Abstract
Background
The mesolimbic dopamine (DA) system is implicated in the development and maintenance of alcohol drinking; however, the exact mechanisms by which DA regulates human alcohol consumption are unclear. This study assessed the distinct effects of alcohol-related cues and alcohol administration on striatal DA release in healthy humans.
Methods
Subjects underwent 3 PET scans with [11C]raclopride (RAC). Subjects were informed that they would receive either an IV Ringer’s lactate infusion or an alcohol (EtOH) infusion during scanning, with naturalistic visual and olfactory cues indicating which infusion would occur. Scans were acquired in the following sequence: (1) Baseline Scan: Neutral cues predicting a Ringer’s lactate infusion, (2) CUES Scan: Alcohol-related cues predicting alcohol infusion in a Ringer’s lactate solution, but with alcohol infusion after scanning to isolate the effects of cues, and (3) EtOH Scan: Neutral cues predicting Ringer’s, but with alcohol infusion during scanning (to isolate the effects of alcohol without confounding expectation or craving).
Results
Relative to baseline, striatal DA concentration decreased during CUES, but increased during EtOH.
Conclusion
While the results appear inconsistent with some animal experiments showing dopaminergic responses to alcohol’s conditioned cues, they can be understood in the context of the hypothesized role of the striatum in reward prediction error, and of animal studies showing that midbrain dopamine neurons decrease and increase firing rates during negative and positive prediction errors, respectively. We believe that our data are the first in humans to demonstrate such changes in striatal DA during reward prediction error.
Keywords: Alcohol, Alcohol Cues, Dopamine, PET, Prediction Error
Conditioned cues associated with alcohol can induce craving in humans (Carter and Tiffany, 1999), which results in alcohol-seeking and consumption (e.g., Cooney et al., 1997; Litt et al., 2000). It has been hypothesized that cue-induced activation of the mesolimbic dopamine (DA) system leads to drinking behavior (Weiss et al., 1993). Animal studies of alcohol self-administration have shown that extracellular DA concentration ([DA]) in the nucleus accumbens (NAc) increases while animals wait in operant chambers for access to alcohol. (Gonzales and Weiss, 1998; Katner et al., 1996; Melendez et al., 2002; Weiss et al., 1993). While these studies observed the effects of environmental cues on DA levels, Doyon et al. (2003, 2005) concluded that the initial perception of the olfactory/gustatory properties of alcohol transiently elevates NAc [DA]. Similarly, others have shown that NAc [DA] increases as a result of exposure to conditioned stimuli associated with cocaine (Ito et al., 2000; Phillips et al., 2003; Weiss et al., 2000). Taken together, these studies support a relationship between dopaminergic activity in the NAc and presentation of alcohol- and drug-related cues (Berridge, 2007; Robinson and Berridge, 1993).
In humans, brain areas associated with reward are activated during cues that elicit craving. Using fMRI, we showed that alcoholic drink odors increased blood oxygen level dependent (BOLD) responses in the NAc of risky drinkers (Bragulat et al., 2008; Kareken et al., 2004a). Myrick and colleagues (2004) found that images of alcoholic beverages increased ventral striatal BOLD signals in alcoholic patients (see also Braus et al., 2001; Wrase et al., 2007). Cue-induced limbic, cortical, and striatal activity have also been correlated with craving (Modell and Mountz, 1995; Myrick et al., 2004) and alcohol intake (Grusser et al., 2004).
The neurochemical basis of cue reactivity and craving in humans is less clear. In parallel with the animal studies cited above, Boileau et al. (2007) reported that conditioned contextual cues of amphetamine administration lead to increased ventral striatal [DA] release. Although several studies have examined the relationship between DA and drug craving, the literature is equivocal. In detoxified alcoholics, low DA synthesis capacity and low DA receptor availability are associated with higher craving (Heinz et al., 2005; Heinz et al., 2004). Nicotine-induced striatal DA release is associated with diminished urge to smoke in nicotine-dependent subjects (Brody et al., 2004), which similarly suggests an underlying hypoactive DA system. In contrast, both Wong et al. (2006) and Volkow et al. (2006b) reported that the intensity of cue-induced craving is correlated with cue-induced increases in [DA] in the dorsal striatum of cocaine users.
We used [11C]raclopride (RAC) PET to study the relationship between striatal [DA] and the sight and smell of preferred alcoholic drinks, which were used to predict intravenous (IV) alcohol administration. As PET methodology cannot resolve the individual contributions of multiple stimuli (e.g., anticipation of administration, physiological effects of alcohol) to changes in [DA], we isolated the effects of cue-induced anticipation from the pharmacological effects of alcohol by using separate scan sessions. Generalizing from the animal literature and our work with IV alcohol (Yoder et al., 2005, 2007), we initially hypothesized that conditioned cues predicting alcohol would increase striatal [DA], while alcohol administration would not. However, ventral striatal [DA] instead varied systematically in response to alcohol cues and unexpected alcohol exposure in a manner consistent with the hypothesized role of the dopamine system in detecting reward prediction errors (Mirenowicz and Schultz, 1994; Pan et al., 2005; Schultz et al., 1993, 2000).
MATERIALS AND METHODS
Subjects
Eight healthy Caucasian subjects (Table 1) signed informed consent statements agreeing to participate in the study, which was approved by the Indiana University Institutional Review Board. None had any history of psychiatric or neurological disease as determined by interview, and none were drug or alcohol dependent according to the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994). Two subjects had a family history of alcoholism. Five subjects (2 female) surpassed the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993) threshold of 8 for hazardous drinking (Conigrave et al., 1995). All were screened with the University of Pennsylvania Smell Identification Test to rule out problems smelling the olfactory cues (Doty et al., 1984, 1989). All subjects completed the Timeline Followback Interview (Sobell et al., 1986) for assessment of drinking habits.
Table 1.
Subject Characteristics
| Mean | SD | n | % | |
|---|---|---|---|---|
| Age | 23.8 | 4.03 | ––– | ––– |
| Male | ––– | ––– | 5 | 62.5 |
| AUDIT | 7.0 | 2.88 | ––– | ––– |
| Drinks/weeka | 11.14 | 8.18 | ––– | ––– |
| Drinks/montha | 46.5 | 34.4 | ––– | ––– |
| Drinks/drinking daya | 4.57 | 1.76 | ––– | ––– |
| Subjects reporting FHAb | ––– | ––– | 4 | 50.0 |
| # Relatives | 2.25 | (Range: 1–4) | ––– | ––– |
SD, standard deviation; AUDIT, Alcohol Use Disorders Identification Test; FHA, family history of alcoholism; includes report of any first- or second degree relatives with alcohol use disorders.
Assessed by the Timeline Followback Interview (Sobell et al., 1986).
Two subjects were “unambiguously” family-history positive for alcoholism (defined as reporting at least 2 relatives, with at least one being a first-degree relative). One subject reported only 1 first-degree relative; the other reported 2 second-degree relatives.
Scanning Procedures
[11C]raclopride (RAC, a selective DA D2/D3 receptor antagonist) was synthesized as reported previously (Fei et al., 2004). Subjects underwent 3 RAC PET scans (EXACT HR+, CTI; Knoxville, TN) over 2 days. PET data were acquired with septa retracted (3D mode). Full width half maximum (FWHM) was 9 mm when images were reconstructed with a 5 mm Hanning filter. Radiochemical purity was > 99%. Scans were initiated with the IV injection of (mean ± SD) 14.1 ± 0.99 mCi of RAC; total mass injected was 15.1 ± 5.69 nmol per subject per scan. Dynamic data acquisition lasted 45 minutes [An analysis of ventral striatal time-activity curves (TACs) from a previous study (Yoder et al., 2005) demonstrated that BPND values from 45 minutes of scan data were only 2.5% lower than BPND values estimated from 60 minutes of data. BPND values from the 45- and 60-minute datasets were highly correlated (R2 = 0.99; p < 3.4 × 10−14)]. A heavily T1-weighted, spoiled gradient recalled (SPGR) magnetic resonance image (MRI; 1.5T GE Echospeed LX, GE; Waukesha, WI) was acquired in each subject for subsequent spatial normalization of image data into Montreal Neurological Institute (MNI) stereotactic space.
Behavioral Paradigm
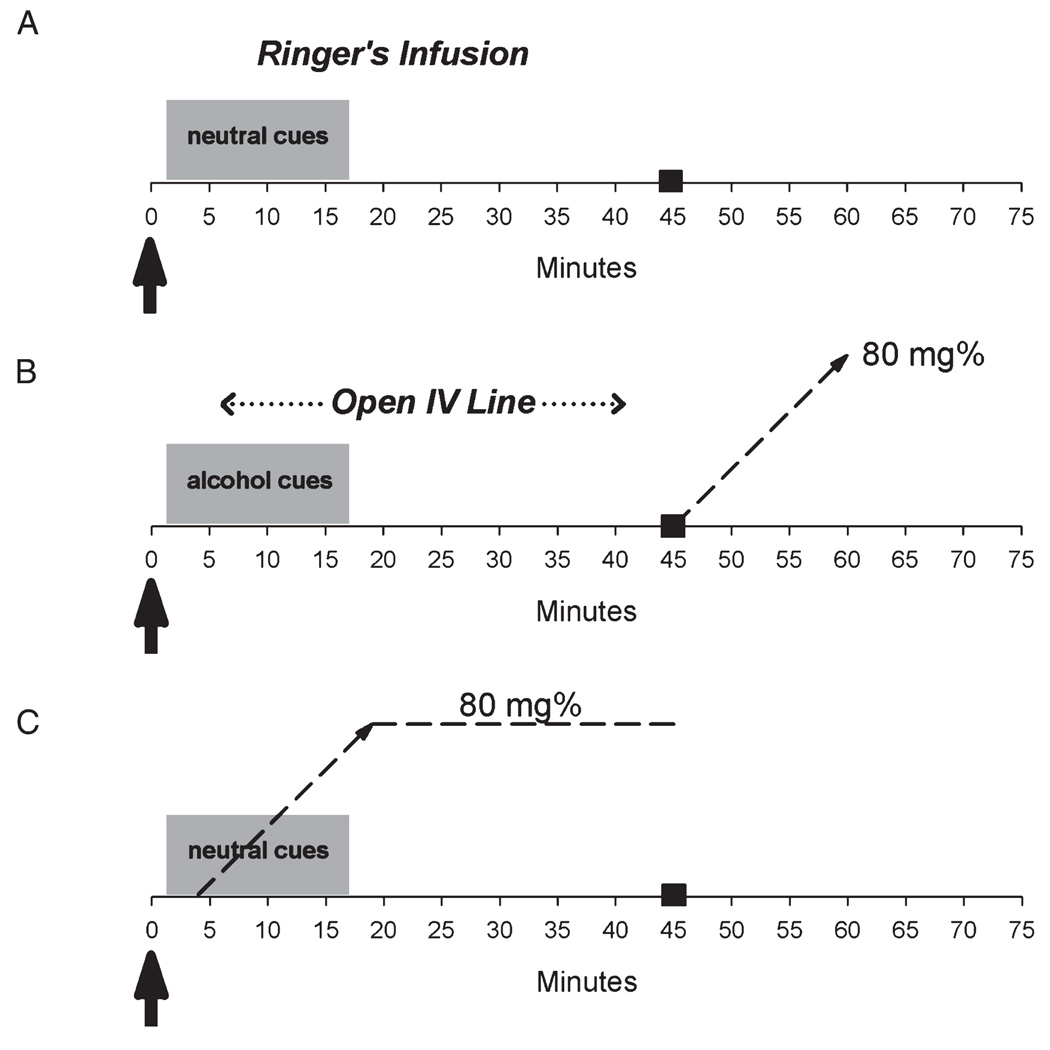
A schematic of the 3 conditions is presented in Fig. 1. Subjects were informed that what they saw and smelled would predict what would happen to them during scanning. Specifically, they were instructed that if they saw and smelled leather and lilac, they would receive an infusion of Ringer’s lactate (no alcohol), and that if they saw and smelled their favorite alcoholic beverages, they would receive an alcohol infusion to an intoxicating level.
Fig. 1.
Graphic depiction of scanning protocols. See text for details. Arrows indicate [11C]raclopride injection and beginning of image acquisition. Dotted lines represent the alcohol infusion profiles, with black box at 45 minutes indicating scan end time. (A) Baseline (BL) scan; Ringer’s solution was infused using the same infusion rate parameters determined for each subject’s alcohol scan. (B) Olfactory and visual alcohol cues (CUES) scan; the IV line was kept open during scanning, alcohol infusion was started after scanning. (C) Alcohol (EtOH) infusion scan.
Cue Stimulation
Neutral or alcohol cues were started 2 minutes after RAC injection, and were maintained for 15 minutes. Visual cues were placed on a rotating table behind the scanner gantry (viewed through mirror goggles). Two sets of objects (neutral cues: scraps of tanned leather and plastic lilac flowers; alcohol cues: a subject’s 2 favorite alcoholic beverages, e.g., a filled glass of beer next to a beer bottle, a glass of wine next to a wine bottle) were set on the table and separated by an opaque divider. The table rotated every 75 seconds, and each side was displayed 6 times. Visual displays were accompanied by presentation of the corresponding olfactory stimulus.
Olfactory stimuli were delivered with a computer-controlled olfactometer (Kareken et al., 2004a, b) through a polytetrafluoroethylene (PTFE) nasal cannula that was mounted on the scanner gantry and positioned approximately one inch in front of the subject’s nose. The cannula delivered a constant airflow of 2.0 liters per min of airflow throughout the imaging session, with odors injected into the constant air-stream during two 10 second odor periods during each visual display period. The first odor in the 75-second display period began 3 seconds after the visual display came into view; the second odor began 27 seconds later. Lilac and leather odors were provided by International Flavors and Fragrances (Union Beach, NJ). Alcohol-related odors were produced by bubbling air from the olfactometer through each subject’s 2 most preferred alcoholic beverages.
The 3 scan conditions occurred in the following fixed order: (1) Baseline (BL): Neutral cues with a lactated Ringer’s infusion; (2) Alcohol cues (CUES): Alcohol cues, with alcohol infusion after scanning to fulfill the promise of alcohol delivery; (3) Alcohol (EtOH): Neutral cues, but with alcohol infusion during scanning (thus violating the subject’s expectation and avoiding anticipation of alcohol). A fixed-order design was used for several reasons. The last scan, the alcohol condition, involved a direct deception. A critical factor in execution of this experiment was that the subjects maintain faith in the veracity of the predictive stimuli. Had a randomized scan order been used, the deception condition could have come either first or second. In that scenario, a subject would have been deceived before completing the entire protocol, and would have been highly unlikely to believe anything that the stimuli were supposed to have predicted in subsequent conditions, thus confounding the remaining scan(s).
Alcohol Infusion
The physiologically-based pharmacokinetically (PBPK) modeled IV alcohol clamp (O’Connor et al., 1998; Ramchandani et al., 1999) was used to control the exact timing of alcohol delivery and minimize the experimental variation in the brain’s exposure to alcohol across subjects. Oral ingestion of alcohol causes highly variable rates and concentrations of brain alcohol exposure as a result of inter-subject differences in stomach pH, volume of stomach contents, age, gender, and first-pass metabolism. Different brain alcohol concentrations are likely to cause different magnitudes of dopamine responses across subjects. In addition, the timing of alcohol exposure is likely to affect the timing of dopamine release. We have shown that if the timing of DA release is not held constant, then the outcome measure of [11C]RAC binding potential could be confounded (Yoder et al., 2004). The PBPK model was therefore used to specify individual alcohol infusion profiles based on each subject’s height, weight, and gender. Profiles were calculated such that an IV infusion of alcohol (6% vol/vol in lactated Ringer’s) would achieve a breath alcohol concentration (BrAC) of 80 mg% (0.08) for both scans involving alcohol infusion. This infusion profile was also used for the Ringer’s lactate infusion during Scan 1. For alcohol infusion following the CUES condition (scan 2), infusion began immediately after PET image acquisition was completed, continued for 15 minutes (the time at which BrAC was calculated to reach the 80 mg% target), and then stopped. For scan 3 (EtOH), alcohol infusion began 4 minutes after RAC injection, ascended over 15 minutes to the target based on model calculations, and was then clamped to maintain the 80 mg% target throughout the remaining 25 minutes of image acquisition (Fig. 1). Following imaging in scans 2 and 3, a BrAC sample was taken using a Dräger Alcotest® 7410 handheld breath meter to determine the subject’s actual breath alcohol level. Subjects were thoroughly debriefed following scan 3 about the need for the experimental deception.
Subjective Impressions
Subjects were assessed for desire to drink (craving) using the Alcohol Urge Questionnaire (AUQ; Bohn et al., 1995) before scanning. After scanning, subjects completed a second AUQ, with the items modified to refer retrospectively to how they felt “while in the scanner, and while seeing and smelling the items on the table.”
During scanning, subjects rated how “high” (up, stimulated, feeling good) and how “intoxicated” (drunk, inebriated, tipsy) they felt during imaging, using a modified Subjective High Assessment Scale (SHAS; Schuckit and Gold, 1988). In our modified version, subjects spoke a number ranging from 0 (same as before infusion) to 100 (most high or intoxicated ever experienced). Subjects were prompted for responses every 10 minutes during imaging. Subjects were informed that they would be assessed for their feelings of “high” and “intoxication” throughout all 3 scan sessions, regardless of the kind of infusion (alcohol or saline) they received.
Subject Expectations
After each scan, subjects were asked to rank their subjective expectations about what they believed would occur during scanning. Subjects rated 2 statements (“It was clear that I was about to get drunk” and “I knew that I was not about to get drunk”) on a visual-analog scale anchored by 1 (strongly disagree) to 7 (strongly agree).
Image Processing
Image processing procedures were as previously described (Yoder et al., 2007). MRI and PET images were converted to Analyze format using MRIcro software (http://www.sph.sc.edu/comd/rorden/mricro.html). All subsequent data processing steps were performed with SPM2 software (http://www.fil.ion.ucl.ac.uk/spm/). For each scan, a summed image was created from the first 10 minutes of dynamic [11C]RAC data using the Realign function in SPM2. These summed images contained a mixture of blood flow and specific striatal D2/D3 binding, permitting accurate registration of all time frames to a single image. The summed image was co-registered to the individual subject’s MRI scan using SPM2. Motion correction was achieved by coregistering individual PET frames to the coregistered, summed PET image. Each subject’s MRI was normalized into Montreal Neurological Institute (MNI) stereotactic space using SPM2’s default normalization parameters. The transformation matrix obtained from this normalization step was applied to the motion-corrected, coregistered PET images from each subject, thus placing all dynamic PET data in MNI stereotactic space.
Parametric Binding Potential Images
The binding potential of RAC is an index of the number of DA D2/D3 receptors that are available for binding, and is operationally defined as Bavail/KD. Binding potential will be denoted herein as BPND, that is, binding potential calculated as bound tracer concentration relative to nondisplaceable tracer concentration (Innis et al., 2007). Changes in BPND can be used as indices of change in [DA] (Innis et al., 2007; Laruelle, 2000). If RAC BPND values from an experimental scan condition are different from baseline BPND values, the changes in BPND are presumed to be caused by changes in endogenous [DA] (Dewey et al., 1992, 1993; Seeman et al., 1989; Young et al., 1991). Increases in BPND relative to baseline indicate decreases in [DA], and decreases in BPND relative to the baseline BPND indicate increases in [DA].
Parametric BPND images were generated as described previously (Yoder et al., 2007), using a multilinear reformulation of the Logan reference region graphical analysis (Ichise et al., 2002; Logan et al., 1996). The parametric whole brain BPND images were smoothed with an 8 mm Gaussian kernel (Costes et al., 2005; Picard et al., 2006; Ziolko et al., 2006). We restricted the search area for the voxel-wise paired t-tests to the striatum, as (1) our sole focus was the striatum, and (2) the striatum has the highest density of D2/D3 receptors in the brain, and is the only brain structure with high enough signal-to-noise ratio to support quantification of D2/D3 receptor availability with [11C]RAC. A bilateral striatal binary mask (created from basal ganglia ROIs from MARINA http://www.bion.de/index.php?title=MARINA&lang=eng) was applied to the whole brain parametric images to create striatal parametric images. The striatal parametric images were used for SPM analysis.
Dopamine responses, which include increases and decreases in [DA], can be indexed by change in BPND (ΔBPND), defined here as (BPND1−BPND2)/BPND1. BPND1 refers to the BL scan, and BPND2 refers either to the CUES or EtOH condition. Positive ΔBPND values indicate increases in [DA], and negative ΔBPND values reflect decreases in DA levels. Striatal ΔBPND maps were created from the parametric BPND images using the ImCalc function in SPM2 for each subject. These maps of changes in [DA] were used as the dependent measures for the CUES and EtOH conditions.
Voxel-Wise Statistics
To test for effects of CUES and EtOH conditions on [DA] via changes in BPND, one-sample voxel-wise t-tests were conducted on the striatal ΔBPND maps in SPM2 to test the null hypothesis that ΔBPND = 0 at each voxel.
Other Statistics
Repeated-measures analysis of variance (ANOVA) was used to test for: (1) changes in pre-scan and postscan AUQ, (2) changes in SHAS scores during the respective scanning periods, and (3) changes in subject expectations. One-way ANOVA was used to test for differences between scan conditions in mCi injected and mass dose injected. Pearson’s correlation coefficient was used to test for exploratory relationships among variables. Statistical significance was set at p <0.05.
RESULTS
Cue Paradigm Validity
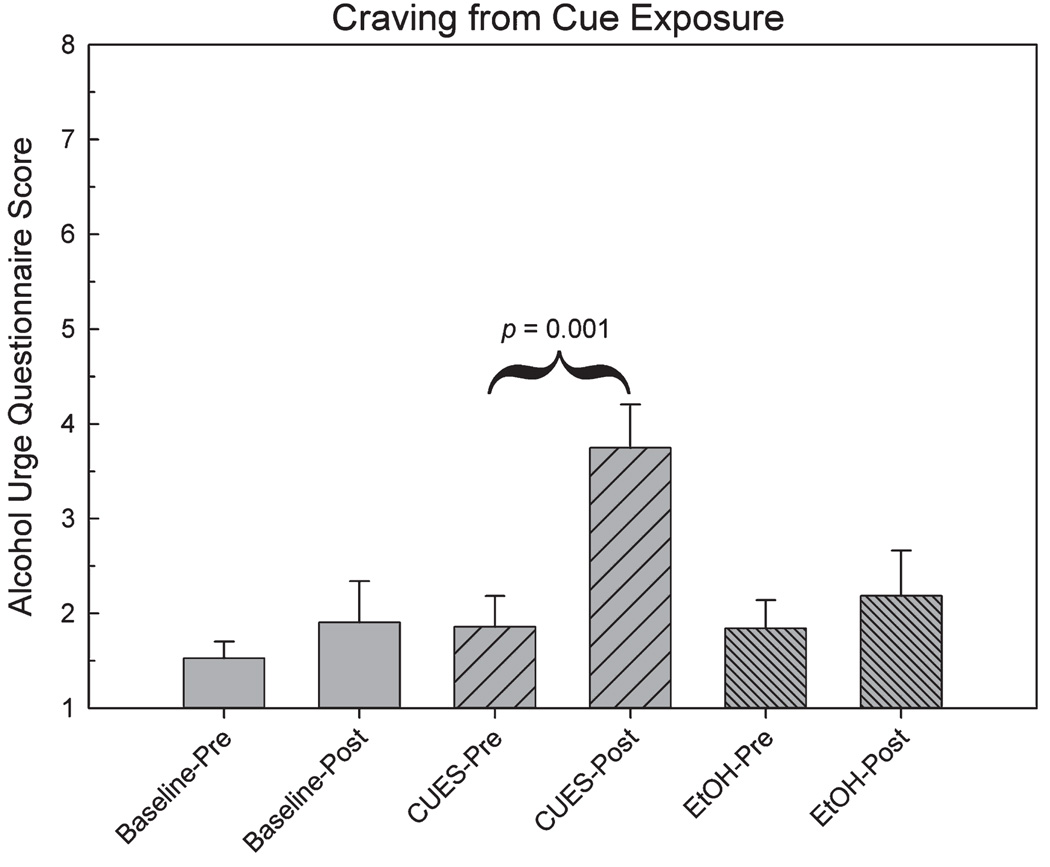
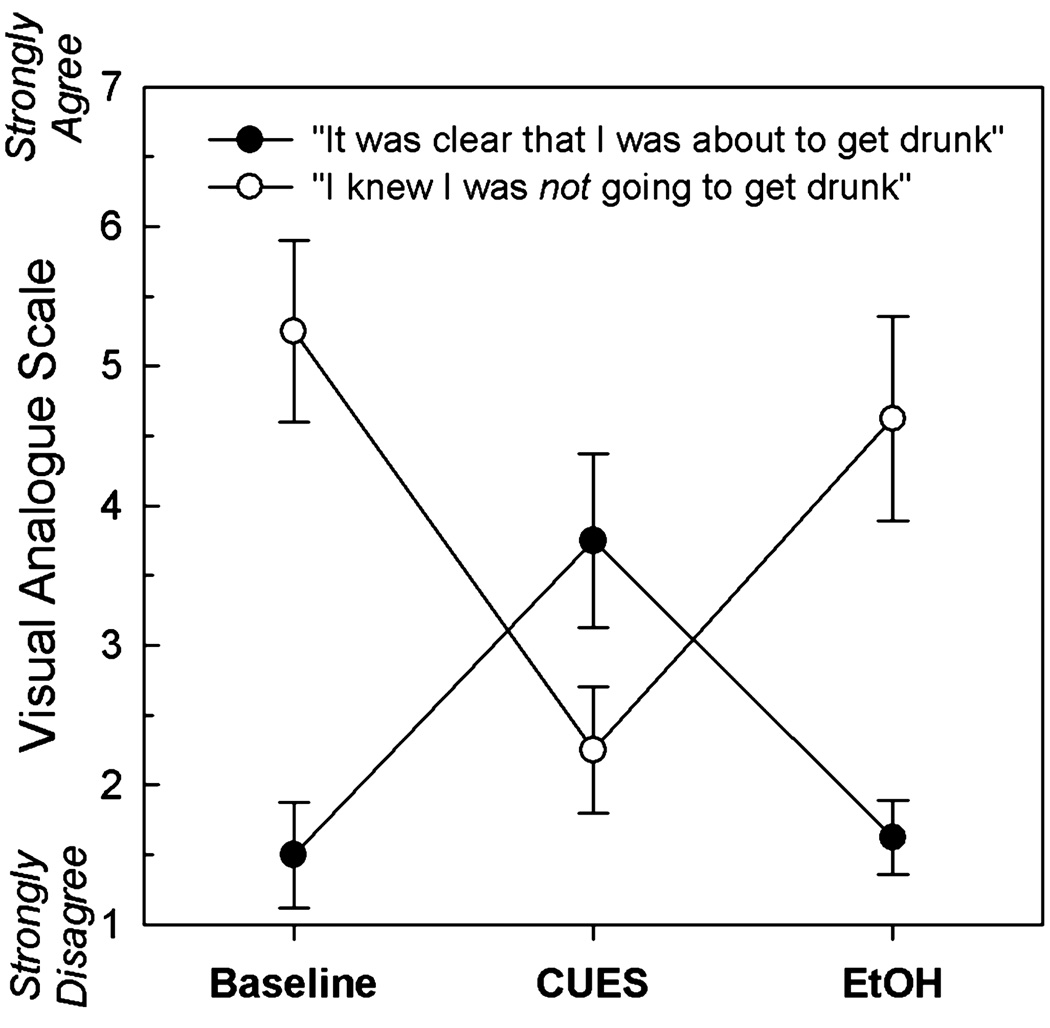
To test the validity of the behavioral paradigm, we tested for differences in pre- and postscan AUQ craving scores across all scan sessions using a repeated-measures ANOVA. The omnibus test revealed significant differences across all time points (F5,35 = 17.90, p < 0.001). A planned contrast showed a significant difference in AUQ scores between measurements made before and after the CUES scan (F1,7 = 33.67, p = 0.001, Fig. 2), in which the visual and olfactory stimuli signified impending alcohol infusion. This result suggests that the multi-sensory paradigm was successful in evoking a significant desire for alcohol. The cues also successfully directed subjects’ expectations in the direction intended by the cues, with significant changes across scans for the questions: “It was clear that I was about to get drunk” (F2,14 = 9.38, p = 0.003) and “I knew that I was not about to get drunk” (F2,14 = 7.81, p = 0.005). For each question, planned comparisons showed that expectations at baseline and during EtOH (both of which involved neutral cues) were significantly different than during the CUES scan, which involved the alcohol cues (p’s < 0.05; Fig. 3).
Fig. 2.
Alcohol Urge Questionnaire (AUQ) scores before and after each scan condition. Alcohol-related cues significantly increased the postscan AUQ score.
Fig. 3.
Subject expectations after cue presentation.
Subjective Effects of Alcohol
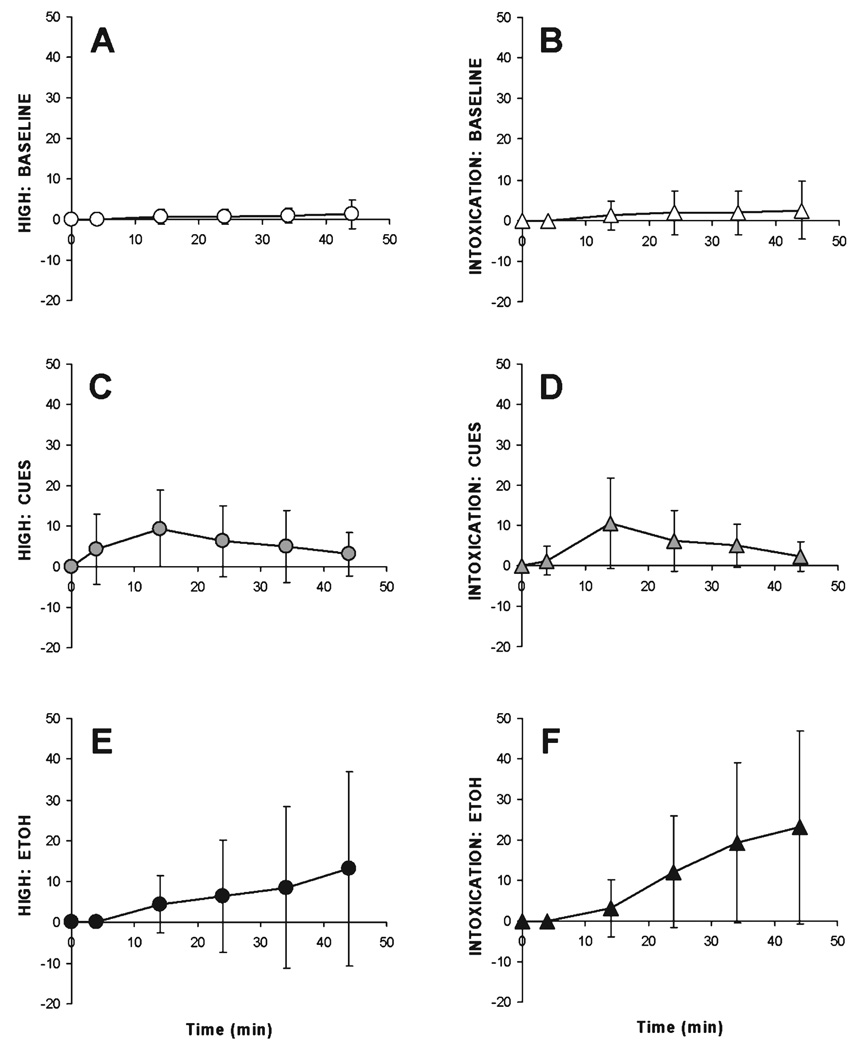
Following alcohol infusion during scan 3, the actual mean BrAC as measured was 79 ± 11 mg%, which compares favorably with the pharmacokinetic model target of 80 mg%. SHAS data for perceived “High” and “Intoxication” were analyzed separately for each scan with a 2(SHAS) × 6(Time) repeated-measures ANOVA, using polynomial contrasts to test for linear trends across the duration of the scan (see Fig. 4). There were no significant changes in subjective impressions of the effects of alcohol during the course of BL image acquisition. While there were some significant changes in perceived effects during the course of the CUES scan (omnibus F1,7 = 7.56, p < 0.05, Greenhouse-Geisser correction), the changes were not highly linear over time (p = 0.1). During the EtOH scan, there were significant changes in subjective ratings over time (omnibus F5,35 = 4.96, p < 0.005), as well as a significant interaction between “High” and “Intoxication” (F5,7 = 5.18, p = 0.001), with “Intoxication” showing a more distinct linear trend over time (F1,7 = 7.53, p < 0.05). “High” showed a borderline level of omnibus significance (p = 0.051), but did not exhibit a significant linear trend (p = 0.14).
Fig. 4.
Subjective High Assessment Score (SHAS) scores (mean ± SD) for high (circles, left panels) and intoxication (triangles, right panels) during the 3 scan conditions. For all scans, cue presentation began 2 minutes after [11C]raclopride injection. Ringer’s infusion (Baseline) or alcohol infusion (ETOH) began 4 minutes after [11C]raclopride injection. (Panels A, B) Baseline. (C, D) CUES. (E, F) EtOH.
Imaging Results
There were no differences in either mCi injected or mass dose injected between scan conditions. mCi injected for baseline, CUES, and ETOH was 13.9 ± 1.30, 14.4 ± 0.64, and 14.1 ± 1.00, respectively. Mass dose injected (nmol/subject) was 14.6 ± 4.20, 14.5 ± 6.78, and 16.4 ± 6.33 for baseline, CUES, and ETOH conditions, respectively.
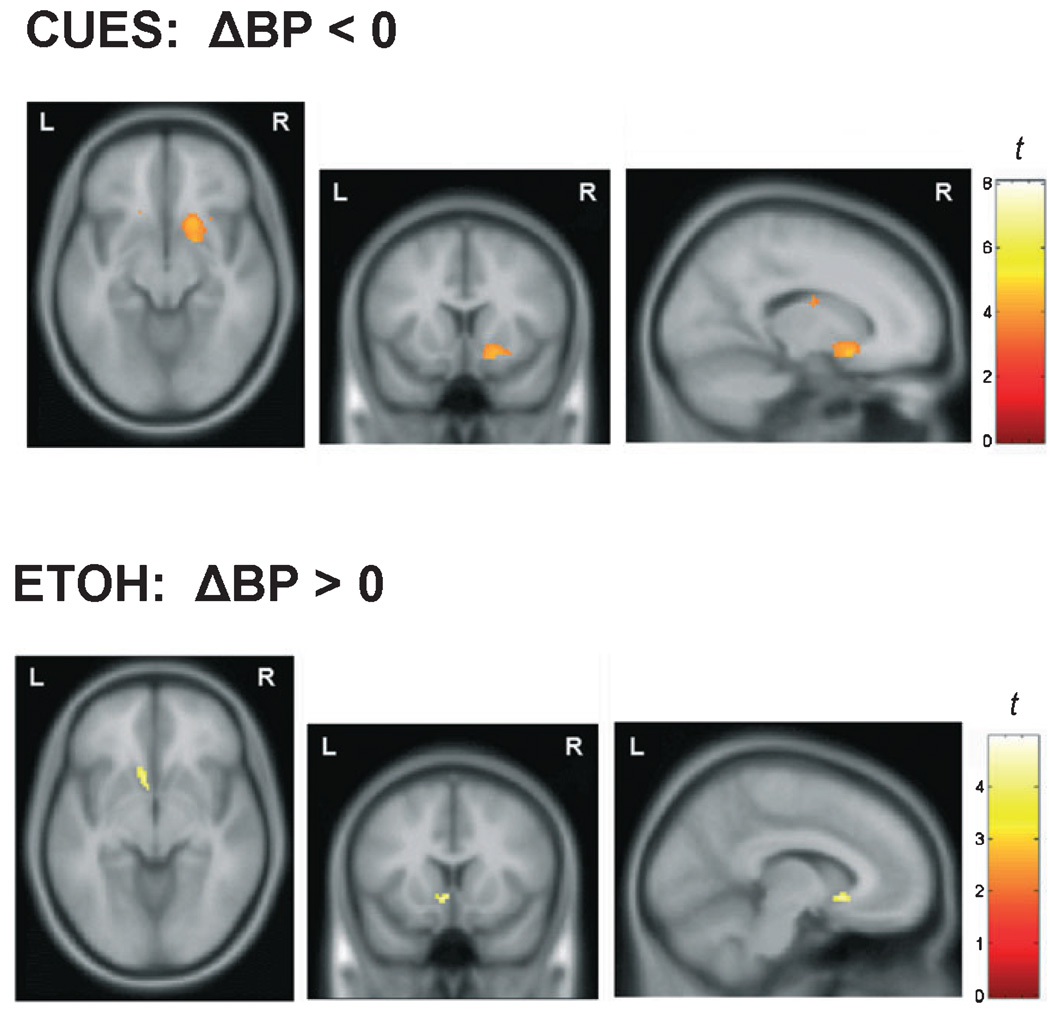
Contrary to the hypotheses, striatal DA concentration did not increase during the CUES condition relative to BL. Instead, the voxel-wise analysis showed a cluster of voxels in the right ventral striatum where ΔBPND was significantly negative (Fig. 5, top), reflecting a decrease in DA concentration. The average ΔBPND value of the voxels in this cluster was −0.20 ± 0.13 (i.e., a 20% increase in BPND).
Fig. 5.
Axial (left), coronal (middle), and sagittal (right) maps of t-values from voxel-wise statistical testing. Top: Voxel-wise 1-sample t-test to determine if ΔBPND at each voxel was significantly < 0. Dopamine levels were significantly lower during the alcohol-related cues (CUES) condition relative to the baseline (neutral cues) condition (display threshold p < 0.005). Bottom: Voxel-wise 1-sample t-test to determine if ΔBPND at each voxel was significantly >0. Dopamine levels were significantly higher during the unanticipated alcohol (EtOH) condition compared with the baseline condition (display threshold p < 0.005).
The voxel-wise t-test of the unexpected EtOH ΔBPND map showed a cluster of voxels in the left NAc with significantly positive ΔBPND values, indicating an increase in DA concentration (Fig. 5, bottom). The average ΔBPND value of the voxels in this cluster was 0.12 ± 0.08 (i.e., a 12% decrease in BPND).
The average BPND values from the significant clusters are presented in Table 2.
Table 2.
Mean ± SD BPND Values From the Baseline and Challenge Scans (CUES or ETOH) for the Significant SPM Clusters (see Fig. 5)
| Cluster | Scan | |
|---|---|---|
| Baseline | Challenge | |
| CUES-R | 1.38 ± 0.46 | 1.59 ± 0.37 |
| ETOH-L | 1.23 ± 0.33 | 1.08 ± 0.30 |
CUES-R: voxel data from the right ventral striatal cluster in the CUES contrast (ΔBPND < 0). ETOH-L: voxel data from the left ventral striatal cluster in the ETOH contrast (ΔBPND > 0).
Effects of Subject-Specific Variables
Subjects classified as “hazardous drinkers” (AUDIT scores > 8, n = 5) did not differ significantly from social drinkers (n = 3) in the average ΔBP from significant clusters in either experimental condition. Neither SHAS nor AUDIT score were significantly correlated with the average ΔBP from the CUES or ETOH clusters.
DISCUSSION
Rodent studies suggest that conditioned cues or contexts that accompany or precede alcohol administration result in increased [DA] in the NAc (Gonzales and Weiss, 1998; Katner et al., 1996; Melendez et al., 2002; Weiss et al., 1993). However, in this human PET study, the sights and smells of subjects preferred alcoholic drinks, intended to explicitly create anticipation of alcohol administration, did not cause any increases in [DA] in the NAc or other striatal areas. Instead, we found an effect in the opposite direction, such that alcohol-related cues decreased ventral striatal [DA]. This is consistent with microdialysis data from animals that were first trained to respond for alcohol and then subsequently underwent extinction training: Olfactory cues that reinstated operant responding for alcohol (without the alcohol delivery predicted by the cues) also decreased NAc [DA] (Katner and Weiss, 1999). Moreover, unexpected alcohol administration increased ventral striatal [DA]. This is similar to Boileau and colleagues (2003), but not to work from our laboratory (Yoder et al., 2005, 2007). In particular, we previously demonstrated that, in subjects who were aware that they would receive alcohol, intravenous alcohol administration did not cause detectable dopamine release (however, see Yoder et al., 2007, for alternative methods of analysis for quantifying EtOH-induced DA release).
When the data from the CUES and EtOH conditions are examined in a different context, they appear to match Schultz et al.’s work on dopamine neuron function in reward prediction error (Mirenowicz and Schultz, 1994; Schultz, 2002; Schultz et al., 1993): (A) If reward is expected but not delivered (negative prediction error), the activity of midbrain DA neurons is greatly reduced (Morris et al., 2004; Schultz et al., 2000). In our CUES condition, alcohol was promised but not delivered during scanning, with a resulting decrease in NAc [DA]. (B) The unexpected delivery of a reward (positive prediction error) increases DA neuronal activity (Mirenowicz and Schultz, 1994; Pan et al., 2005; Schultz et al., 2000). During our EtOH scan condition, alcohol was delivered unexpectedly, and [DA] consequently increased in the NAc. Given the strength of the midbrain dopaminergic innervation of the striatum, it seems likely that the firing rates of DA neurons would correspond to changes in striatal [DA]. We therefore suggest that our present in vivo human PET data are consistent with the aforementioned preclinical electrophysiological findings: (A) [DA] is reduced when alcohol was promised but not delivered, (B) [DA] increased with unexpected alcohol administration.
Our results are consistent with and complementary to the work of other human neuroimaging investigations. Several fMRI studies have reported changes in striatal brain activity during conditions that involved prediction errors. Specifically, negative prediction errors resulted in lower ventral (Abler et al., 2006) and dorsal striatal (Davidson et al., 2004; McClure et al., 2003) activity, while unpredictable delivery of stimuli (positive prediction error) caused increased activity in the ventral striatum (Berns et al., 2001) and putamen (McClure et al., 2003). Although fMRI does not provide information about changes in specific neurotransmitter systems, these reported changes in local brain activity resulting from prediction errors parallel our data regarding the effects of prediction error on striatal dopamine activity. The data in the present work are also analogous to that of Pappata and colleagues (2002), who detected striatal DA release during unexpected monetary gain; however, this group did not detect any change in striatal [DA] in response to unexpected monetary loss.
It is possible that our observed increase in [DA] during alcohol administration resulted from pharmacological effects of alcohol on dopaminergic neurons (Brodie and Appel, 2000; Brodie et al., 1999; Gessa et al., 1985) rather than prediction error, per se. Using the same radioligand as in the present work, Boileau and colleagues (2003) demonstrated that oral alcohol induced DA release in the ventral striatum of human subjects. However, when we used a similar analysis, our group could not show increased [DA] from IV alcohol administration (Yoder et al., 2005, 2007). Were the effect of alcohol on DA release strictly pharmacological, increased [DA] would be expected with either IV or oral alcohol. We instead propose that the discrepancy between the Yoder and colleagues (2005, 2007) and Boileau and colleagues (2003) studies stems from the mode of administration of alcohol (oral versus IV). In particular, IV alcohol administration does not provide conditioned gustatory and olfactory cues (which are present in oral administration), which may themselves induce DA release (Doyon et al., 2003, 2005).
Our present work also parallels an emerging view that the NAc DA release observed following investigator-administered alcohol was provoked by novelty, unexpectedness, and/or aversiveness of the administration (Bradberry, 2002; Gonzales et al., 2004; Heidbreder and De Witte, 1993; Imperato and Di Chiara, 1986; Joseph et al., 2003; Marinelli et al., 2003; Philpot and Kirstein, 1998; Yan, 1999; Yim et al., 1998, 2000; Yoshimoto et al., 1992). Although several studies have documented increases in NAc [DA] during oral self-administration of alcohol (Doyon et al., 2005; Gonzales and Weiss, 1998; Melendez et al., 2002; Weiss et al., 1992, 1993, 1996), Doyon and colleagues (2003, 2005) showed that increases in NAc [DA] were dissociated from changes in brain ethanol concentration: NAc DA levels peaked 5 minutes after the onset of oral alcohol self-administration, then gradually tapered off over the next 30 minutes as the animals continued to drink alcohol and brain ethanol levels continued to rise. These authors suggested that the sensory properties of alcohol (e.g., scent, taste, intraoral sensation) associated with subsequent alcohol administration/intoxication are the sources of increased accumbal DA levels seen at the beginning of alcohol consumption. Likewise, in the 2003 Boileau study, subjects began drinking alcohol 30 minutes prior to scanning, at which time they also became aware of the drink’s contents. If the properties of oral alcohol cause effects in humans similar those observed in Doyon and colleagues’ work, it may have been the case that the intraoral sensory properties of alcohol raised ventral striatal dopamine levels high enough and long enough to produce a measurable decrease in RAC binding. Even if NAc [DA] tapered off after drinking (Doyon et al., 2003, 2005) and before the start of the PET scan, modestly elevated DA levels at the time of RAC injection may have been sufficient to cause a measurable decrease in RAC binding potential relative to the control scan. This possibility is supported by the fact that the measure of BPND is especially sensitive to early perturbations of endogenous dopamine (Morris et al., 1996; Yoder et al., 2004). Future studies are needed to further explore and reconcile these findings.
We detected unilateral effects in each prediction error condition. Asymmetries in human neurotransmitters (including dopamine) were first documented several decades ago (Glick et al., 1982), and asymmetries in rodent dopaminergic systems can have functional relevance at molecular and behavioral levels (e.g., Adrover et al., 2007; Besson and Louilot, 1995; Louilot and Le Moal, 1994). Particularly relevant to the present work is a study suggesting that the NAc dopaminergic response to appetitive odors is differentially lateralized as a function of the animal’s conditioning history (Besson and Louilot, 1995). There are also a growing number of human studies reporting that the left and right dopaminergic systems subserve qualitatively different aspects of cognitive functions (Badgaiyan et al., 2007; Cheesman et al., 2005; Tomer and Aharon-Peretz, 2004; Tomer et al., 2008). Tomer and colleagues (2008) recently reported that motivated behavior is related to higher DA receptor availability in the left putamen relative to the right. This same group found that novelty-seeking was decreased in Parkinson’s Disease patients who had initial left-side dopaminergic loss, but not in PD subjects with initial right-side DA loss. Badgaiyan and colleagues (2007) hypothesized that the anterior left caudate was responsible for detecting rule changes during an implicit learning task. Further study is required to determine if positive and negative prediction errors recruit hemisphere-specific dopaminergic circuitry consistently across populations and paradigms.
Although very little work exists on prediction error and addiction, both positive and negative prediction errors could conceivably contribute to development of alcohol addiction. Berridge suggests that increases in DA code incentive salience, or “wanting,” the component of reward that motivates seeking and consumption (Berridge, 2007). Equally interesting is the manner in which prediction errors may drive excessive alcohol consumption (Lapish et al., 2006). For example, early drinking experiences may be more rewarding than initially thought, creating the equivalent of a positive prediction error. With repeated drinking, and with the transition from alcohol abuse to addiction, the perceived reward value of alcohol may diminish from factors such as tolerance, effectively creating a negative prediction error. In the latter case, the perceived effects of alcohol do not match the effects expected from earlier experiences, and more alcohol is consumed in an attempt to reproduce the desired (“expected”) effects (Lapish et al., 2006). It is therefore possible that alcoholics code alcohol-related “prediction errors” differently than social drinkers, and that the relative strength of these signals mediates the destructive drinking behavior in alcoholism. The dopaminergic responses to prediction errors may constitute potential biomarkers that represent components of the neurochemical basis for alcohol addiction, and may be predictive of how likely individuals are to respond to treatment. Further research in both addicted and nonaddicted populations would be required to test such hypotheses.
There is an important consideration that could temper our interpretation that the decrease in DA we observed during the CUES condition is indicative of a negative prediction error. Specifically, we did not assess subjects’ beliefs as to whether they had actually received alcohol during scanning, and the group data suggests a transient (although highly variable) change in subjective effects at the third time point of measurement (~12 minutes after cue exposure). Thus, we cannot rule out the contribution of a “placebo” effect (i.e., the errant belief that alcohol was being administered). However, this interpretation of a placebo effect runs counter to reports that placebo administration increases striatal DA concentration (de la Fuente-Fernandez et al., 2001, 2002; Kaasinen et al., 2004)—an effect opposite to what we observed during the CUES condition. Nevertheless, further study is indicated to better dissociate these phenomena.
Although none of our subjects were dependent drinkers, our sample was heterogeneous with respect to recent drinking and family history of alcoholism. We did not find any effects of drinking history on our results. However, it is possible that a larger sample would reveal relationships between drinking and the dopamine response to prediction error. As only 2 subjects had an unambiguous positive family history of alcoholism (see Table 1), we were unable to assess the effects of family history. Given that family history-positive subjects without alcoholism may possess protective factors in the dopamine system (Volkow et al., 2006a; but see Munro et al., 2006), it will be important to understand how dopaminergic responses to prediction error may differ across alcoholic and nonalcoholic subjects who vary according to family history.
To the best of our knowledge, this is the first report to provide in vivo human evidence that striatal dopamine concentration varies bi-directionally as a function of violations of reward expectation. Our results can be explained in the context of preclinical electrophysiological data which show that the firing rates of midbrain dopamine neurons change during prediction errors—errors which themselves may play a role in addiction by heightening differences between original reward experiences and a tolerance-driven inability to recapture that original experience (Lapish et al., 2006). The results of this study provide further rationale for using PET to study the dopaminergic signals associated with alcohol-related stimulus processing and learning in humans.
ACKNOWLEDGMENTS
This work was supported by P60 AA07611 (Pilot 44) and R01 AA014605-02 to D.A. Kareken, the human research component of the Indiana Alcohol Research Center (P60 AA07611), and the General Clinical Research Center at the Indiana University School of Medicine (MO1 RR000750). We gratefully acknowledge Steven Warrenburrg and International Flavors and Fragrances for supplying the odorants used in this work, Mario Dzemidzic for analysis assistance and helpful comments, Robert Kessler for fruitful scientific discussion, Kevin Perry for assistance with PET image acquisition and reconstruction, Bruce Mock and Qi-Huang Zheng for radiochemistry support, Alex Radnovich for help with paradigm programming, Daniel Albrecht and Melissa Walker for assistance with data analysis, and Regat Seyoum and Cari Cox for subject recruitment and technical support.
REFERENCES
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accum-bens. Neuroimage. 2006;31(2):790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Adrover E, Berger MA, Perez AA, Tarazi FI, Antonelli MC. Effects of prenatal stress on dopamine D2 receptor asymmetry in rat brain. Synapse. 2007;61(6):459–462. doi: 10.1002/syn.20389. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release in sequential learning. Neuroimage. 2007;38(3):549–556. doi: 10.1016/j.neuroimage.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21(8):2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Besson C, Louilot A. Asymmetrical involvement of mesolimbic dopaminergic neurons in affective perception. Neuroscience. 1995;68(4):963–968. doi: 10.1016/0306-4522(95)00255-h. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27(15):3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Dose-dependent effect of ethanol on extracellular dopa-mine in mesolimbic striatum of awake rhesus monkeys: comparison with cocaine across individuals. Psychopharmacology (Berl) 2002;165(1):67–76. doi: 10.1007/s00213-002-1233-9. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin Exp Res. 2008;32:1124–1134. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108(7):887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24(7):1120–1124. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23(11):1848–1852. [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76(9):1204–1210. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave KM, Saunders JB, Whitfield JB. Diagnostic tests for alcohol consumption. Alcohol Alcohol. 1995;30(1):13–26. [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46(12):1980–1989. [PubMed] [Google Scholar]
- Davidson MC, Horvitz JC, Tottenham N, Fossella JA, Watts R, Ulug AM, Casey BJ. Differential cingulate and caudate activation following unexpected nonrewarding stimuli. Neuroimage. 2004;23(3):1039–1045. doi: 10.1016/j.neuroimage.2004.07.049. [DOI] [PubMed] [Google Scholar]
- Dewey S, Smith G, Logan J, Brodie J, Fowler J, Wolf A. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synap-tic dopamine levels. Synapse. 1993;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Dewey S, Smith G, Logan J, Brodie J, Yu D, Ferrieri R, King P, MacGregor R, Martin T, Wolf A, Volkow N, Fowler J, Meller E. GABAergic inhibition of endogenous dopamine release measure in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. 1989;45(5):381–384. doi: 10.3758/bf03210709. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93(6):1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consumma-tory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27(10):1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Fei X, Mock BH, DeGrado TR, Wang JQ, Glick-Wilson BE, Sullivan ML, Hutchins GD, Zheng QH. An improved synthesis of PET dopamine D2 receptors radioligand [11C]raclopride. Synthetic Communications. 2004;34(10):1897–1907. [Google Scholar]
- de la Fuente-Fernandez R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ, Stoessl AJ. Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res. 2002;136(2):359–363. doi: 10.1016/s0166-4328(02)00130-4. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348(1):201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982;234(1):53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18(24):10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, De Witte P. Ethanol differentially affects extracellular monoamines and GABA in the nucleus accumbens. Pharmacol Biochem Behav. 1993;46(2):477–481. doi: 10.1016/0091-3057(93)90383-5. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser-Sinopoli SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopa-mine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22(10):1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239(1):219–228. [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20(19):7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MH, Datla K, Young AM. The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neurosci Biobehav Rev. 2003;27(6):527–541. doi: 10.1016/j.neubiorev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Nagren K, Rinne JO. Expectation of caffeine induces dopaminergic responses in humans. Eur J Neurosci. 2004;19(8):2352–2356. doi: 10.1111/j.1460-9568.2004.03310.x. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004a;28(4):550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Sabri M, Radnovich AJ, Claus E, Foresman B, Hector D, Hutchins GD. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage. 2004b;22(1):456–465. doi: 10.1016/j.neuroimage.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (P) but not Wistar rats. Behav Pharmacol. 1996;7(7):669–674. [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23(11):1751–1760. [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Judson C, handler L. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30(9):1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95(6):889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Louilot A, Le Moal M. Lateralized interdependence between limbicotemporal and ventrostriatal dopaminergic transmission. Neuroscience. 1994;59(3):495–500. doi: 10.1016/0306-4522(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169(1):60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26(3):318–325. [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72(2):1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Modell JG, Mountz JM. Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatry Clin Neurosci. 1995;7(1):15–22. doi: 10.1176/jnp.7.1.15. [DOI] [PubMed] [Google Scholar]
- Morris ED, Heidbreder CA, Shippenberg TS, Ernst M, Christian BT, London ED. Can PET Detect Changes in Intrasynaptic Dopamine? Computer Simulations can Optimize the Experimental Design. Washington, D.C.: Society for Neuroscience; 1996. [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43(1):133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Oswald LM, Wong DF, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Striatal dopamine release and family history of alcoholism. Alcohol Clin Exp Res. 2006;30(7):1143–1151. doi: 10.1111/j.1530-0277.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29(2):393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22(1):202–210. [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25(26):6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappata S, Dehaene S, Poline JB, Gregoire MC, Jobert A, Delforge J, Frouin V, Bottlaender M, Dolle F, Di Giamberardino L, Syrota A. In vivo detection of striatal dopamine release during reward: a PET study with [(11)C]raclopride and a single dynamic scan approach. Neuroimage. 2002;16(4):1015–1027. doi: 10.1006/nimg.2002.1121. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Kirstein CL. The effects of repeated alcohol exposure on the neurochemistry of the periadolescent nucleus accumbens septi. Neuroreport. 1998;9(7):1359–1363. doi: 10.1097/00001756-199805110-00020. [DOI] [PubMed] [Google Scholar]
- Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schollhorn-Peyronneau MA, Roumenov D, Brodtkorb E, Zuberi S, Gambardella A, Steinborn B, Hufnagel A, Valette H, Bottlaender M. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129(Pt 8):2047–2060. doi: 10.1093/brain/awl156. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23(4):617–623. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry. 1988;45(3):211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10(3):272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Niznik HB. Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: implications for positron emission tomography of the human brain. Synapse. 1989;3(1):96–97. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11(2):149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Tomer R, Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson’s disease: effects of asymmetric dopamine deficiency. J Neurol Neurosurg Psychiatry. 2004;75(7):972–975. doi: 10.1136/jnnp.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77(1):98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006a;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006b;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Hurd YL, Ungerstedt U, Markou A, Plotsky PM, Koob GF. Neurochemical correlates of cocaine and ethanol self-administration. Ann NY Acad Sci. 1992;654:220–241. doi: 10.1111/j.1749-6632.1992.tb25970.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97(8):4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16(10):3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharma-cology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yan QS. Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol. 1999;19(1):1–7. doi: 10.1016/s0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA. Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol Clin Exp Res. 2000;24(6):781–788. [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22(2):367–374. [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopa-mine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31(6):965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29(6):965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Wang C, Morris ED. Change in binding potential as a quantitative index of neurotransmitter release is highly sensitive to relative timing and kinetics of the tracer and the endogenous ligand. J Nucl Med. 2004;45(5):903–911. [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9(1):17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Young LT, Wong DF, Goldman S, Minkin E, Chen C, Matsumura K, Scheffel U, Wagner HN., Jr Effects of endogenous dopamine on kinetics of [3H]N-methylspiperone and [3H]raclopride binding in the rat brain. Synapse. 1991;9(3):188–194. doi: 10.1002/syn.890090305. [DOI] [PubMed] [Google Scholar]
- Ziolko SK, Weissfeld LA, Klunk WE, Mathis CA, Hoge JA, Lopresti BJ, DeKosky ST, Price JC. Evaluation of voxel-based methods for the statistical analysis of PIB PET amyloid imaging studies in Alzheimer’s disease. Neuroimage. 2006;33(1):94–102. doi: 10.1016/j.neuroimage.2006.05.063. [DOI] [PubMed] [Google Scholar]