Abstract
P50, N100, and P200 auditory sensory gating reflect distinct mechanisms involved in protecting the integrity of higher-order functions. They have been implicated in multiple psychiatric disorders. Recent studies showed the (limited) effects of age and gender on sensory gating in control subjects, suggesting there may be other sources of variance. Two potential sources may be education and intelligence (intellectual capability), variables that frequently differ across studies and across experimental groups. We explored potential effects of age, gender, education, and intelligence (Shipley intelligence scale) on P50, N100, and P200 sensory gating measured with the paired-click paradigm in 60 healthy subjects recruited from the general population. Increased intellectual capability related to stronger N100 and P200 gating and more pronounced N100 and P200 amplitudes. In addition, increased age related to weaker P200 gating and smaller P200 amplitudes. Gender had negligible effects. Intellectual capability or age could contribute to variation in N100 or P200 auditory sensory gating and should be controlled for when studying sensory gating in clinical and control groups.
Keywords: evoked potentials, sensory gating, age, education, intelligence
Introduction
P50 auditory sensory gating, measured as the degree of reduction in P50 amplitude in response to stimulus repetition (Freedman, Waldo, Bickford-Wimer, & Nagamoto, 1991; Fruhstorfer, Soveri, & Järvilehto, 1970), could reflect a preattentional filter mechanism (Jerger, Biggins, & Fein, 1992) protecting the integrity of cognitive functioning by inhibiting irrelevant stimuli before information is transferred to higher-order functions for more elaborate processing (Boutros, Korzyukov, Jansen, Feingold, & Bell, 2004; Freedman et al., 1991; Kisley, Noecker, & Guinther, 2004; Wan, Friedman, Boutros, & Crawford, 2008). Previous studies showed P50 gating impairments in subjects with Alzheimer dementia (Thomas et al., in press), childhood-onset antisocial personality disorder (Lijffijt, Moeller, Boutros, Burroughs, et al., in press), posttraumatic stress disorder (Karl, Malta, & Maercker, 2006), panic disorder (Ghisolfi et al., 2006), schizotypal personality disorder (Cadenhead, Light, Geyer, & Braff, 2000; Wan, Crawford, & Boutros, 2007; Wang, Miyazato, Hokama, Hiramatsu, & Kondo, 2004), cocaine abuse (Boutros, Gooding, Sundaresan, Burroughs, & Johanson, 2006; Fein, Biggins, & MacKay, 1996), schizophrenia (Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Brockhaus-Dumke, Schultze-Lutter et al., 2008; De Wilde, Bour, Dingemans, Koelman, & Linszen, 2007; Patterson et al., 2008), and bipolar I disorder (Lijffijt, Moeller, Boutros, Steinberg, et al., in press; Olincy& Martin, 2005; Schulze et al., 2007), suggesting a common underlying filter deficit across a wide range of disorders.
Recent meta-analyses showed that the degree of P50 impairment in schizophrenia may vary considerably across individual studies (Bramon et al., 2004; De Wilde et al., 2007; Patterson et al., 2008), partly because of differences in filter settings that were used to analyze the data or differences in intensity of the auditory stimulus (De Wilde et al., 2007; Patterson et al., 2008). De Wilde et al. (2007) further showed that the influence of these variables may affect gating measures more among healthy controls than among subjects with schizophrenia, which illustrates the importance of investigating the potential moderator variables of P50 gating more carefully as outcomes could help in interpreting differences between studies and interpreting the generalizability of results.
In addition to effects of methodological variables on sensory gating, studies in healthy adults also reported weaker P50 gating with increased age (Patterson et al., 2008; Turetsky et al., in press), and weaker P50 gating in females compared to males (Hetrick et al., 1996; Patterson et al., 2008). However, age and gender seem to account for only a small part of the variance in P50 gating (Freedman, Adler, & Waldo, 1987; Patterson et al., 2008; Thomas et al., in press; Waldo, Graze, de Graff Bender, Adler, & Freedman, 1987; Wang et al., 2004; White, Kanazawa, & Yee, 2005). This raises the question of whether variation in P50 gating in controls, and the potential effect on P50 gating differences between clinical and control groups, may relate to other subject characteristics that have so far not been taken into account.
Although it may be a challenge to select subjects to match for age or gender, most studies have done so at least at the group level. However, the same studies frequently showed clear differences in level of education between clinical and comparison groups, with controls having significantly higher levels of education than clinical groups (Boutros et al., 2006; Brockhaus-Dumke, Mueller, Faigle, & Klosterkoetter, 2008; Brockhaus-Dumke, Schultze-Lutter et al., 2008; Cadenhead et al., 2000; Fresán et al., 2007; Lijffijt, Moeller, Boutros, Burroughs et al., in press; Lijffijt, Moeller, Boutros, Steinberg et al., in press; Olincy & Martin, 2005; Turetsky et al., in press). Across samples of healthy subjects a similar difference in education seems to exist as reflected by whether subjects were recruited among students and university or hospital staff (e.g., Hetrick et al., 1996; Thoma et al., 2006; Thomas et al., in press;Wang et al., 2004) or among the general population (e.g., Lijffijt, Moeller, Boutros, Burroughs et al., in press; Lijffijt, Moeller, Boutros, Steinberg et al., in press; Turetsky et al., in press). Furthermore, even in studies that did match groups for education (Schulze et al., 2006; Thomas et al., in press) significant differences could still remain in intelligence (Thoma et al., 2006). Thus, it is possible that higher levels of education or intelligence could relate to better P50 gating, which would be consistent with the idea that P50 gating mechanisms are involved in protecting the integrity of cognitive functions (Freedman et al., 1991; Wan et al, 2008). Considering the numerous studies that did not control for education or intelligence, more information is needed on whether and how these two variables might influence P50 gating.
Similar questions can be asked for variation in sensory gating of the N100 and P200 auditory evoked potentials. Sensory gating of the auditory N100 and P200 evoked potentials have received increased interest. N100 and P200 gating could relate to inhibition of irrelevant information (Sable, Low, Maclin, Fabiani, & Gratton, 2004) affecting early attention or working memory (Lijffijt, Lane et al., in press), supporting findings that N100 and P200 gating reflect biological substrates distinct from those involved in P50 gating (Brockhaus-Dumke, Mueller et al., 2008; Hanlon et al., 2005; Kisley et al., 2004; Oranje, Geyer, Bocker, Kenemans, & Verbaten, 2006).
Recent studies revealed impairments in N100 and P200 sensory gating in subjects with schizophrenia (Boutros, Korzyuko, Oliwa, et al., 2004; Brockhaus-Dumke, Schultze-Lutter et al., 2008; Turetsky et al., in press), cocaine abuse (Boutros et al., 2006), and bipolar I disorder (Lijffijt, Moeller, Boutros, Steinberg, et al., in press). However, in contrast to P50 gating, nothing is known about potential effects of methodological or subject variables on N100 and P200 gating.
In the current study we investigated effects of age, gender, education, and intelligence on P50, N100, and P200 auditory sensory gating in 60 healthy control subjects who were recruited from the general population. Based on findings for P50 gating we hypothesized (1) diminished P50, N100 and P200 gating as a function of age, (2) diminished gating in female compared to male subjects, and (3) diminished gating with lower levels of education or intelligence. More information on these relationships could be valuable in study design, sample selection, interpreting differences between studies, and in establishing the degree of generalizability of findings. Similarly, outcomes may provide information on how sensory gating, an important aspect in efficient information processing, is related to intelligence and the process of aging.
Materials and Methods
Subjects
The study complied with the Declaration of Helsinki and was approved by the Committee for the Protection of Human Subjects, IRB for the University of Texas Health Science Center at Houston, TX, USA. Subjects received thorough descriptions of the study, with full opportunity for questions, and signed informed consent before any research-related procedures.
Subjects were recruited from the general population through advertisements in the local press. A total of 60 subjects (26 males, 34 females) were included. Age ranged from 18–54 years (M = 31.55, SD = 10.13); highest level of completed education ranged from 11–20 years (M = 14.47, SD = 2.24). All subjects were free of current and past axis-I or axis-II disorders as assessed by the Structured Clinical Interview for DSM-IV axis-I and axis-II disorders (SCID-I and SCID-II) (First, Spitzer, Gibbons,&Williams, 1996), which were administered by fully trained staff members. Subjects had good hearing by self-report, normal or corrected-to-normal vision, no history of head trauma or epilepsy, and no criminal history or history of alcohol or drug abuse. Of the 11 subjects who were current smokers, significantly more were male (n = 8) than female (n = 3) (χ2 = 4.74, df = 1, p = .03).
Finally, 48 subjects (19 males and 29 females) completed the Shipley Institute of Living Scale (Shipley, 1940; Zachary, 1986) assessing intelligence. For the current study age-corrected T-scores were used for the verbal, abstraction, and combined (total) scales. Verbal score ranged from 26 to 66 (M = 48.50, SD = 10.61); abstraction score ranged from 39 to 66 (M = 56.52, 6.45); total score ranged from 37–68 (M = 53.73, SD = 8.54).
Measures
Paired-Click Paradigm
Subjects were administered two to three blocks of the paired-click paradigm. Each block contained 40 pairs of two identical 40 ms 80 dB (SPL) clicks (1000 Hz, 4 ms raise-fall) presented binaurally through headphones using STIM software (Neuroscan, Inc., El Paso, TX, USA). The interstimulus interval between the first (S1) and second click (S2) was 500 ms. The interval between two consecutive pairs was randomized between 8–10 s, allowing full recovery of the P50 component (Zouridakis & Boutros, 1992).
Shipley Institute of Living Scale
The Shipley Institute of Living Scale (Shipley, 1940; Zachary, 1986) was used to estimate overall intelligence as well as verbal and abstract intellectual skills (Zachary, 1986, p. 54). The Shipley contains 40 vocabulary and 20 abstraction items assessed by two separate scales that had to be completed within 10 min each. For the vocabulary scale, subjects choose the best description of a given word out of four alternatives. For the abstraction scale, subjects completed combination of letters or digits. The test generates a vocabulary, abstraction, and total raw score, which were converted into age-adjusted T-scores. Test-retest reliability for the three scales is fair to good (α = 0.60–0.78).
Procedure
Prior to any experimental procedure, subjects were required to have negative screens for drugs (RediCup®, Redwood Biotech, Santa Rosa, CA, USA) and alcohol (Alco-Sensor III, Intoximeters Inc., Saint Louis, MO, USA). Subjects were also required to refrain from consuming caffeinated products for at least 8 h and from smoking for at least 1 h prior to testing. The Shipley was completed on the day that subjects signed informed consent; 12 subjects choose not to complete the Shipley Intelligence Scale. Electrophysiologic testing was completed in the morning. Subjects were seated in a chair in a hospital room without additional safeguards against noise or electrical interference, and were instructed to listen passively to the clicks, to relax, and to sit quietly with their eyes fixated on a fixation-cross mounted on the wall. Blinking was allowed.
Data Recording and Analysis
Raw EEG was recorded from 32 electrodes attached in a Quik-cap (Neuromedics Neuroscan, El Paso, TX, USA), arranged according to the international 10–20 system (Jasper, 1958). Data were recorded using Acquire 4.3 (NeuroScan, El Paso, TX, USA). Signals were sampled at 1000 Hz, filtered between 0.1 and 100 Hz (AC mode), and amplified ×10,000 through SynAmps amplifiers. Signals were referenced to linked electrodes attached to the mastoids. Vertical and horizontal electrooculograms were assessed with electrodes placed above and below the right eye, and at the right and left canthi, respectively. The ground electrode was attached in the cap anteriorly to F3 and F4, extending the midline. Impedances were kept below 5 kΩ.
Procedures have been described in detail in Lijffijt, Moeller, Boutros, Steinberg et al. (in press). Signals were filtered off-line between 1 and 50 Hz (48 dB/oct roll-off) and corrected for eye blinks with a regression algorithm (see Semlitsch, Anderer, Schuster, & Presslich, 1986). The data were epoched from 100 ms preceding (baseline) to 400 ms following S1 and S2, and corrected for baseline activity. Trials with artifacts were detected manually and rejected from further analysis. Trials were always rejected as a pair, i.e., if S1 was rejected, the accompanying S2 trial was also rejected. Signals for S1 and S2 were averaged separately per block. Next, averages were made for each stimulus across blocks. The mean number of retained pairs across blocks was 80.9 (SD ± 14). Prior to averaging signals were filtered with a 10 Hz high-pass filter to optimize scoring of the P50, or with a 20 Hz low-pass filter to optimize scoring of the N100 and P200 (Jerger et al., 1992). N100 and P200 gating measures had higher reliability compared to P50 gating measures (Fuerst, Gallinat, & Boutros, 2007; Rentzsch, Jockers-Scherübl, Boutros, & Gallinat, 2008).
Following the general convention, P50, N100, and P200 peaks were scored at Cz relative to their preceding troughs (Boutros, Korzyuko, Oliwa et al., 2004). This was generally the P50 for the N100, and was always the N100 for the P200 component. All peaks and preceding troughs were detected automatically (Neuroscan, El Paso) using preset intervals (Boutros, Korzyuko, Oliwa et al., 2004). The N100 was identified first as the most pronounced peak between 80 and 150 ms. If no N100 peak could be identified within that time-window, the time-window between 0–300 ms was taken into account in which the most pronounced negativity with a clear fronto-central scalp distribution was considered the N100. The N100 had to be distinguishable from baseline activity and visible on more than one lead for subjects to be included in the analysis. P50 components were scored by two investigators (NNB and SB) unaware of the purpose of the study. The P50 was defined as the most pronounced negativity between 35 and 85 ms. If no P50 was detected in that window, the P50 was identified as the most positive peak preceding the N100. The P200 was detected last and defined as the most pronounced positivity between 150 and 250 ms. If no P200 peak was found in that window, the P200 was identified as the most positive peak following the N100 as long as it peaked before 300 ms. In this study, all P50 peaks were detected between 38 and 86 ms, N100 peaks were detected between 79 and 156 ms, and P200 peaks were detected between 142 and 273 ms. Scalp distributions were taken into account to identify components that were ambiguous due to multiple peaks or small amplitudes, in which peaks were selected with a central or fronto-central scalp distribution. For S2 evoked components, additional constraints included: S2 P50 activity peaking within 10 ms of the S1 P50 (Nagamoto, Adler, Waldo, Griffith, & Freedman, 1991); S2 N100 and P200 activity peaking within 40 and 80 ms of the S1 N100 and P200, respectively. If no peaks occurred within those windows, the component was considered completely attenuated, unless S2 elicited clear components falling outside the windows as revealed by iso-potential maps (P50: n = 4, window to 15 ms; N100: n = 4, window to 82 ms; P200: n = 1, window to 98 ms).
Statistical Analysis
We collected data on P50, N100, and P200 peak amplitudes and latencies for S1 and S2 at Cz. Gating ratio was calculated as (S2amplitude/S1amplitude)*100; difference score was calculated as S1amplitude-S2amplitude (Smith, Boutros, & Schwarzkopf, 1994). A lower ratio or higher difference score indicated stronger sensory gating. Distributions were tested for normality using the Shapiro-Wilk test. An initial exploration of outcomes obtained with normalized and nonnormalized data analyzed with parametric and nonparametric tests revealed only marginal differences in outcome. Therefore, and for ease of interpreting the data, analyses were conducted with parametric statistics on the original, nonnormalized data. To test the effect of stimulus redundancy on P50, N100, and P200 amplitudes, repeated measures ANOVA were used with stimulus (S1, S2) as within-subjects factor. Gender effects were tested with independent-samples t-tests. Pearson’s r was used to calculate correlations among electrophysiology, age, education, and intelligence.
Initial stepwise regression analyses revealed a high collinearity between education and Shipley scores, but also showed that effects of these two variables on electrophysiological measures were comparable. This suggested that education and Shipley scores could both reflect intellectual capability. These findings let to the decision to only use education in analyses examining the unique contributions of age, intellectual capability, and gender on gating, amplitudes, and latencies, thus using the larger sample. To calculate unique contributions of these three independent variables general linear measure (GLM) analyses were performed for each electrophysiological measure with age, education and gender as independent variables.
Results
Across the total sample (n = 60), age did not correlate significantly with years of education (r = −.08). Across the sample that completed the Shipley (n = 48), age correlated with Shipley verbal, abstraction, and total scores (r between −.41 and −.49, p < .004). Highest level of completed education correlated significantly with Shipley verbal (r = .44, p = .002), abstraction, (r = .59, p < .001), and total (r = .55, p < .001) scores. Age, education, and Shipley scores did not differ between genders (t < 0.99).
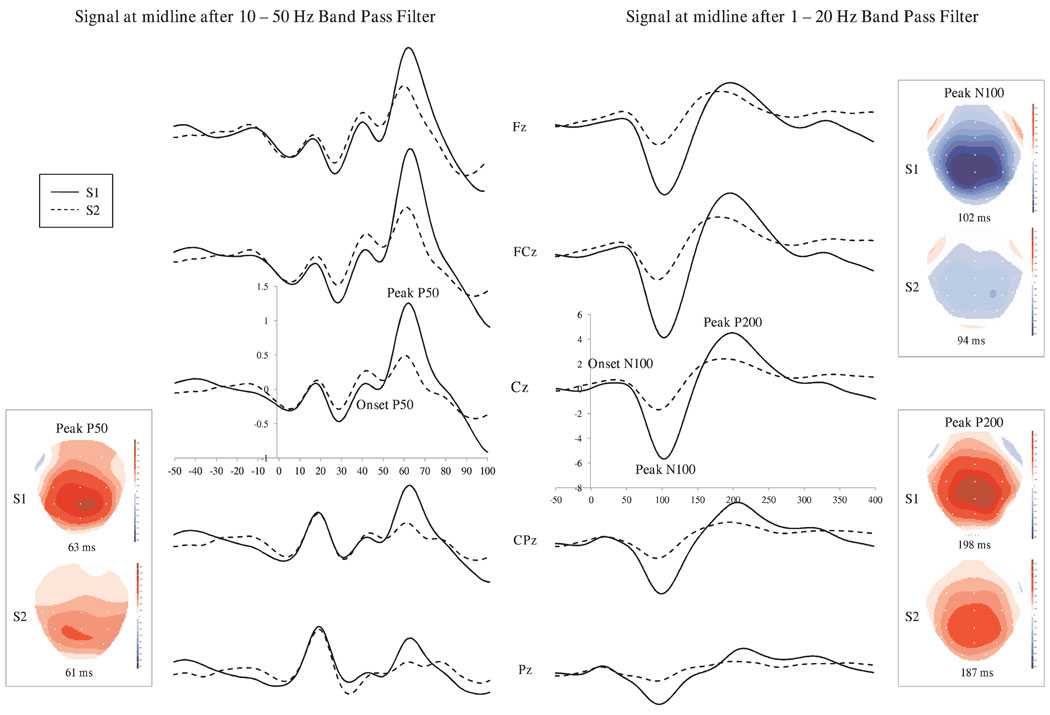
Table 1 provides the means and standard deviations for P50, N100, and P200 gating measures, amplitudes, and latencies. Figure 1 shows the grand averages for S1 and S2 at Fz, FCz, Cz, CPz, and Pz and include the associated topographies for P50, N100 and P200 peaks for S1 and S2. Signals were filtered between 10–50 Hz and 1–20 Hz to emphasize the P50 component and the N100 and P200 components, respectively. Repeated measures ANOVA for data measured at Cz showed a significant attenuation in S2 amplitude compared to S1, F(1, 59) > 65.36, p < .001, for all three components, indicating sensory gating. Finally, N100 and P200 components peaked earlier for S2 than for S1, F(1, 59) > 19.33, p < .001.
Table 1.
Descriptives (mean [SD]) for P50, N100, and P200 ratio, difference score, amplitudes, and latencies across the total sample (n = 60)
| P50 | N100 | P200 | |
|---|---|---|---|
| Ratio | 53.99 (40.80) | 48.62 (22.68) | 47.50 (19.80) |
| Difference score | 1.41 (1.32) | 4.05 (2.89) | 6.77 (4.78) |
| Amplitude S1 | 2.76 (1.53) | 7.40 (3.73) | 11.98 (6.20) |
| Amplitude S2 | 1.36 (1.18) | 3.35 (2.04) | 5.21 (2.63) |
| Latency S1 | 62.72 (13.65) | 105.29 (14.25) | 202.09 (27.43) |
| Latency S2 | 60.65 (14.46)a | 94.69 (14.05) | 183.72 (29.01) |
Note. an = 52 due to no P50 latency for S2 with absolute suppression (ratio = 0).
Figure 1.
Averages at midline leads for signals evoked by S1 and S2. Left panel: Signal filtered 10–50 Hz to accentuate the P50, and topographies for P50 for S1 and S2. Right panel: Signal filtered 1–20 Hz to accentuate the N100 and P200, and topographies for N100 and P200 for S1 and S2.
P50 Component
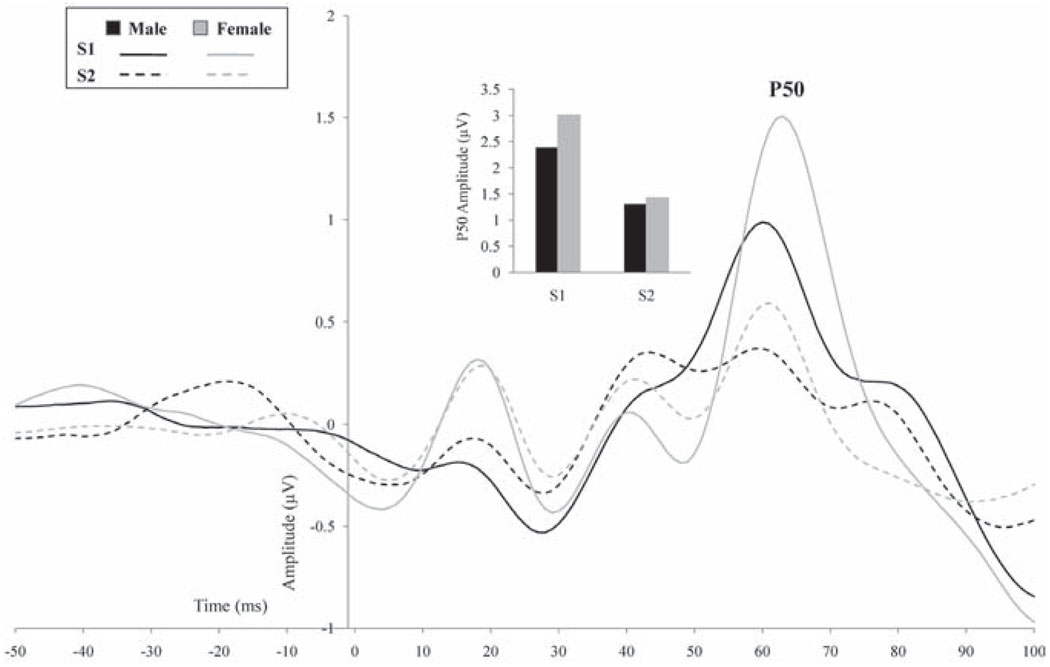
Table 2 summarizes the correlations among P50, N100, and P200 gating and amplitude measures with gender, age, education, and Shipley scores. Figure 2 presents the ERP data for males and females for S1 and S2 at Cz after the signal was filtered with a 10–50 Hz band pass filter. As shown in Table 2, P50 ratio, difference score, S1 and S2 amplitudes, and S1 and S2 latencies were comparable between both genders, and did not correlate significantly with age, level of education, or Shipley scores.
Table 2.
Relationships between gender, age, highest level of completed education (all n = 60), Shipley vocabulary, abstraction, and total scores (n = 48) with P50, N100, and P200 ratio, difference score, and S1 and S2 amplitudes
| P50 | N100 | P200 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | ΔS1S2 | S1 | S2 | Ratio | ΔS1S2 | S1 | S2 | Ratio | ΔS1S2 | S1 | S2 | |
| T-tests (df = 58) | ||||||||||||
| Gender | 0.97 | −1.50 | −1.58 | −0.36 | 1.06 | 0.88 | 0.73 | 0.10 | 1.63 | −1.13 | −0.88 | −0.04 |
| Correlations (Pearson’s r, df = 58) | ||||||||||||
| Age | .11 | −.12 | −.06 | .05 | −.01 | .07 | .13 | .15 | .44 | −.38 | −.28 | .05 |
| Education | −.08 | .21 | .10 | −.10 | −.37 | −.35 | −.31 | −.08 | .01 | .30 | .37 | .32 |
| T-score (df = 46) | ||||||||||||
| Vocabulary | −.23 | .10 | −.08 | −.21 | −.07 | −.17 | −.23 | −.20 | −.29 | .35 | .29 | .06 |
| Abstraction | −.15 | .20 | .11 | −.07 | −.23 | −.32 | −.33 | −.16 | −.26 | .47 | .47 | .26 |
| Total | −.22 | .20 | .04 | −.17 | −.18 | −.30 | −.33 | −.19 | −.32 | .48 | .44 | .19 |
Note. ΔS1S2: Difference score. p < .05, p < .005.
Figure 2.
P50 at Cz (f iltered 10–50 Hz) for S1 and S2 for males (black) and females (gray). The inserted graph presents the P50 peak amplitudes for S1 and S2 for males and females.
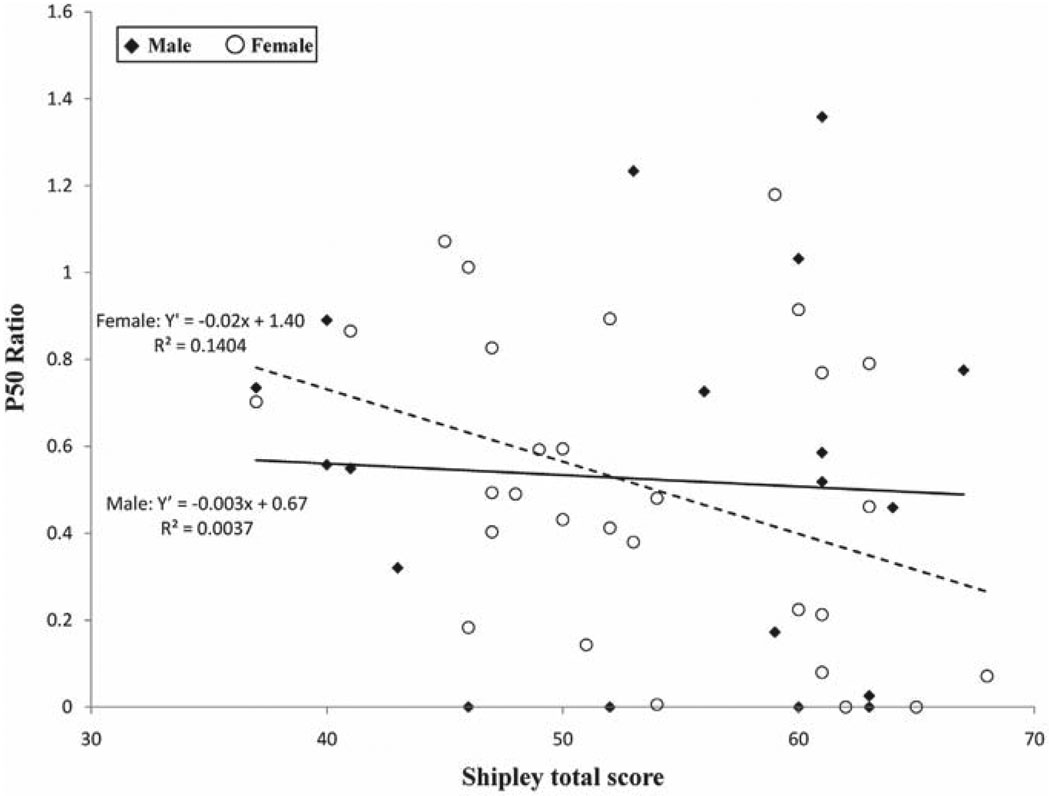
Additional correlation analyses separately for males and females revealed significant correlations among females between age and P50 latency for S1 (r = −.41, p = .02), and between Shipley total score and P50 ratio (r = −.38, p = .05). The male group did not have significant correlations (r = .03 and −.06, respectively). Figure 3 illustrates this relationship. However, if looked at closely, the relationship in females seems to be explained by one or two subjects with lower Shipley scores and higher P50 ratios. Z-tests revealed no significant differences in correlations between genders (z < 1.66, p > .10, two-tailed)
Figure 3.
Scatter plot of relationship between P50 ratio and Shipley total score for male and female subjects.
GLM analyses revealed no unique contributions of age, education, or gender on P50 ratio, difference score, S1 and S2 amplitudes, or S1 latency, although P50 peaking time for S2 was delayed in females (M = 62.23, SD = 10.98) compared to males (M = 55.68, SD = 11.27). Thus, P50 measures seem to be unaffected by age, but could be affected by intellectual capability in females.
N100 Component
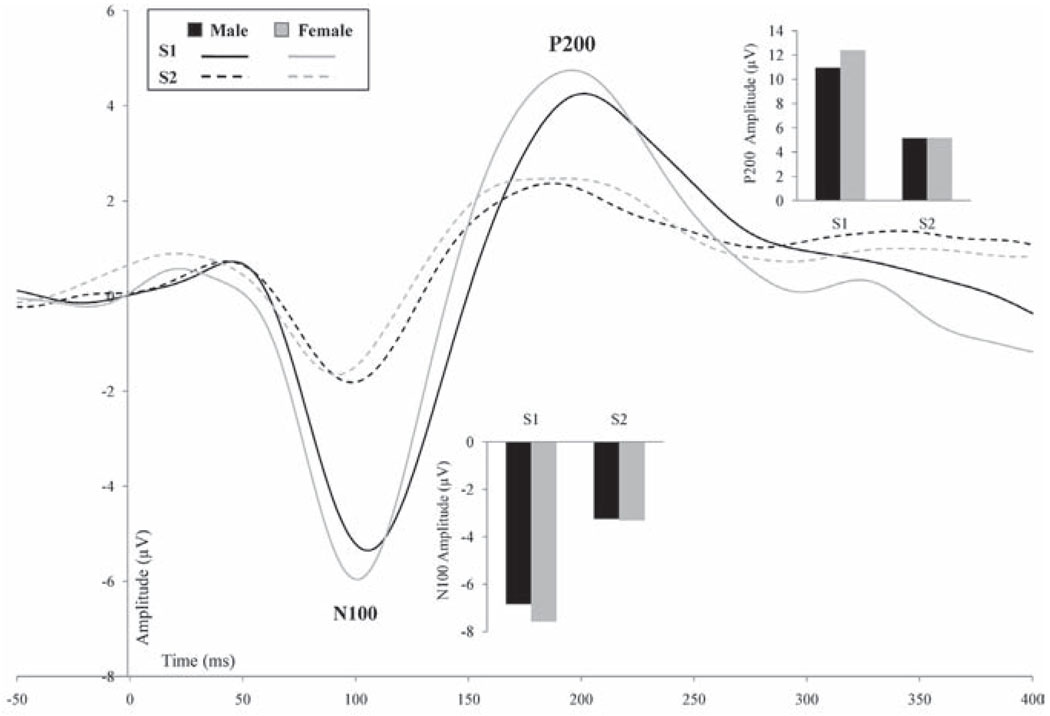
Figure 4 presents the N100 and P200 for males and females for S1 and S2 at Cz. As shown in Table 2 N100 ratio, difference score, and S1 amplitude correlated significantly with education. N100 difference score and S1 amplitude further correlated significantly with Shipley abstraction and total scores. No other significant relationships were found.
Figure 4.
N100 and P200 at Cz (filtered 1–20 Hz) for S1 and S2 for males (black) and females (gray). The inserted graphs present the N100 and P200 peak amplitudes for S1 and S2 for males and females.
Additional correlation analyses revealed a significant correlation for age with N100 ratio in males (r = −.51, p = .008), but not in females (r = −.22), whereas education correlated with N100 difference score and S1 amplitude in females (r = −.40 and −.44, respectively, p < .02) but not in males (r = −.31 and −.18, respectively, p > .13). Z-tests revealed that the gender differences for these correlations were not significant (z < 1.46, p > .14, two-tailed).
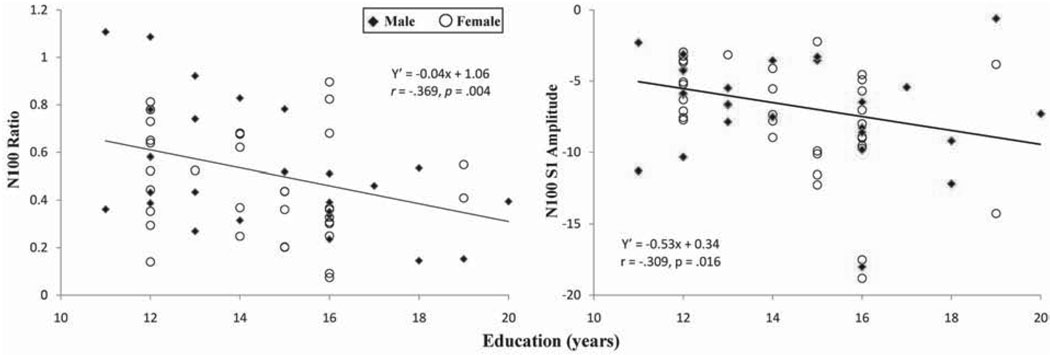
Table 3 presents the outcomes of GLM analyses for N100 measures. Only education uniquely contributed to N100 ratio, difference score, and S1 amplitude. Figure 5 presents the relationships between education with N100 ratio and with N100 S1 amplitude. The outcomes suggest better N100 gating due to enhanced N100 amplitudes for S1 with a higher level of intellectual capability, whereas age and gender do not affect N100 measures.
Table 3.
GLM analysis for N100 measures with age, education, and gender as independent variables
| Age | Education | Gender | ||||
|---|---|---|---|---|---|---|
| F(1, 56) | p | F(1, 56) | p | F(1, 56) | p | |
| Ratio | 0.10 | .76 | 9.14 | .004 | 1.30 | .30 |
| Difference score | 0.08 | .77 | 7.72 | .007 | 0.81 | .37 |
| Amplitude S1 | 0.69 | .41 | 5.68 | .02 | 0.48 | .49 |
| Amplitude S2 | 1.18 | .28 | 0.25 | .62 | 0.01 | .98 |
| Latency S1 | 1.24 | .27 | 0.73 | .40 | 0.04 | .83 |
| Latency S2 | 0.32 | .57 | 2.79 | .10 | 0.01 | .96 |
Figure 5.
Scatter plots of relationships between N100 ratio (left panel) and S1 amplitude (right panel) with highest level of completed education.
P200 Component
As shown in Table 2 P200 ratio, difference score, and S1 amplitude correlated significantly with age. P200 difference score, S1 amplitude, and S2 amplitude further correlated significantly with education. Finally, P200 ratio, difference score, and S1 amplitude correlated significantly with Shipley abstraction and total scores, but less so with verbal score. No other significant relationships were found.
Additional correlation analyses showed significant correlations for age with P200 ratio, difference score, and S1 amplitude in males (r = .61, −.59, and −.47, respectively, p < .016), but not in females (r < .29), whereas education correlated with P200 difference score and S1 amplitude in females (r = .44 and −.47, respectively, p < .009) but not in males (r = .15 and .26, respectively). In contrast, Shipley verbal score correlated more strongly in males than females with P200 difference score (r = .69, p = .001 and r = .28, respectively) and S1 amplitude (r = .55, p = .014 and r = .28, respectively). Correlations between Shipley abstraction or total scores with difference score and S1 amplitude were significant in both genders. z-tests revealed that the gender differences for these correlations were not significant (z < 1.46, p > .14, two-tailed).
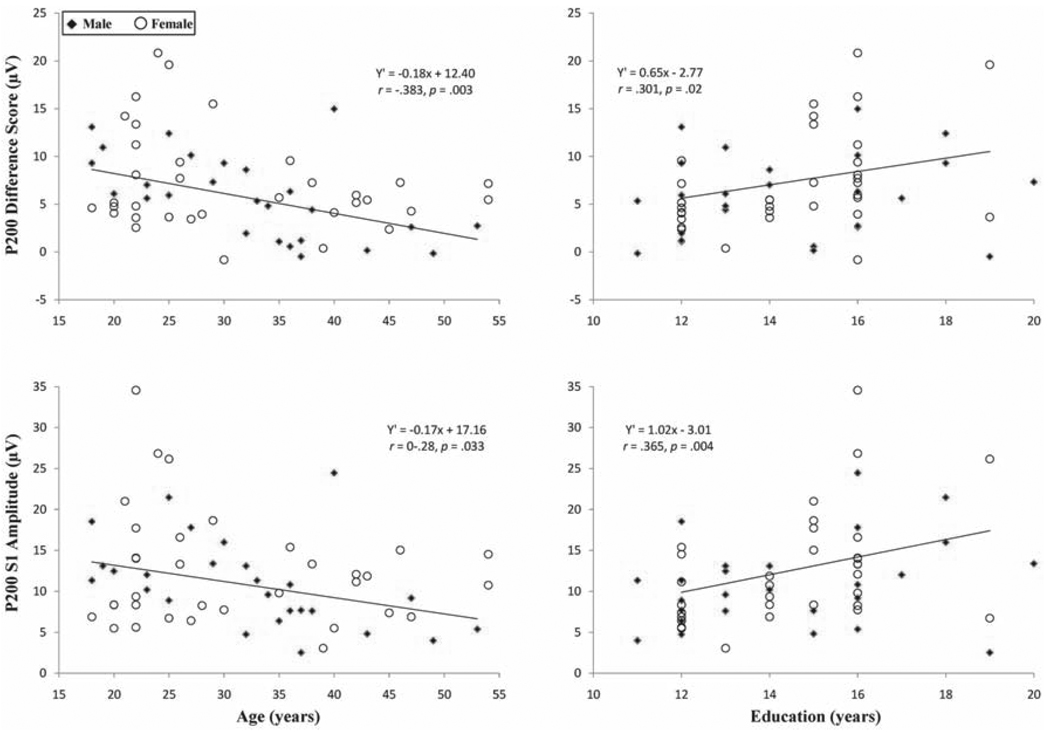
Table 4 presents the outcomes of GLM analyses for P200 measures. Both age and education uniquely contributed to P200 difference score, and S1 amplitude, whereas age contributed to P200 ratio and education contributed to S2 amplitude. Figure 6 presents the relationships for age and education with P200 difference score and with P200 S1 amplitude. The outcomes suggest weaker P200 gating with increased age and stronger P200 gating with a higher level of intellectual capability seemingly related to enhanced S1 amplitudes.
Table 4.
GLM analysis for P200 measures with age, education, and gender as independent variables
| Age | Education | Gender | ||||
|---|---|---|---|---|---|---|
| F(1, 56) | p | F(1, 56) | p | F(1, 56) | p | |
| Ratio | 13.34 | < .001 | 0.14 | .71 | 2.39 | .13 |
| Difference score | 9.10 | .004 | 5.43 | .02 | 1.12 | .29 |
| Amplitude S1 | 4.08 | .05 | 8.35 | .005 | 0.69 | .41 |
| Amplitude S2 | 0.34 | .56 | 6.67 | .01 | 0.01 | .94 |
| Latency S1 | 2.01 | .16 | 0.01 | .99 | 4.38 | .04 |
| Latency S2 | 0.48 | .49 | 1.07 | .31 | 0.79 | .38 |
Figure 6.
Scatter plots of relationships between P200 difference score (top panel) and S1 amplitude (bottom panel) with age (left panel) and highest level of completed education (right panel).
Posthoc Analysis: Smoking
To explore effects of smoking on sensory gating, the 11 subjects who smoked were compared to 11 subjects who did not smoke. The groups were matched for gender, age, education, and Shipley total score. Despite slightly higher ratios and lower difference scores for all three components for smokers, differences were not significant, F(1, 20) < 2.94, p > .10 (see Table 5).
Table 5.
Mean (SD) for P50, N100, and P200 ratio and difference score for smokers (n = 11) and nonsmokers (n = 11)
| P50 | N100 | P200 | ||||
|---|---|---|---|---|---|---|
| Smoking | Non | Smoking | Non | Smoking | Non | |
| Ratio | 66.32 (35.72) |
53.07 (32.34) |
56.46 (24.22) |
44.14 (21.30) |
62.77 (23.65) |
45.58 (28.23) |
| Difference score |
0.77 (0.63) |
1.66 (1.60) |
−2.78 (2.08) |
−4.59 (3.85) |
4.56 (4.11) |
7.20 (5.10) |
Note. The two groups were matched at the group level for gender, age, education, and Shipley total score.
Discussion
We explored effects of age, gender, education, and intelligence on variation in P50, N100, and P200 sensory gating in a sample of healthy subjects. Subject characteristics (Hetrick et al., 1996), together with methodological variables (De Wilde et al., 2007; Patterson et al., 2008), may explain variance in sensory gating in control subjects, and may contribute to differences in sensory gating between clinical and control samples. Consistent with earlier findings for effects of age (Patterson et al., 2008; Turetsky et al., in press) and gender (Hetrick et al., 1996; Patterson et al., 2008) on P50 gating, we expected weaker P50, N100, and P200 gating with increased age, in women, and in subjects with less education or lower intelligence.
Gender and Sensory Gating
Healthy females could have weaker P50 gating than healthy males (Hetrick et al., 1996; Patterson et al., 2008), although this was not found in other studies (Freedman et al., 1987; Waldo et al., 1987; White et al., 2005).We found no difference in P50 gating or in N100 and P200 gating between male and female subject.
Age and Sensory Gating
The relationship between age and sensory gating remains unclear. Some studies reported no changes in P50 gating with age (De Wilde et al., 2007;Wang et al., 2004),whereas others reported a low, but significant, positive correlation but only with a 10 Hz high-pass filter and not with other filter setting (Patterson et al., 2008), indicating that an effect of age on P50 gating may be weak. We found no significant relationship between age and P50 gating.
In contrast, age correlated significantly with P200 ratio, difference score, and S1 amplitude, even when the effect of intellectual capability is taken into account. These findings suggest weaker P200 gating due to attenuated P200 amplitudes as a function of age, replicating a trend toward weaker P200 gating in healthy subjects around 65 years of age compared to subjects around 50 years of age (Boutros et al., 2000).
Education, Intelligence, and Sensory Gating
We hypothesized that because of the small contributions of age and gender to sensory gating, other subject characteristics should be taken into account. Previous studies frequently did not match subjects for education or intelligence, which could have resulted in lower levels of education or intelligence in clinical compared to control groups, thus potentially distorting intergroup differences in P50, N100 and P200 sensory gating. Furthermore, generalizations across studies of data obtained from healthy subjects could also be impacted by education or intelligence considering differences in whether subjects were recruited from the general population or from university students or staff.
Because of the overlap between education and Shipley scores in the current study, analyses were performed with education only. However, initial regression analyses showed that outcomes were comparable if education or Shipley scores were used, suggesting that any relationship between education and electrophysiology could be interpreted as a relationship between electrophysiology with intellectual capability.
We found no relationship between intellectual capability with P50 gating across the total group. However, a significant relationship was found for increased intellectual capability and stronger P50 gating for females only, partly corroborating the central theory for P50 gating of its protective function of the integrity of higher-order functions (Boutros, Korzyukov, Jansen, et al., 2004; Freedman et al., 1991; Wan et al., 2008), which would have predicted a relation between P50 gating and education or intelligence. For males, however, such a relationship was not found. These findings suggest that the potential for a relationship between intellectual capability and P50 gating, although this topic needs further investigation as the data (Figure 3) suggest that the relationship could be due to one or two subjects with low Shipley scores who had a high P50 ratio.
In contrast, we found relations among N100 and P200 gating measures, S1 amplitudes, and education and Shipley scores, suggesting stronger N100 and P200 gating with increased level of education and abstract thinking, flexibility, and set-shifting (Zachary, 1986). This suggests that intellectual capability could relate to N100 and P200 gating through an increased N100 and P200 amplitude for S1. These results seem consistent with our recent finding that N100 and P200 gating and amplitude for S1, but not P50 gating may relate to attention and working memory (Lijffijt, Lane et al., in press).We recommend that education or intelligence should be controlled for in future studies of sensory gating.
Other Potential Variables Affecting Sensory Gating
Sensory gating may be affected by methodological variables including filter settings (Patterson et al., 2008), whether subjects sit or lie down, and loudness of the stimulus (De Wilde et al., 2007). However, those two studies have only addressed P50 sensory gating, and no information is available about potential effects of those variables on N100 or P200 gating.
Smoking could also affect gating, although results have been inconsistent. Some studies reported stronger gating in subjects who smoked compared to those who did not (Crawford, McClain-Furmanski, Castagnoli, & Castagnoli, 2002; Schulze et al., 2007). Other studies, in contrast, reported weaker P50 gating, but better P200 gating, in smokers compared to nonsmokers, although this was found only for subjects who scored low on schizotypy rating scales (Wan et al., 2007). In a posthoc analysis we found no differences in P50, N100, or P200 gating between smokers and nonsmokers if both groups were matched for gender, age, and intellectual capability. However, our sample of subjects contained only 11 active smokers, limiting the power to explore effects of smoking on sensory gating in our sample effectively.
Conclusions
Our findings showed stronger N100 and P200 gating with more education and higher intelligence, whereas age related to weaker P200 gating. Stronger P50 gating related to higher intelligence in females only, although this finding needs to be interpreted with caution. Our results underscore the importance of measuring education and intelligence and to minimize differences between experimental groups on these two variables, or at least to measure these variables and control for them statistically. Finally, further study should explore whether education, intelligence, and age influence gating in clinical samples, and how these variables may affect the degree of difference in gating between clinical and control groups.
Acknowledgments
This study was supported in part by the Pat R. Rutherford, Jr. Chair in Psychiatry (ACS) and by NIH grants RO1-MH 69944 (ACS), RO1-DA08425 (FGM), KO2-DA00403 (FGM), RO1-MH58784 (NNB), and UL1-RR024148 (CTSA) (General Clinical Research Center UT Houston). Thanks are due to Hima Bogagala, Blake Cox, Sherine Kurian, Stacey L. Meier, Leslie Paith, Irshad Prasla, Tammy Souter, and Tony G. Zamudio for their help with recruitment of subjects, and the General Clinical Research Center for providing research facilities and excellent nursing support.
References
- Boutros NN, Carrington Reid M, Petrakis I, Campbell D, Torello M, Krystal J. Similarities in the disturbances in cortical information processing in alcoholism and aging: A pilot evoked potential study. International Psychogeriatrics. 2000;12:513–525. doi: 10.1017/s1041610200006621. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson C. Cocaine-dependence and cocaine-induced paranoia and midlatency auditory evoked responses and sensory gating. Psychiatry Research. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the midlatency phase of information processing in medicated schizophrenic patients. Psychiatry Research. 2004;126:203–215. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyuko O, Oliwa G, Feingold A, Campbell D, McClain-Furmanski D, et al. Morphological and latency abnormalities of the midlatency auditory evoked responses in schizophrenia: A preliminary report. Schizophrenia Research. 2004;70:303–313. doi: 10.1016/j.schres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: Relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biological Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. American Journal of Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr, Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neuroscience Letters. 2002;317:151–155. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- De Wilde OM, Bour LJ, Dingemans PM, Koelman JHTM, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: Differences in methodology between research groups. Schizophrenia Research. 2007;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins C, MacKay S. Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biological Psychiatry. 1996;39:955–965. doi: 10.1016/0006-3223(95)00299-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders – patient edition. New York: Biometrics Research Institute, New York State Psychiatric Institute; 1996. [Google Scholar]
- Freedman R, Adler LE, Waldo M. Gating of the auditory evoked potential in children and adults. Psychophysiology. 1987;24:223–227. doi: 10.1111/j.1469-8986.1987.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunction in schizophrenia. Schizophrenia Research. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Fresán A, Apiquian R, García-Anaya M, de la Fuente-Sandoval C, Nicolini H, Graff-Guerrero A. The P50 auditory evoked potential in violent and nonviolent patients with schizophrenia. Schizophrenia Research. 2007;97:128–136. doi: 10.1016/j.schres.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Soveri P, Järvilehto T. Short-term habituation of the auditory evoked response in man. Electroencephalography and Clinical Neurophysiology. 1970;28:153–161. doi: 10.1016/0013-4694(70)90183-5. [DOI] [PubMed] [Google Scholar]
- Fuerst DR, Gallinat J, Boutros NN. Range of sensory gating values and test-retest reliability in normal subjects. Psychophysiology. 2007;44:620–626. doi: 10.1111/j.1469-8986.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Ghisolfi ES, Heldt E, Zanardo AP, Strimitzer IM, Jr, Prokopiuk AS, Becker J, et al. P50 sensory gating in panic disorder. Psychiatry Research. 2006;40:535–540. doi: 10.1016/j.jpsychires.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Miller GA, Thoma RJ, Irwin J, Jones A, Moses SN, et al. Distinct M50 and M100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. doi: 10.1111/j.1469-8986.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Hetrick WP, Sandman CA, Bunney WE, Jr, Jin Y, Potkin SG, White MH. Gender differences in gating of the auditory evoked potential in normal subjects. Biological Psychiatry. 1996;39:51–58. doi: 10.1016/0006-3223(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical Neurophysiology. 1958;10:370–371. [Google Scholar]
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biological Psychiatry. 1992;31:365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in posttraumatic stress disorder. Biological Psychology. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Noecker TL, Guinther PM. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 2004;41:604–612. doi: 10.1111/j.1469-8986.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, et al. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00845.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Moeller FG, Boutros NN, Burroughs S, Steinberg JL, Lane SD, et al. A pilot study revealing impaired P50 gating in antisocial personality disorder. Journal of Neuropsychiatry and Clinical Neurosciences. doi: 10.1176/appi.neuropsych.21.3.328. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Moeller FG, Boutros NN, Steinberg JL, Meier SL, Lane SD, et al. Diminished P50, N100 and P200 auditory sensory gating in bipolar I disorder. Psychiatry Research. doi: 10.1016/j.psychres.2008.04.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R. Gating of the auditory response in schizophrenics and normal controls. Schizophrenia Research. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. American Journal of Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Oranje B, Geyer MA, Bocker KBE, Kenemans JL, Verbaten MN. Prepulse inhibition and P50 suppression: Commonalities and dissociations. Psychiatry Research. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, et al. P50 sensory gating ratios in schizophrenics and controls: A review and data analysis. Psychiatry Research. 2008;158:226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Jockers-Scherübl MC, Boutros NN, Gallinat J. Test-retest reliability of the P50, N100, and P200 auditory sensory gating in healthy subjects. International Journal of Psychophysiology. 2008;67:81–90. doi: 10.1016/j.ijpsycho.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Sable JJ, Low KA, Maclin EL, Fabiani M, Gratton G. Latent inhibition mediates N1 attenuation to repeating sounds. Psychophysiology. 2004;41:636–642. doi: 10.1111/j.1469-8986.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall M, McDonald C, Marshall N, Walshe M, Murray RM, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biological Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliability and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. Journal of Psychology. 1940;9:371–377. [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB. Reliability of the P50 auditory event-related potential indices of sensory gating. Psychophysiology. 1994;31:495–502. doi: 10.1111/j.1469-8986.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 2nd ed. New York: Harper & Row; 1989. [Google Scholar]
- Thoma RJ, Hanlon FM, Miller GA, Huang M, Weisend MP, Sanchez FP, et al. Neuropsychological and sensory gating deficits related to remote alcohol abuse history in schizophrenia. Journal of the International Neuropsychological Society. 2006;12:34–44. doi: 10.1017/S1355617706060097. [DOI] [PubMed] [Google Scholar]
- Thomas C, Vom Berg I, Rupp A, Seidl U, Schröder J, Roesch-Ely D, et al. P50 gating deficit in Alzheimer dementia correlates to frontal neuropsychological function. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2008.05.002. (in press) [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Greenwoord TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, et al. Abnormal auditory N100 amplitude: A heritable endophenotype in first-degree relatives of schizophrenia probands. Biological Psychiatry. doi: 10.1016/j.biopsych.2008.06.018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo MC, Graze K, de Graff Bender S, Adler LE, Freedman R. Premenstrual mood changes and gating of the auditory evoked potential. Psychoneuroendocrinology. 1987;12:35–40. doi: 10.1016/0306-4530(87)90020-5. [DOI] [PubMed] [Google Scholar]
- Wan L, Crawford HJ, Boutros N. Early and late auditory sensory gating: Moderating influences from schizotypal personality, tobacco smoking status, and acute smoking. Psychiatry Research. 2007;151:11–20. doi: 10.1016/j.psychres.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Wan L, Friedman BH, Boutros NN, Crawford HJ. P50 sensory gating and attentional performance. International Journal of Psychophysiology. 2008;67:91–100. doi: 10.1016/j.ijpsycho.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Miyazato H, Hokama H, Hiramatsu K, Kondo T. Correlation between P50 suppression and psychometric schizotypy among nonclinical Japanese subjects. International Journal of Psychophysiology. 2004;52:147–157. doi: 10.1016/j.ijpsycho.2003.06.001. [DOI] [PubMed] [Google Scholar]
- White PM, Kanazawa A, Yee CM. Gender and suppression of mind-latency ERP components during stress. Psychophysiology. 2005;42:720–725. doi: 10.1111/j.1469-8986.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley institute of living scale: Revised manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- Zouridakis G, Boutros NN. Stimulus parameter effects on the P50 evoked response. Biological Psychiatry. 1992;32:839–841. doi: 10.1016/0006-3223(92)90088-h. [DOI] [PubMed] [Google Scholar]