nm in diameter, that in some planes of section appear to radiate from the underlying chromatin (Fig. 3, white arrows). In contrast to conventionally fixed preparations, in which the kinetochore plate can be recognized over a wide range of sectioning angles (see above), because of its nature the corresponding mat could only be clearly recognized when the plane of section passed through both sister kinetochores on the opposing sides of the primary constriction (which occurred ~50% of the time). The two sister kinetochores (K1 and K2) in Fig. 2 illustrate how lightly stained the mat is before digital-contrast enhancement, even when optimally oriented. Since the mat structure is difficult to recognize in obliquely sectioned kinetochores (e.g., K3, K4, and K5 in Fig. 2), these kinetochores were defined by their position within the primary constriction and by the fact that they excluded ribosomes from their region.
Fig. 2.
Low magnification view of a Colcemid-treated PtK1 cell prepared via HPF/FS. Kinetochores are most readily recognized by the adjoining clear zones that exclude ribosomes and other cytoplasmic particles. Longitudinally sectioned kinetochores (K1, K2) also show faintly staining mats (black arrows) that are not evident in obliquely sectioned kinetochores (K3-K5). The mottled appearance of centromeric heterochromatin (white arrows) is a general feature of chromosomes prepared via HPF/FS. Bar represents 500 nm
The most conspicuous feature of the Colcemid-treated kinetochore in HPF cells is that ribosomes are excluded from a zone radiating from the mat component on the surface of the primary constriction (Figs. 1B, 2, 3). This zone extends 120–150 nm beyond the mat component (i.e., a total distance of 150–250 nm from the heterochromatin), compared with 100–120 nm for the width of the corona material in conventional preparations. Since the prominent, tightly packed filaments of the conventional corona (Fig. 1A) are not readily apparent in the exclusion zone, the kinetochore appears to be embedded in a homogenous, structure-free, region of cytoplasm that excludes stain when viewed at low magnification (Fig. 2). At higher magnifications the exclusion zone appears to contain an array of extremely fine, lightly staining filaments that exclude ribosomes (Figs. 1B, 3, 4). These filaments are not nearly as tightly packed as those within the mat. A particularly informative example of the exclusion zone is shown in Fig. 4 where a kinetochore is cut slightly oblique to an en face view. The first section (4 A) contains the corona and/or part of the mat material from which ribosomes are excluded. The adjacent section (4B) contains a nice example of the mat/corona complex in cross section at the periphery of the kinetochore.
Additional fine structure detail is revealed by tomographic reconstructions of HPF Colcemid-treated kinetochores, two of which are illustrated in Fig. 5. In some views the mat appears as a string of blocks or beads (Fig. 5D), particularly in volume renderings from en face and oblique viewing directions (Fig. 5E). Often linear fiber-like elements are observed oriented parallel to the chromatin surface (Fig. 5B, see also Fig. 3, black arrows). In some cases these elements form parallel tracks that delineate a tripartite structure (Fig. 5D) reminiscent of that seen in serial thin sections and tomographic reconstructions of conventionally fixed material (e.g., Bernat et al. 1991; McEwen et al. 1993), particularly since the tripartite structure is often separated from the heterochromatin by a lighter staining zone. However, in contrast to conventional preparations, the cytoplasm and the lightly stained zone are unextracted in reconstructions of HPF/FS preparations, leaving a high level of background gray. In addition, the mat structure is much less densely stained than the outer plate of conventional preparations. As a result, the whole kinetochore structure shows low contrast relative to the cytoplasmic background (e.g., Figs. 5B, D). The exclusion zone contains a very lightly stained, fine filamentous material (Figs. 5B, D). Thicknesses of the putative fibers are probably <9 nm but unresolvable due to low contrast.
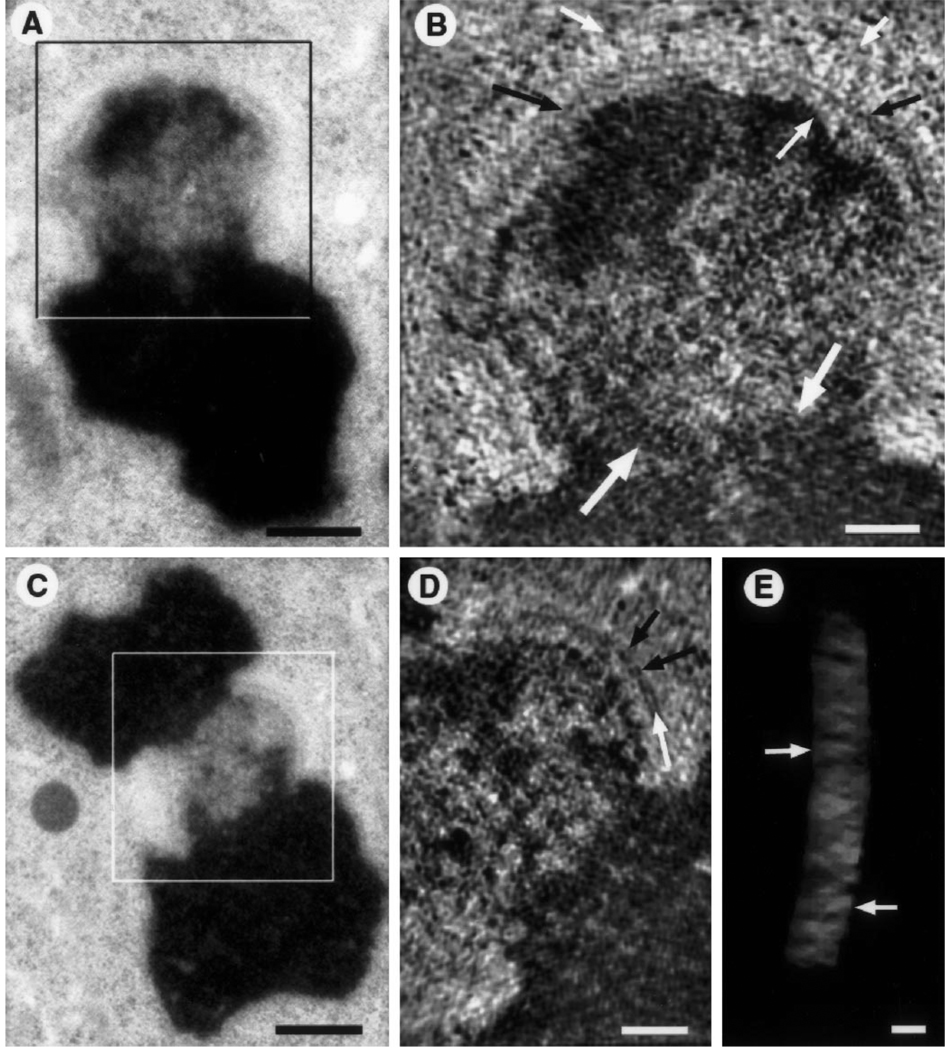
Fig. 5.
A–E. Tomographic reconstructions of colcemid-treated, HPF/FS kinetochores. A Low magnification view of sister kinetochore used for tomographic reconstruction (box indicates the approximate area of the reconstruction shown in B). As in Fig. 3, the chromosome is oriented with a slight rotation into the section plane. Bar represents 500 nm. B A 9 nm thick slice (average of three successive 2.9 nm thick tomograms) from the three-dimensional reconstruction of the boxed area in A. Fibrous elements are evident radiating from the heterochromatin and from the surface of the mat (small white arrows) but low contrast and inherent resolution limits preclude exact measurements of diameters and lengths. In this view the mat structure appears as a long, thin, fiber-like element running parallel to the chromatin surface (black arrows). A striking difference in texture between euchromatin and centromeric heterochromatin is evident along the border of the two domains (large white arrows). Bar represents 200 nm. C Low magnification view of the kinetochore used for the tomographic reconstruction illustrated in D and E (box delineates the reconstructed area). Bar represents 500 nm. D A 9 nm thick slice (like B) from the three-dimensional reconstruction of the boxed area in D. The mat shows a block-like arrangement (black arrows) and some unit blocks having a tripartite organization (white arrows). Bar represents 200 nm. E Tilted view of the mat structure. The mat has been isolated from the remainder of the three-dimensional reconstruction and is displayed as a semi-transparent subvolume using masking tools and volume rendering software (see McEwen et al. 1993; McEwen and Marko 1998). The block-like arrangement of putative unit structures is particularly evident in this view (arrows). Bar represents 50 nm
In conventionally fixed mitotic cells both the chromosome arms and the primary constriction stain relatively uniformly electron opaque, and no definable staining boundary exists between the two (Fig. 1A). However, without exception, after HPF/FS the centromeric heterochromatin immediately below the kinetochores appeared mottled in its contrast, even though the adjoining chromosome arms were uniformly and densely stained (Figs. 1B, 2, 3). The reason for this nonuniform staining is unknown (see Discussion) but it was also seen in tomographic reconstructions (Figs. 5A–D), and to a lesser extent in the heterochromatin of non-Colcemid-treated mitotic cells fixed by HPF/FS (Figs. 6B, C). Unlike the centromere region, the telomere regions at the ends of the chromosomes in these cells were indistinguishable in their staining properties from the chromosome arms. However, the euchromatin in prophase cells frozen in the process of chromosome condensation showed the same mottled appearance as the centromere region in prometaphase/metaphase cells (data not shown).
We also examined the structure of attached kinetochores in HPF mitotic cells not treated with Colcemid. In conventionally fixed cells a typical trilaminar kinetochore (see Introduction) was seen with associated Mts that terminate in its outer plate (Fig. 6A). By contrast the attached kinetochores in metaphase or early anaphase cells fixed by HPF/FS consisted of a lightly staining mat of fibrillar material, ~30–50 nm thick, that was associated directly with the surface of the underlying heterochromatin (Figs. 6B, C). Since the K-fiber Mt ends terminated directly in this mat it must correspond to the outer kinetochore plate seen in conventional preparations. As noted above, even in these HPF/FS preparations of non-Colcemid-treated cells, the pericentric heterochromatin subjacent to the kinetochore region had a mottled staining appearance relative to the rest of the chromosome (Fig. 6B, C). Finally, as with the corona of conventionally prepared specimens, the cytoplasmic exclusion zone is not visible where Mts are bound. However, the exclusion zone can still be detected in regions of attached kinetochores that lack Mts (Fig. 6B, large black arrows).
Discussion
Rapid vitreous freezing followed by FS is generally considered to be the method of choice for faithfully preserving cell structure at the highest possible resolution (reviewed in Moor 1987; Dahl and Staehelin 1989; Studer et al. 1989; McDonald 1994, 1998). During the freezing process internal and external cellular constituents, including their soluble components, are immobilized in their native structure and position in just a few milliseconds. During the FS step an organic solvent (methanol or acetone) is then substituted for the vitreous ice at −90°C, which minimizes the surface tension-mediated collapse and shrinkage that occurs when water is rapidly and progressively removed at room temperature (see Hippe-Sanwald 1993). By incorporating a fixative into the FS denydrant, the structure, location, and spatial relationships of components are preserved from distortions that occur in conventional preparations owing to a fixation front that sweeps through the cell at room temperature. The superior preservation produced by HPF/FS is clear from the observations that: (1) vesicles appear circular and their surrounding membrane is smooth and continuous rather than broken and rippled; (2) the cytoplasm is free from signs of extraction and coagulation; (3) ribosomes are evenly distributed and not clumped or extracted; and (4) cytoskeleton elements, particularly actin and Mts, appear as straight linear elements or smooth arcs (see McDonald 1994).
Although the peripheral edges of cells grown in monolayer are sufficiently thin (less than 1.5 µm) to be vitreously frozen by simpler means, including freeze-slamming or plunging, vitreous freezing of the thicker nuclear regions requires HPF even when cryoprotectants are used. The same is true for dividing cells, which tend to round and thus become appreciably thicker during mitosis.
Many of our findings are consistent with the predicted differences in structural preservation between conventional chemical fixation and HPF/FS. In HPF specimens, unattached kinetochores have a delicate fibrillar mat-like structure that is 25%–100% more extended (i.e., thicker) than the corresponding outer plate of conventional preparations. It is reasonable to assume that conventional protocols induce the components of this mat to coagulate and collapse, to produce a thinner, denser, and more heavily stained outer plate. Similarly, the electron-lucent zone seen to separate this outer plate from the chromosome in conventional preparations is absent or much less prominent in HPF/FS specimens (Figs. 1B, 2, 3). The translucence of this zone appears to be an artifact caused by extraction of soluble components, disruption of fine fibrillar material, and a differential shrinkage between the pericentromeric heterochromatin and the surrounding cytoplasm as water is removed during dehydration at room temperature (e.g., see Jensen and Bajer 1969). Under such a condition the kinetochore “plate” could remain cross-linked to, and embedded, in the surrounding cytoplasm as the chromatin/chromosome complex shrinks away from it to produce a relatively large, non-staining, clear zone lacking appreciable structure. Finally, the fine, light-stained material comprising the corona of HPF/FS kinetochores appears to be coagulated into thicker, shorter fibers in conventional preparations. Thus, since the resolution ultimately required to understand how the kinetochore is structured at the molecular level approaches the limits attainable in plastic sections (1–2 nm), even subtle differences in fixation and preparation protocols can be expected to produce a structure of substantially different appearance.
The alterations induced in kinetochore structure by conventional fixation protocols force a reexamination of those current models that attempt to explain how this organelle works based on its structure. For example, our observation that the outer plate and the electron-translucent layer are not separate domains in HPF/FS preparations calls into question models that postulate different roles for each zone (e.g., see Hyman and Mitchison 1992; Inoue and Salmon 1995; Rieder and Salmon 1998). However, even with HFP/FS the kinetochore is seen to consist of three defined domains that are intimately related: the corona that radiates from the surface of a mat of material, which is intimately associated with the chromosome surface. Thus in most cases those components demonstrated by immuno-EM studies to be located in the heterochromatin/inner plate (e.g., CENP A, B, C and G) or outer plate/corona region (CENP-E, cytoplasmic dynein) would likely have a comparable location in HFP/FS-prepared material, which may itself ultimately provide a superior method for detailing the location of many kinetochore antigens at the highest possible resolution (see Kiss and McDonald 1993). By contrast the location of those molecules reported to be in the electron-lucent middle layer, including the 3F3/2 epitopes (Campbell and Gorbsky 1995), needs to be reevaluated.
In conventionally prepared specimens the outer plate is connected to the underlying heterochromatin by a loose network of 10–20 nm fibers and its staining is similar to that of the underlying pericentric heterochromatin (Ris and Witt 1981; McEwen et al. 1993). These and other observations led to the hypothesis that the outer plate contains a unique DNA/chromatin fiber that is continuous with and arises from the underlying heterochromatin (e.g., Ris and Witt 1981; reviewed in Cooke et al. 1993). However, in our images the texture and staining properties of the mat, which corresponds to the outer plate of conventional preparations, does not resemble that of the underlying chromatin. This lends support to the more recent view, based on electron energy loss spectra, antibody labeling, and specific stains (Cooke et al. 1993), that the kinetochore outer plate is constructed from proteins and lacks DNA.
In HPF/FS samples the centromere region between the sister kinetochores appears mottled (Figs. 1B, 2, 3, 5) whereas in conventional preparations it is stained evenly and similarly to the chromosome arms. This mottling is specific to the centromere region since it is not seen along the chromosome arms or telomeres of HPF/FS samples. We have no explanation for why the centromere region of HPF/FS cells is selectively mottled but note that a similar mottled appearance of the centromere region, as well as the chromosome arms, is seen in PtK1 cells exposed to 6°C for prolonged periods (Rieder and Borisy 1981). It is not an artifact induced by Colcemid treatment since a similar mottling is seen beneath the kinetochores in untreated HPF/FS cells. Also, it is highly unlikely that this unusual pattern of stain intensity reflects structural changes induced during HPF because vitreous freezing occurs within several milliseconds and there is no evidence of the reticulation that always accompanies ice-induced damage. It is possible that mottling arises somehow during our FS protocol. This can be evaluated in the future by comparing our current results with those obtained using other FS protocols. Alternatively, it is possible that this staining pattern reflects the fact that the centromere region contains a unique chromatin structure as well as numerous proteins that are not located in the chromosome arms (reviewed in Lamond and Earnshaw 1998; Rieder and Salmon 1998). Tomographic reconstructions support this view since they show pronounced staining differences between euchromatin and centromeric heterochromatin throughout the three-dimensional reconstruction (Figs. 5B, D).
Future studies will attempt to resolve whether the unique staining property of centromeric heterochromatin is an artifact, or reflects a reality normally masked by conventional fixation protocols. We will also endeavor to optimize staining conditions to provide greater contrast in the tomographic reconstructions, and thereby gain a more comprehensive understanding of kinetochore architecture.
Acknowledgements
We thank Mary Morphew for helpful advice and for sharing her unpublished FS protocol, Dr. Kent McDonald for invaluable advice on HPF and FS procedures and for critique of initial results, Michael Marko for help with tomographic reconstructions, Rita Barnard and Muniba Naqi for help preparing figures, Rita Barnard and Cindy Hughes for growing PtK1 cells, and Richard Cole for advice concerning operation of the Balzer’s HPF. We also thank Dr. Alexey Khodjakov for helpful comments on the manuscript. This work was supported, by NSF MCB9420772 and MCB 9808879 to B.F.M., NIH GMS R01-40198 to C.L.R., and by NIH NCRR/BTP P41-RR01219, which supports part of the Wadsworth Center’s Biological Microscopy and Image Reconstruction facility as a National Biotechnological Resource. This work also made use of the Wadsworth Center’s electron microscopy core facility.
References
- Bernat RL, Delannoy MR, Rothfield NF, Earnshaw WC. Disruption of centromere assembly during interphase inhibits kinetochore morphogenesis and function in mitosis. Cell. 1991;66:1229–1238. doi: 10.1016/0092-8674(91)90045-z. [DOI] [PubMed] [Google Scholar]
- Brinkley BR, Stubblefield E. Fine structure of the kinetochore in Chinese hamster cells in vitro. Chromosoma. 1966;19:28–43. doi: 10.1007/BF00332792. [DOI] [PubMed] [Google Scholar]
- Campbell MS, Gorbsky GJ. Microinjection of mitotic cells with the 3F3/2 antiphosphoepitope antibody delays the onset of anaphase. J Cell Biol. 1995;129:1195–1204. doi: 10.1083/jcb.129.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L, Rieder CL, Rupp G, Salmon ED. Stability of microtubule attachment to metaphase kinetochores in PtK1 cells. J Cell Sci. 1990;96:9–15. doi: 10.1242/jcs.96.1.9. [DOI] [PubMed] [Google Scholar]
- Chen R-H, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component xMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Cooke CA, Bazett-Jones DP, Earnshaw WC, Rattner B. Mapping DNA within the mammalian kinetochore. J Cell Biol. 1993;120:1083–1091. doi: 10.1083/jcb.120.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CA, Schaar B, Yen T, Earnshaw WC. Localization of CENP-E in fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma. 1997;106:446–455. doi: 10.1007/s004120050266. [DOI] [PubMed] [Google Scholar]
- Dahl R, Staehelin LA. High-pressure freezing for the preservation of biological structure: theory and practice. J Electron Microsc Tech. 1989;13:165–174. doi: 10.1002/jemt.1060130305. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Zeng C, Woods K, Zhong L, Turner D, Busch RK, Brinkley BR, Busch H. CENP-G: a new centromeric protein that is associated with the alpha-1 satellite DNA subfamily. Chromosoma. 1998;107:189–197. doi: 10.1007/s004120050296. [DOI] [PubMed] [Google Scholar]
- Hippe-Sanwald S. The impact of freeze-substitution on biological electron microscopy. Microsc Res Tech. 1993;24:400–422. doi: 10.1002/jemt.1070240506. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ. Molecular basis of chromosome movement. Curr Opin Struct Biol. 1992;2:275–279. [Google Scholar]
- Inoue S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C, Bajer A. Effects of dehydration on the microtubules of the mitotic spindle. J Ultrastruct Res. 1969;26:367–386. doi: 10.1016/s0022-5320(69)90044-6. [DOI] [PubMed] [Google Scholar]
- Jokelainen PT. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967;19:19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, McDonald K. Electron microscopy immunecytochemistry following cryofixation and freeze substitution. Methods Cell Biol. 1993;37:311–341. doi: 10.1016/s0091-679x(08)60256-3. [DOI] [PubMed] [Google Scholar]
- Lamond AI, Earnshaw WC. Structure and function in the nucleus. Science. 1998;280:547–553. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN, Morphew MK, McIntosh JR. HVEM tomography of PtK cells shows that the plus ends of kinetochore microtubules flare outward in prometaphase, metaphase, and anaphase. Mol Biol Cell. 1997;8:171a. [Google Scholar]
- McDonald KF. Electron microscopy and EM immunocytochemistry. Methods Cell Biol. 1994;44:411–444. doi: 10.1016/s0091-679x(08)60926-7. [DOI] [PubMed] [Google Scholar]
- McDonald KF. High pressure freezing for preservation of high resolution fine structure and antigenicity for immunolabeling. Methods Mol Biol. 1998 doi: 10.1385/1-59259-201-5:77. (in press) [DOI] [PubMed] [Google Scholar]
- McDonald KF, Morphew MK. Improved preservation of ultrastructure in difficult-to-fix organisms by high pressure freezing and freeze substitution. Microsc Res Tech. 1993;24:465–473. doi: 10.1002/jemt.1070240603. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Marko M. Three-dimensional transmission electron microscopy and its application to mitosis research. Methods Cell Biol. 1998;61:81–111. doi: 10.1016/s0091-679x(08)61976-7. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Arena JT, Frank J, Rieder CL. Structure of the colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J Cell Biol. 1993;120:301–312. doi: 10.1083/jcb.120.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BF, Ding Y, Heagle AB. Relevance of kinetochore size and microtubule-binding capacity for stable chromosome attachment during mitosis in PtK1 cells. Chromosome Res. 1998;6:123–132. doi: 10.1023/a:1009239013215. [DOI] [PubMed] [Google Scholar]
- Moor H. Theory and practice of high pressure freezing. In: Steinbrecht RA, Zierold K, editors. Cryotechniques in biological microscopy. Berlin Heidelberg New York: Springer; 1987. pp. 175–199. [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–638. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC. The centromere: hub of chromosomal activities. Science. 1995;270:1591–1594. doi: 10.1126/science.270.5242.1591. [DOI] [PubMed] [Google Scholar]
- Rieder CL. Localization of ribonucleoprotein in the trilaminar kinetochore of PtK1. J Ultrastruct Res. 1979;66:109–119. doi: 10.1016/s0022-5320(79)90128-x. [DOI] [PubMed] [Google Scholar]
- Rieder CL. Thick and thin serial sectioning for the three-dimensional reconstruction of biological ultrastructure. Methods Cell Biol. 1981;22:215–249. doi: 10.1016/s0091-679x(08)61879-8. [DOI] [PubMed] [Google Scholar]
- Rieder CL. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–59. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Borisy GG. The attachment of kinetochores to the pro-metaphase spindle PtK1 cells: recovery from low temperature treatment. Chromosoma. 1981;82:693–716. doi: 10.1007/BF00285776. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Rupp G, Bowser SS. Electron microscopy of semi-thick sections: advantages for biomedical research. J Electron Microsc Tech. 1985;2:11–28. [Google Scholar]
- Ris H, Witt PL. Structure of the mammalian kinetochore. Chromosoma. 1981;82:153–170. doi: 10.1007/BF00286101. [DOI] [PubMed] [Google Scholar]
- Roos U-P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma. 1973;41:195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- Rudner AD, Murray AW. The spindle assembly checkpoint. Curr Opin Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Williams BC, Li Z, Etmad-Moghadam B, Daw RK, Goldberg ML. Conservation of the centromere/kinetochore protein ZW10. J Cell Biol. 1997;138:1289–1301. doi: 10.1083/jcb.138.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer D, Michel M, Müller M. High pressure freezing comes of age. Scanning Microsc. 1989;3:253–259. [PubMed] [Google Scholar]
- Studer D, Michel M, Wohlwend M, Hunziker EB, Buschmann MD. Vitrification of articular cartilage by high-pressure freezing. J Microsc. 1995;179:321–332. doi: 10.1111/j.1365-2818.1995.tb03648.x. [DOI] [PubMed] [Google Scholar]
- Taylor SS, McKeon F. Kinetochore localization of murine BUB1 is required for normal mitotic timing and checkpoint response to unattached kinetochores. Cell. 1997;89:727–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- Tomkiel J, Cooke CA, Saitoh H, Bernat RL, Earnshaw WC. CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J Cell Biol. 1994;125:531–545. doi: 10.1083/jcb.125.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton PE, Cooke CA, Bourassa S, Vafa O, Sullivan BA, Stetten G, Gimelli G, Warburton D, Tyler-Smith C, Cullivan KF, Poirier GG, Earnshaw WC. Immunolocalization of CENP-A suggests a distinct nucleosome structure at the inner kinetochore plate of active centromeres. Curr Biol. 1997;7:901–904. doi: 10.1016/s0960-9822(06)00382-4. [DOI] [PubMed] [Google Scholar]
- Wood K, Sakowicz R, Goldstein LSB, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–105. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Anderson KL, Cleveland DW. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J Cell Biol. 1997;139:435–447. doi: 10.1083/jcb.139.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]