Abstract
Caffeine is an antagonist at A1 and A2A adenosine receptors and epidemiological evidence suggests that caffeine consumption reduces the risk of Alzheimer's and Parkinson's diseases. Neuroinflammation plays a role in the etiology of these diseases and caffeine may provide protection through the modulation of inflammation. Adenosine has a known role in the propagation of inflammation and caffeine may reduce microglia activation directly by blocking adenosine receptors on microglia. Chronic neuroinflammation is associated with an increase in extracellular levels of glutamate and drugs that limit the effects of glutamate at neuronal receptors have been shown to indirectly reduce the neuroinflammatory response of microglia cells. A1 and A2A receptors have been shown to regulate the pre-synaptic release of glutamate, therefore, caffeine may also reduce neuroinflammation via its ability to regulate glutamate release. Caffeine was administered at various doses to young rats with experimentally induced neuroinflammation by chronic infusion of lipopolysaccharide (LPS) over two or four weeks into the 4th ventricle and to aged rats with naturally elevated levels of microglia activation. Caffeine attenuated the number of activated microglia within the hippocampus of animals with LPS-induced and age-related inflammation.
Chronic neuroinflammation is associated with a cascade of self-propagating cellular events including blockade of glutamate uptake by glia [38] and enhanced release of glutamate [2]. Neurons can cope with the short lasting high influx of calcium ions seen during physiological activation; however, compensatory mechanisms are not sufficient in the case of lower, but prolonged ion influx as likely occurs in the presence of chronic neuroinflammation [4, 24]. There is a clear need for a pharmacological strategy to attenuate the dysregulation of glutamate neurotransmission and the subsequent increase in calcium influx into vulnerable neurons [25, 39]. We have previously demonstrated that the selective antagonism of N-methyl-d-aspartate (NMDA) receptors by memantine can reduce significantly the number of activated microglia within the hippocampus of young rats chronically infused with LPS into the 4th ventricle and restore glutamate-dependent calcium signaling via NMDA channels [35, 37]. Because microglia in the hippocampus do not express voltage-gated NMDA channels and memantine does not influence the effects of LPS upon cultured microglia [35] we speculated that the ability of memantine to reduce the activation of resident microglia was related to a general blockade of the synaptic effects of glutamate between neurons [37]. In order to further investigate the role of glutamate in the regulation of microglia activation we manipulated adenosine receptor function in the presence of chronic neuroinflammation.
Adenosine may regulate aspects of the brain's inflammatory processes in multiple and complementary ways [8, 17, 34]. Additionally, adenosine modulates the pre-synaptic release of glutamate via A1 (inhibition) or A2A (facilitation) [11, 13, 41] and corresponding NMDA receptor function [10, 32]. Adenosine type A1 and A2A receptors on glutamatergic terminals play a critical role in the control of presynaptic glutamate release [9, 26, 27, 30, 31, 33] particularly within the hippocampus [9, 10, 26, 27]. The relationship between glutamate and adenosine neural systems may be reciprocal: a recent report concluded that increased levels of extracellular glutamate are able to convert the anti-inflammatory influence of A2A receptor activation to proinflammatory [7].
Caffeine is an antagonist at both adenosine type A1 and A2A receptors and epidemiological evidence suggests that caffeine consumption is inversely correlated with the risk of Alzheimer's and Parkinson's diseases [12, 28, 40] which are associated with chronic neuroinflammation [18, 19]. Likewise, protection by caffeine has been demonstrated in animal models of Parkinson's disease [3, 5, 6, 23]. The current study investigated the ability of caffeine to reduce the number of activated microglia associated with experimentally induced neuroinflammation in young rats by exposure to lipopolysaccharide (LPS).
The subjects were sixty, 3 month old male F-344 (Harlan Sprague-Dawley, Indianapolis, IN) rats. LPS (250 ng /h) or artificial CSF (aCSF) was chronically infused through a cannula implanted into the 4th ventricle that was attached to an osmotic minipump (model 2004; 0.25 ul/hr) as previously described [15, 36]. Beginning the day after surgery, caffeine was administered by daily intraperitoneal (i.p.) injection in order to accurately control the dosage. Rats were assigned to one of eleven different groups in which they were infused with either aCSF or LPS into the 4th ventricle and injected (i.p.) daily with either 0.9% saline (Sal, 1ml/kg) or caffeine (Caf, at 0.5, 5, 10, 20 or 40 mg/kg/day) over a period of either 2 or 4 weeks; groups sizes ranged from three-eight rats. Histological analyses were performed to confirm the effects of LPS and caffeine treatment on the number of activated microglia in three regions of the hippocampus, the dentate gyrus (DG) and areas CA1 and CA3. The monoclonal antibody OX-6 (final dilution 1:400, Pharmigen, San Diego, CA) was used to visualize activated microglia cells as previously described [15, 36]. This antibody is directed against Class II major histocompatibility complex (MHC II) antigen. Areas of interest were determined as previously reported [36], their surface area measured, and the immunoreactive cells numerated in two sections and both hemispheres from each rat allowing us to determine the average number of immunoreactive cells per millimeter square.
Immunostaining for OX-6 revealed numerous highly activated microglia cells distributed throughout the brain of LPS-infused young rats. The activated microglia had a characteristic bushy morphology with increased cell body size and contracted and ramified processes. Rats infused with aCSF had very few mildly activated microglia evenly scattered throughout the brain. The results are consistent with our previous studies [15, 16, 29, 35-37].
A two-way ANOVA of the data revealed an overall main effect of group (F10, 308=10.4, p<0.001), an overall main effect of hippocampal region (F2,308=47.3, p<0.001) and a significant interaction between experimental group and hippocampal region (F20,308=3.5, p<0.001). Post-hoc analyses (Fisher LSD) indicated that the infusion of LPS for both two (Figure 1) and four (Figure 2) weeks significantly (p<0.001) increased the number of activated microglia within the DG and CA3 regions, but not the CA1. In all rats infused with LPS, the DG and CA3 region had significantly (p<0.05) more activated microglia than did the CA1 region; consistent with our previous reports [24, 25]. Caffeine administration significantly (p<0.001) reduced the number of activated microglia in the DG. These results indicate that caffeine treatment is effective against the activation of microglia induced experimentally by chronic infusion of LPS in young rats. Surprisingly, 40 mg/kg/d of caffeine was effective after administration for two weeks, but not after four weeks. These results are consistent with the hypothesis that chronic administration of caffeine produces a time-dependent “effect inversion” with regard to its neuroprotective actions within the brain [20] that might be related to changes in the density and affinity of A1 receptors [21, 22].
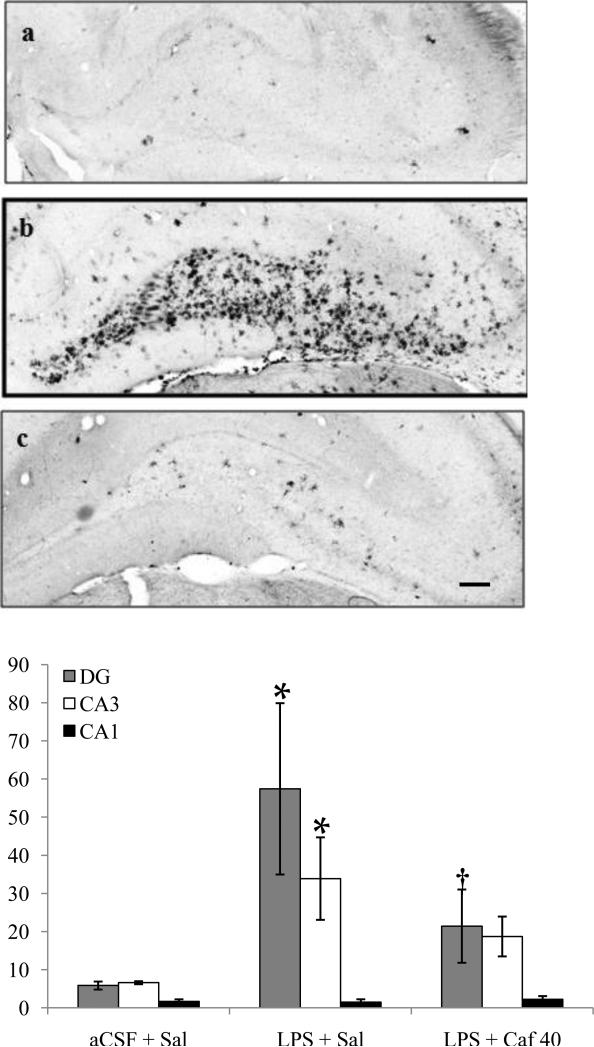
Figure 1.
(Top) Photomicrographs of dorsal hippocampus showing MHCII IR microglia within the hippocampus after two weeks of aCSF (a) or LPS infusion into the 4th ventricle and treated with saline (b) or caffeine (c, 40 mg/kg/d, i.p.), scale bar = 150 μm. (Bottom) Quantification (microglia/mm2, mean ± SEM) of activated microglia were determined in two sections from each rat in both hemispheres and from three different hippocampal regions after two weeks of saline or caffeine (40 mg/kg/d, i.p.) administration. LPS + Sal had significantly (*p<0.001) more MHCII-IR microglia in the DG than did aCSF + Sal. LPS + Caf 40 had significantly (†p<0.001) fewer MHCII-IR microglia in the DG than did LPS + Sal, and did not differ significantly from aCSF + Sal.
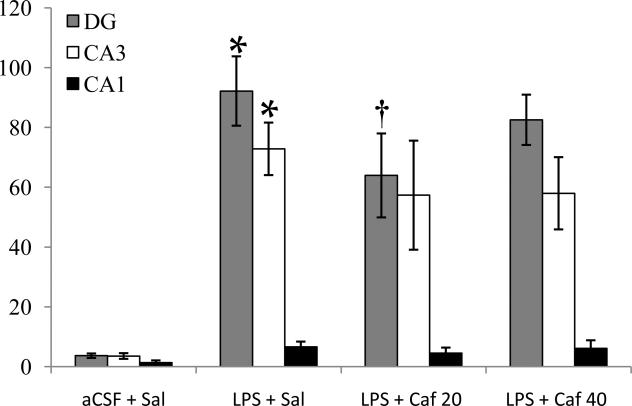
Figure 2.
Quantification (microglia/mm2, mean ± SEM) of activated microglia (MHC II IR) in three different regions after four weeks of saline or caffeine (20 or 40 mg/kg/d, i.p.) administration. LPS + Sal had significantly (*p<0.001) more MHCII-IR microglia in the DG and CA3 than did aCSF + Sal. LPS + Caf 20 had significantly (†p=0.031) fewer MHCII-IR microglia in the DG than did LPS + Sal.
Because recent evidence suggests that caffeine's effectiveness may be related to age [12] we subsequently administered caffeine (40 mg/kg/d, i.p.) or saline (1 ml/kg, i.p.) for two weeks to a group of aged (24 mo) F-344 male rats. Normal aging was associated with a significant (p<0.05) increase in the number of activated microglia within the CA3 region and DG, but not within CA1 (Figure 3). Caffeine administration significantly (p<0.05) reduced the number of activated microglia within the CA3 hippocampal region of the aged rats.
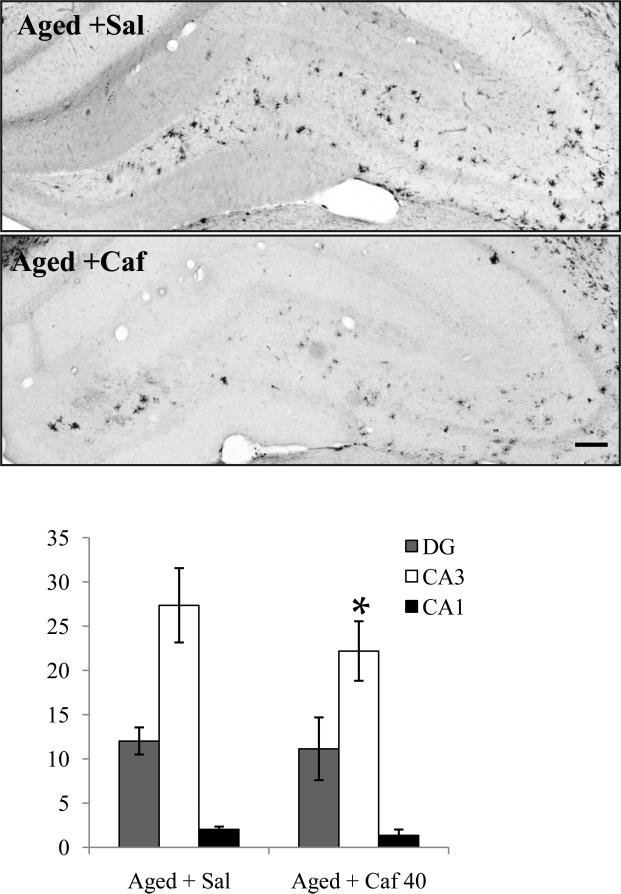
Figure 3.
(Top) Photomicrographs of dorsal hippocampus showing MHCII IR microglia within the hippocampus of aged rats treated with saline or caffeine (40 mg/kg/d, i.p.) over two weeks, scale bar = 150 μm. (Bottom) Quantification of activated microglia (microglia/mm2, mean ± SEM) in three hippocampal regions. Aged + Caf 40 had significantly (*p<0.05) fewer MHCII-IR microglia in the CA3 region than did Aged + Sal.
The current study demonstrated the ability of caffeine to significantly reduce LPS-induced microglia activation in young rats in a manner that was influenced by the duration of treatment and dose. We estimated that a dose of 40 mg/kg in a rat was roughly equivalent to 3-5 cups of coffee per day, a dose that, according to epidemiological findings, is neuroprotective in humans [1, 6, 28] and, for a 0.3 kg rat, an equivalent dose for a 70 kg human of approximately 400 mg/day (calculated as mg/kg rat × (kg-rat/kg-human)0.33). However, if the metabolic rate of a rat is corrected for, 20 mg/kg is equivalent to approximately 4-6 cups of coffee and 40 mg/kg is the equivalent of 6-9 cups [14]. Consistent with this concept, drinking 3 cups of coffee per day provided the optimal protection against cognitive decline, 4 cups was protective but less so, and drinking 5 or more cups per day was not protective in a large prospective study of the FINE cohort [40]. This discovery may explain why a dose of 40 mg/kg/day was effective in both young and aged rats when administered over a 2 week period, but was not optimal compared to a dose of 20 mg/kg/day when administered over a period of 4 weeks.
Chronic neuroinflammation is associated with dysregulation of glutamate neurotransmission that may contribute to the pathogenesis and cognitive decline associated with age-related neurodegenerative disorders [35, 36]. We have previously demonstrated that the antagonism of glutamate neurotransmission post-synaptically at the NMDA channel, via memantine, significantly reduced LPS-induced microglial activation in young rats [29, 35, 37]. Here we document a reduction in the number of activated microglial within the hippocampus in response to antagonism of adenosine A1 and A2a receptors in the presence of both experimentally induced neuroinflammation in young rats and naturally occurring neuroinflammation that is typically present in aged rats. Taken together with previous results [6, 14] the current study suggests that caffeine, via direct action at microglia and manipulation of glutamate neurotransmission, at an appropriate dose and duration that is yet to be defined, may restore this equilibrium in the brain by reducing the impact of microglial activation that potentially underlies aspects of age-associated neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases, that have a prominent neuroinflammatory component.
Acknowledgments
Supported by the U.S. Public Health Service, RO1 AG030331 to GLW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ascherio A, Zhang SMM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann. Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 2.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNF alpha: amplification by microglia triggers neurotoxicity. Nat. Neurosi. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 3.Bibbiani F, Oh J, Petzer J, Castagnoli NJ, Chen J, Schwarzschild M, Chase T. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson's disease. Exp Neurol. 2003;184:285–294. doi: 10.1016/s0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- 4.Blasko I, Marx F, Steiner E. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Carta AR, Kachroo A, Schintu N, Xu K, Schwarzschild MA, Wardas J, Morelli M. Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neurochem. 2009;111:1478–1489. doi: 10.1111/j.1471-4159.2009.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alz Dis. 2010 doi: 10.3233/JAD-2010-1408. in press. [DOI] [PubMed] [Google Scholar]
- 7.Dai S-S, Zhou Y-G, Li W, An J-H, Li P, Yang N, Chen X-Y, Xiong R-P, Liu P, Zhao Y, Shen H-Y, Zhu P-F, Chen J-F. Local glutamate levels dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci. 2010;30:5802–5810. doi: 10.1523/JNEUROSCI.0268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortex A, Canela EL, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A(1)-A(2A) receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Mendoca A, Sebastiao AM, Ribeiro JA. Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor. NeuroRep. 1995;6:1097–1100. doi: 10.1097/00001756-199505300-00006. [DOI] [PubMed] [Google Scholar]
- 11.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Ann Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Eskelinen M, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 13.Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- 14.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:84–133. [PubMed] [Google Scholar]
- 15.Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- 16.Hauss-Wegrzyniak B, Vraniak P, Wenk GL. The effects of a novel NSAID upon chronic neuroinflammation are age dependent. Neurobiol. Aging. 1999;20:305–313. doi: 10.1016/s0197-4580(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 17.Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol. Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann NY Acad. Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann KW, Schuh AF, Saute J, Townsend R, Fricke D, Leke R, Souza DO, Portela LV, Chaves ML, Rieder CR. Interleukin-6 serum levels in patients with Parkinson's disease. Neurochem Res. 2009;34:1401–1404. doi: 10.1007/s11064-009-9921-z. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson KA, von Lubitz DKJE, Daly JW, Fredholm BB. Adenosine receptor ligands: Differences with acute versus chronic treatment. Tr Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson B, Ahlberg S, Vanderploeg I, Brene S, Lindefors N, Persson H, Fredholm BB. Effect of long-term caffeine treatment on A(1) and A(2) adenosine receptor-binding and on messenger-RNA levels in rat-brain. N-S Arch Pharm. 1993;347:407–414. doi: 10.1007/BF00165391. [DOI] [PubMed] [Google Scholar]
- 22.Lupica CR, Berman RF, Jarvis MF. Chronic theophylline treatment increases adenosine-A1, but not adenosine-A2, receptor-binding in the rat-brain - an autoradiographic study. Synapse. 1991;9:95–102. doi: 10.1002/syn.890090203. [DOI] [PubMed] [Google Scholar]
- 23.Kalda A, Yu L, Oztas E, Chen JF. Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson's disease. J Neurol Sci. 2006;248:9–15. doi: 10.1016/j.jns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi S, Fujishita K, Tsuda M, Inoue K. Neurone-to-astrocyte communication by endogenous ATP in mixed culture of rat hippocampal neurons and astrocytes. Drug Dev. Res. 2003;59:88–94. [Google Scholar]
- 25.Li Y, Cao YP. Changes in intracellular calcium in brain cells of aged rats. Neural Regener. Res. 2008;3:1026–1029. [Google Scholar]
- 26.Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neurosci. 2002;112:319–329. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 27.Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A(1) and A(2A) adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 28.Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer's disease? Eur. J. Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 29.Marchalant Y, Rosi S, Wenk GL. Anti-inflammatory property of the cannabinoid agonist WIN-55212-2 in a rodent model of Alzheimer's disease. Neurosci. 2007;144:1516–1522. doi: 10.1016/j.neuroscience.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M. Effects of adenosine A(1) and A(2A) receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br. J. Pharmacol. 2002;136:434–440. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintor A, Galluzzo M, Grieco R, Pezzola A, Reggio R, Popoli P. Adenosine A(2A) receptor antagonists prevent the increase in striatal glutamate levels induced by glutamate uptake inhibitors. J. Neurochem. 2004;89:152–156. doi: 10.1111/j.1471-4159.2003.02306.x. [DOI] [PubMed] [Google Scholar]
- 32.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Rebola N, Rodrigues RJ, Lopes LV, Richardson PJ, Oliveira CR, Cunha RA. Adenosine A1 and A2A receptors are co-expressed in pyramidal neurons and co-localizd in glutamatergic nerve terminals of the rat hippocampus. Neurosci. 2005;133:79–83. doi: 10.1016/j.neuroscience.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 34.Rosi S, McGann K, Hauss-Wegrzyniak B, Wenk GL. The influence of brain inflammation upon neuronal adenosine A2B receptors. J. Neurochem. 2003;86:220–227. doi: 10.1046/j.1471-4159.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esperanza E, Larkin P, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–2486. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters selective patterns of behaviorally induced Arc expression in the hippocampus. J. Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally- induced gene expression and spatial learning in the rat. Neurosci. 2006;142:1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Rothwell NJ, Allan S, Toulmond S. The role of interleukin-1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J. Clin. Invest. 1997;100:2648–2652. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Gelder B, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur. J. Clin. Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 41.Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J. Cell Physiol. 2005;202:9–20. doi: 10.1002/jcp.20138. [DOI] [PubMed] [Google Scholar]