Abstract
Objective:
To compare cognitive-behavioral therapy (CBT) and standard medical care (SMC) as treatments for psychogenic nonepileptic seizures (PNES).
Methods:
Our randomized controlled trial (RCT) compared CBT with SMC in an outpatient neuropsychiatric setting. Sixty-six PNES patients were randomized to either CBT (plus SMC) or SMC alone, scheduled to occur over 4 months. PNES diagnosis was established by video-EEG telemetry for most patients. Exclusion criteria included comorbid history of epilepsy, <2 PNES/month, and IQ <70. The primary outcome was seizure frequency at end of treatment and at 6-month follow-up. Secondary outcomes included 3 months of seizure freedom at 6-month follow-up, measures of psychosocial functioning, health service use, and employment.
Results:
In an intention-to-treat analysis, seizure reduction following CBT was superior at treatment end (group × time interaction p < 0.0001; large to medium effect sizes). At follow-up, the CBT group tended to be more likely to have experienced 3 months of seizure freedom (odds ratio 3.125, p = 0.086). Both groups improved in some health service use measures and on the Work and Social Adjustment Scale. Mood and employment status showed no change.
Conclusions:
Our findings suggest that cognitive-behavioral therapy is more effective than standard medical care alone in reducing seizure frequency in PNES patients.
Classification of evidence:
This study provides Class III evidence that CBT in addition to SMC, as compared to SMC alone, significantly reduces seizure frequency in patients with PNES (change in median monthly seizure frequency: baseline to 6 months follow-up, CBT group, 12 to 1.5; SMC alone group, 8 to 5).
GLOSSARY
- AED
= antiepileptic drug;
- CBT
= cognitive-behavioral therapy;
- CI
= confidence interval;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- HADS
= Hospital Anxiety and Depression Scale;
- IQR
= interquartile range;
- ITT
= intention-to-treat;
- OR
= odds ratio;
- PNES
= psychogenic nonepileptic seizures;
- RCT
= randomized controlled trial;
- SMC
= standard medical care;
- WASAS
= Work and Social Adjustment Scale.

LOE Classification
Psychogenic nonepileptic seizures (PNES) are paroxysmal episodes of behavior resembling epileptic seizures but lacking organic etiology. Most clinicians agree that in most cases the episodes are involuntary, arising through unconscious psychological mechanisms. They are, therefore, classified as dissociative phenomena (International Classification of Diseases–10)1 or as conversion disorder (DSM-IV),2 and are distinguished from willful attempts to simulate epilepsy. PNES are common, with an estimated prevalence of 2–33 per 100,000.3 The disorder is associated with chronic disability and welfare dependence, especially when the diagnosis is delayed, as it usually is.4
Referral for psychological treatment is common,5 yet there is no reliable evidence for the efficacy of such treatment from randomized controlled trials (RCTs).6 Cognitive-behavioral therapy (CBT) is an effective treatment for other somatoform disorders,7-10 although evidence is lacking for dissociative/conversion disorders.9 We11 and others12,13 have conducted promising open-label trials of CBT in PNES. Developed from our previous work,11,14 we set out to test the superiority of CBT over standard medical care in treating PNES within a pilot RCT.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Ethics Committee at the Institute of Psychiatry, London, UK (Clinicaltrials.gov identifier NCT00688727). Patients gave written informed consent. We followed CONSORT reporting guidelines.15 The methodology provides Class III evidence concerning the use of CBT plus SMC vs SMC alone in the reduction of monthly PNES frequency.
Participants.
Participants were recruited from the Neuropsychiatry Service, South London and Maudsley NHS Foundation Trust, London, UK, from June 2001 through April 2007. Inclusion criteria were as follows: 1) age 18–70 years; 2) clinical diagnosis of PNES primarily confirmed by video-EEG telemetry, and only if this was not feasible by ictal EEG, or where the referrer and consultant neuropsychiatrists involved in the study agreed that there was no doubt about the diagnosis and that further investigation was unjustified (“clinical consensus”). Exclusion criteria were as follows: 1) a coexistent diagnosis (past or current) of epilepsy; 2) <2 seizures per month; 3) current drug or alcohol misuse; 4) benzodiazepine use exceeding the equivalent of 10 mg diazepam/day; 5) IQ <70.
Randomization.
Following an assessment by a consultant neuropsychiatrist, when the patient's diagnosis was restated (table e-1 on the Neurology® Web site at www.neurology.org; see reference 4), patients were randomized to CBT or SMC using an independently prepared sequence of consecutive, randomized treatment assignments. This was developed from a table of random numbers, using unstratified permuted blocks of 4, and concealed in sealed envelopes. The envelopes were then numbered consecutively and given to an independent clinician who allocated them in order as patients gave written informed consent.
Treatments.
Cognitive-behavioral therapy.
Our CBT manual was based on our earlier study11 and was developed from Chalder's14 case report. The underlying model has been described elsewhere.11,14,16
Following an assessment session, participants were offered up to 12 weekly/fortnightly hour-long outpatient sessions of CBT with a CBT-trained nurse therapist with experience in working with PNES patients.
Treatment involved enabling patients to interrupt the behavioral/physiologic/cognitive responses experienced at the start of the seizure, encouraging them to engage in activities they were avoiding, and addressing negative thoughts and illness beliefs maintaining PNES occurrence, low self-esteem, low mood, or anxiety. Sessions incorporated agenda-setting and the planning and review of homework, which included completing seizure records. While the main treatment aim was seizure reduction and improved psychosocial functioning, the approach was formulation-based; therefore, issues raised in sessions that might be perpetuating seizures were addressed. We considered that patients receiving at least 9 sessions of CBT would receive the key elements of the therapy package (table e-2).
Therapy sessions were audio-recorded. Two independent raters evaluated the content of sessions 4 and 9. Overall ratings of therapeutic alliance and CBT were calculated.17 Higher scores represented either more extensive therapeutic alliance or higher-quality CBT.
Standard medical care.
Patients in both groups were offered ongoing clinic review by a neuropsychiatrist. Appointment frequency was determined by clinical need. Patients in the SMC group received SMC only. Sessions were supportive in nature, and provided explanations about the psychological basis of the seizures and supervised withdrawal of antiepileptic drugs (AEDs). Instructions were given not to include any CBT-based techniques (e.g., relaxation, breathing exercises, distraction methods) or structured cognitive techniques (e.g., identifying negative thoughts and dysfunctional thinking styles). As we intended the CBT sessions to be completed within a 4-month period, SMC sessions occurred over a 4-month period and end-of-treatment measures were then taken.
Both patient groups were followed up for 6 months after the end of treatment. During this period, patients were offered follow-up sessions at 1, 3, and 6 months.
Outcome measures.
The primary outcome measure was monthly seizure frequency, measured at start and end of treatment and at 6-month follow-up. Patients were encouraged to keep seizure diaries and their seizure frequency was recorded at appointments.
Secondary outcomes included 1) seizure freedom, defined as having no seizures during the 3-month period immediately prior to the 6-month follow-up point; 2) Work and Social Adjustment Scale (WASAS) scores18,19; 3) Hospital Anxiety and Depression Scale (HADS) scores20-22; and 4) a modified Client Service Receipt Inventory23 measuring patients' health service use and employment during the 6 months prior to treatment and in the 6-month follow-up period. Table e-3 provides information about these measures.
Sample size calculation.
Based on previous experience, we assumed a true mean change in monthly seizure frequency of 7.3 in the CBT group, no change in the SMC group, and a common SD of 9.5 seizures. Therefore, 28 per group were required to detect a difference with this effect size (Cohen Δ = 0.768)24 with 80% power at p = 0.05. Allowing for a 20% dropout rate, we sought to recruit 35 patients per group; resource limitations resulted in recruitment of 33 per group.
Statistical analyses.
The effect of treatment (CBT vs SMC) over time on the primary and secondary outcomes was investigated in intention-to-treat (ITT) analyses. Seizure frequency and health service measures were analyzed using Poisson mixed models with maximum likelihood estimation, in STATA v10.0; analyses assumed missing at random with missingness allowed to be driven by variables included in the analyses. Questionnaire data were analyzed using linear mixed models25 in SPSS v 15.0. All models included random intercepts to account for correlations between the repeated measures for each participant. The fixed components of the models included effects of group and time and a group × time interaction. To assess the presence of a group effect, where this may change over time, we first determined the existence of group × time interactions. Where interactions (p < 0.1) were identified, the effect of group at each time point was evaluated. Interactions where p ≥ 0.1 were excluded from the model; main effects of group and time were then estimated from the reduced model. Logistic regression was used to determine the effect of treatment on seizure freedom and employment status.
RESULTS
Participants.
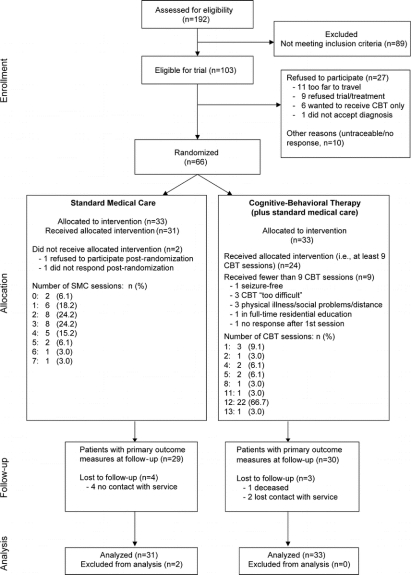
A total of 192 PNES patients were referred to the service (figure 1). Of these, 103 patients met our inclusion criteria; 37 then either refused to participate in the trial or were not contactable. Thirty-three were randomized to each treatment arm (i.e., in total 64% of all those eligible). While the number of SMC sessions varied according to clinical need, 2 patients either refused to participate or did not respond at all following randomization and so received no SMC sessions. Data were included from all 33 CBT participants and from 31 SMC participants when analyzing the primary outcome measure.
Figure 1 Study flow
CBT = cognitive-behavioral therapy.
Figure 1 also shows the number of CBT sessions received. Concurrently, most CBT patients attended SMC sessions. Although 7 CBT patients received no SMC sessions, 12 patients had 1 SMC session, 3 patients had 2 SMC sessions, 9 patients had 3 SMC sessions, and 2 patients (1 of whom also had an in-patient admission) had 4 SMC sessions.
During the CBT patients' follow-up period, 1 person had 1 CBT session, 6 patients had 2 sessions, and 11 patients had 3 sessions. In terms of concurrent SMC sessions during follow-up, 5 CBT patients also had 1 SMC session, 4 people had 2 SMC sessions, and 1 person had 3 SMC sessions. During follow-up, 6 SMC patients were seen once, 10 were seen twice, 7 were seen 3 times, and 3 people received 4 SMC sessions.
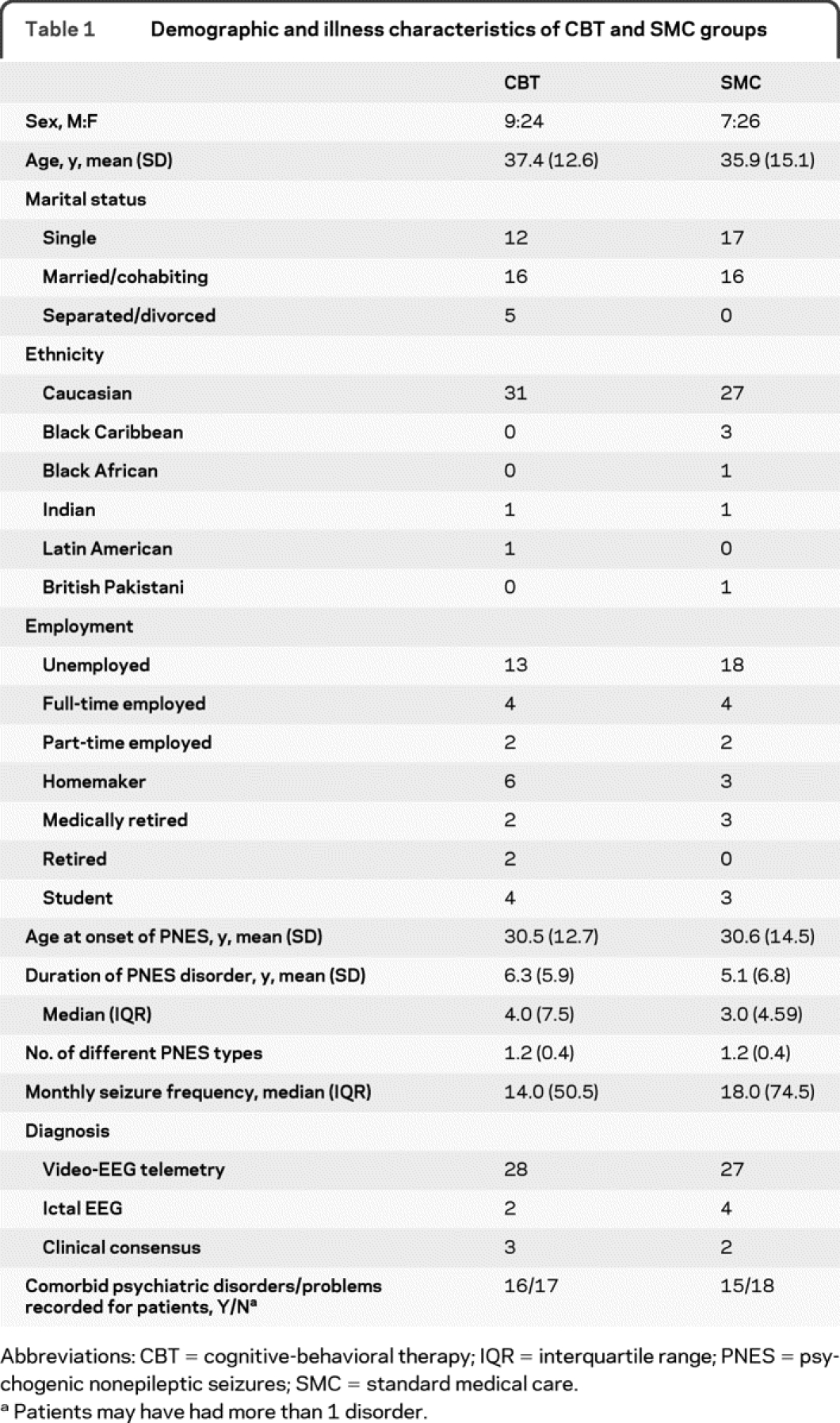
The groups had similar demographic characteristics and seizure histories at enrollment (table 1). The ratio of women to men in both groups was typical of patients with PNES.26 Most people were unemployed: only 6 in each group indicated any employment and only 3 (SMC) or 4 (CBT) reported that they were students. The majority were diagnosed following video-EEG telemetry. The proportions diagnosed using ictal EEG or clinical consensus were similar in each group.
Table 1 Demographic and illness characteristics of CBT and SMC groups
Protocol violations.
Following randomization, 1 person allocated to SMC received CBT due to an administrative error. One person received 13 CBT sessions due to distress expressed during their 12th session. Two further SMC patients commenced CBT before the end of the follow-up period. Due to perceived clinical need, 3 SMC patients received 4 SMC sessions in the 6-month follow-up period. All of these patients are included in the ITT analyses.
Primary outcome: Seizure frequency.
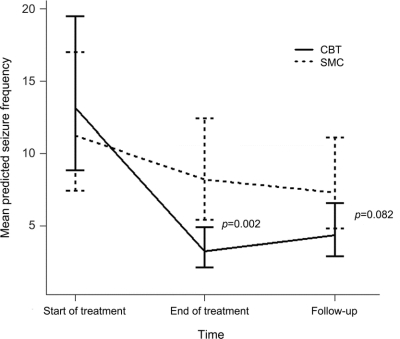
Median monthly seizure frequency at the start and end of treatment and at follow-up is shown in table 2. The presence of missing seizure data was not predicted by current age (p = 0.816), age at PNES onset (p = 0.607), duration of the PNES disorder (p = 0.539), or gender (p = 0.341), so these variables were not included in the regression models. We analyzed seizure frequency data, adjusting for prerandomization seizure frequency. We found a group × time interaction [Wald χ2(2) = 168.06, p < 0.0001]; there was no between-groups difference at start of treatment but significantly lower seizure frequency at treatment end and a trend for lower seizure frequency at follow-up following CBT (figure 2 and table 3). We found a large between-groups effect size (0.75)24 at end of treatment and a medium effect size (0.42) at follow-up.
Table 2 Mean and median scores for outcome measures at each timepoint
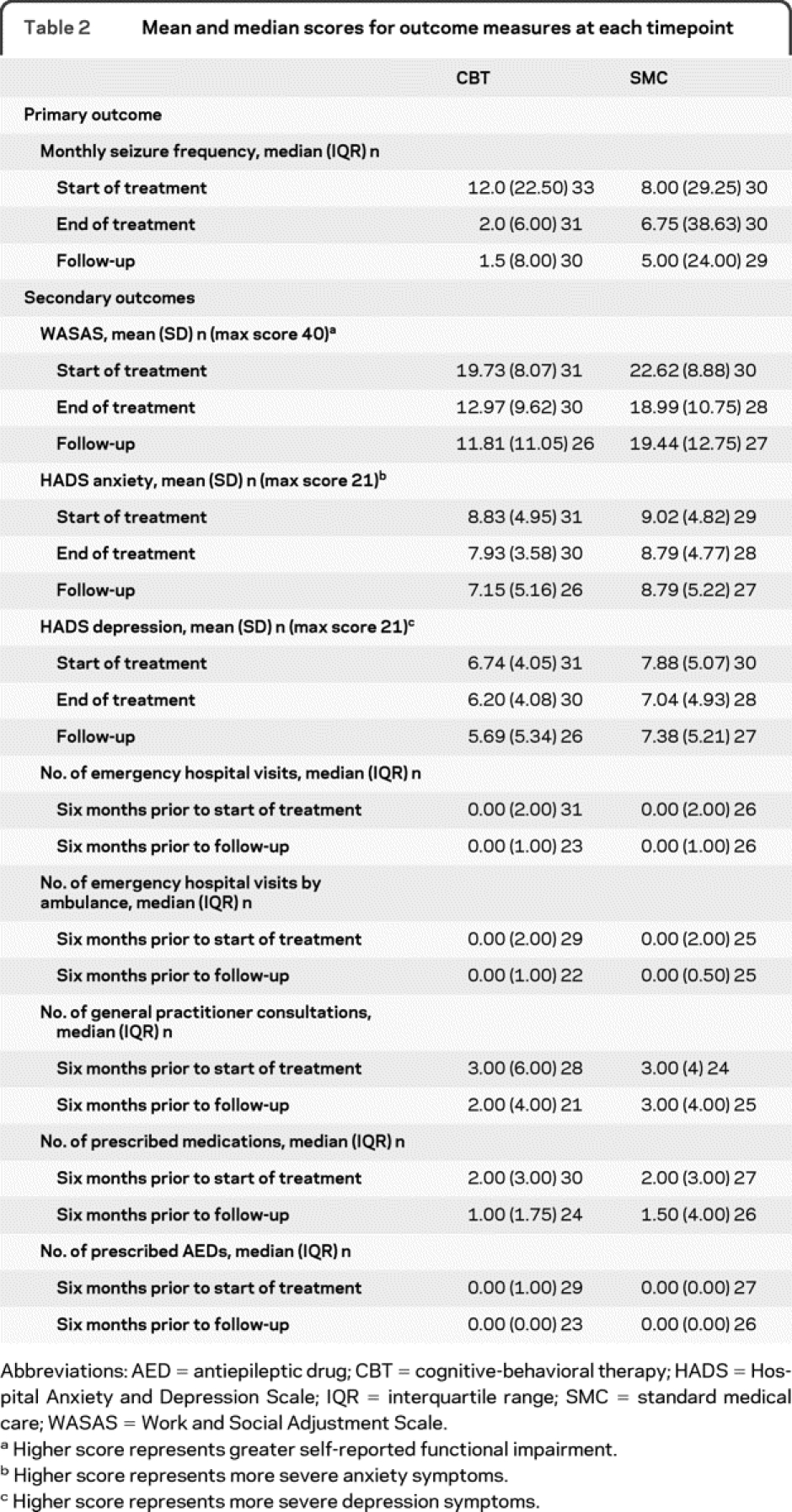
Figure 2 Mean predicted seizure frequency from Poisson mixed model analysis, adjusted for prerandomization seizure frequency
CBT = cognitive-behavioral therapy; SMC = standard medical care. Error bars: 95% confidence interval.
Table 3 Estimates of between-groups differences from fixed-effects component of Poisson mixed model and LMM analyses
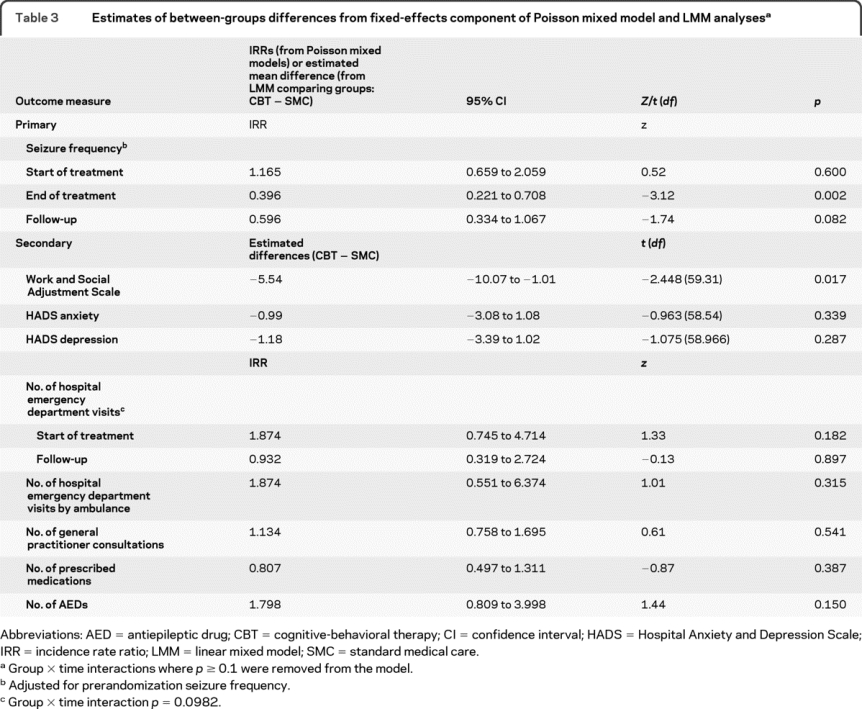
Secondary outcomes.
Seizure freedom.
There was a trend for the CBT group to be more likely to be seizure-free at the 6-month follow-up (odds ratio [OR] = 3.125, 95% confidence interval [CI] 0.852–11.468, p = 0.086). Absolute risk reduction was 19.5%; risk was defined as not being seizure-free at follow-up. The number needed to treat to achieve seizure freedom in 1 patient was 5.13.
Questionnaires.
We report questionnaire scores for start and end of treatment and follow-up in table 2 and estimates of between-groups differences from the linear mixed model analyses in table 3.
On the WASAS, the absence of a group × time interaction (p = 0.120) indicated no differential benefit of CBT over time. However, overall the CBT group rated their PNES as having less functional impact than did the SMC group (F1,59.31 = 5.99, p = 0.017; effect size = 0.65); there was an overall effect of time (F2,111.36 = 12.61, p < 0.001).
For the HADS anxiety and depression subscales, there were no group × time interactions and no main effects of group or time.
Health service use and employment in the 6 months pretreatment and in the 6 months to follow-up (table 2).
For most measures, we found no group × time interactions at p < 0.1. However, for the number of emergency department visits, presence of the group × time interaction [Wald χ2(1) = 2.73, p = 0.098] and the time-specific incidence rate ratios from the Poisson mixed models (table 3) suggested that in the 6-month follow-up period the CBT group tended to show a greater reduction in such visits than the SMC group; there was also a main effect of time (z = −2.10, p = 0.036) on this measure. There were no main effects of group (table 3). There was a reduction over time in the number of emergency department visits made by ambulance (time z = −5.66, p < 0.001).
No reductions were found in the reported number of prescribed medications or, specifically, of AEDs in the 6-month follow-up period compared to before treatment (time: p > 0.1), but most patients were not taking AEDs at the start of treatment (table 2).
Prior to treatment, there was no between-groups difference in terms of whether patients were in any kind of employment/were students or were unemployed/retired (OR 0.913, 95% CI 0.317–2.629, p = 0.866). The pattern was similar at follow-up (OR = 1.219, 95% CI 0.428–3.476, p = 0.711).
Quality assurance.
The median rating for overall therapeutic alliance for sessions 4 and 9 was 5.00 (maximum 7; session 4 interquartile range [IQR] 2.25 and session 9 IQR 2.38). The median overall ratings for CBT quality for these sessions were 5.25 (maximum 7; session 4 IQR 1.38 and session 9 IQR 2.75).
Exploratory analyses for the primary outcome.
We explored whether protocol violations, the inclusion of the 5 patients diagnosed by clinical consensus, and variations in length of treatment and follow-up had an impact on seizure outcome. We again found a group × time interaction [Wald χ2(2) = 208.72, p < 0.0001] indicating similar results to the main analysis. Two therapists delivered the CBT, 1 treating 12 and the other treating 21 patients; there was no therapist × time interaction for seizure outcome [Wald χ2(2) = 1.88, p = 0.390] and no therapist effect (z = 0.66 p = 0.511).
Adverse outcomes.
One patient in the CBT group, with a diagnosis of emotionally unstable personality disorder, committed suicide in the follow-up period. This was considered to be unrelated to her seizure disorder, which had improved during CBT.
DISCUSSION
Building on our previous work,11,14 the results from this pilot RCT suggest that, relative to SMC alone, CBT (plus SMC) produced a greater reduction in PNES frequency at the end of treatment (figure 2 and table 3). There was a tendency for this benefit to be maintained at the 6-month follow-up, when there was also a trend for CBT to be more likely to have resulted in 3 consecutive months without PNES. We also observed some reduction in PNES frequency in our SMC group, suggesting that our standard outpatient neuropsychiatric care offered patients some benefit. There was an overall improvement in self-rated social functioning; CBT was not differentially more effective over time in this respect. While we observed no changes in mood and observed only minimal improvements in health service use, the current study lends weight to the potential contribution of CBT to the management of PNES when compared directly to another treatment.
Our CBT approach is predicated on the assumption that PNES represent dissociative responses to arousal,27 occurring when the person is faced with fearful or intolerable circumstances.11,16 Our treatment model emphasizes seizure reduction techniques especially in the early treatment sessions and most of the CBT group will have been exposed to these techniques. While the usefulness of seizure remission as an outcome measure has been questioned,28 seizures are the reason for patients' referral for treatment. Despite being seizure-free, some patients may remain unemployed or dependent on benefits.28,29
The absence of change in HADS scores was unexpected11 but may reflect low pretreatment scores, providing less scope for change. This finding may also reflect the heterogeneous nature of the patients, who presented with a range of comorbid diagnoses, including somatoform (nonseizure) disorder, eating disorder, and personality disorder, and relate to reports of PNES occurrence in the absence of raised levels of general anxiety.27
Our pilot RCT has a number of limitations. Our SMC condition did not control for therapist contact, which was greater in the CBT group. Another limitation concerns the difficulty of rendering psychological treatment studies such as this blind: whether the patients were receiving CBT could not be concealed from the neuropsychiatrists providing SMC. We cannot determine how this influenced the number or content of the SMC sessions.
Other important limitations concern sample size and selection. Our study was designed to detect a large treatment effect yet we found only a medium effect size at follow-up. Furthermore, in planning the study, our assumption of no improvement in the SMC group was not borne out in our outcome measures. Our clinical service is specialized. Patients referred to us have typically not responded to routine interventions in neurology clinics, which may themselves constitute a highly effective treatment.30 Thus, a selection bias in favor of chronic, more difficult-to-treat patients might exist. However, patients had at least accepted referral to a psychiatric service, which some of the most intractable patients may refuse to do. Willingness to enroll in the study might also indicate motivation to reduce seizure occurrence and explain the SMC group's improvement. Additionally, our SMC is an intervention, so we cannot comment on a potential comparison between the benefits of CBT vs no treatment at all. Although our results are not readily generalizable to nonspecialist settings,31 they are likely to be generalizable to other specialist services with an interest in both neurologic and psychiatric management of PNES. We chose to exclude patients with comorbid epilepsy to facilitate measuring outcome in terms of PNES frequency. It has been suggested that such patients might benefit from psychological interventions13 and our study provides no contradictory evidence. While ideally all patients would have been diagnosed on the basis of video-EEG telemetry, with a low seizure frequency this is not always feasible and our groups were well-matched for diagnostic methods.
Although primary outcomes were analyzed for almost all participants, statistical power may have been reduced: some patients were unwilling to complete the secondary outcome questionnaires (which were also not completed by patients prior to treatment arm assignment) (table 2) and seizure freedom could not be evaluated in 7/66 patients. Finally, our 6-month follow-up may have been too short to observe a change in employment status; indeed, the older age of some participants and financial incentives derived from state benefits may have dissuaded people from seeking work.
Larger multicenter studies would allow comparisons between CBT and other psychological interventions controlling for therapist contact. They would also permit investigations of predictors of treatment response.32 This will help identify patients most likely to benefit from treatment and improve clinical decision-making. Additionally, such studies would permit greater examination of the magnitude of therapist effects.33 Longer posttreatment follow-up would provide a better comparison of treatment outcomes to the natural history of PNES.29
These limitations notwithstanding, our results suggest CBT can reduce PNES occurrence. Further investigation of those who may benefit most from CBT may offer increased opportunities to treat this disabling disorder.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Prof. Goldstein and Dr. Khondoker.
ACKNOWLEDGMENT
Sara Mitchell-O'Malley (South London and Maudsley NHS Foundation Trust) treated the first 12 CBT patients reported here. The authors thank Gillian Watson, Ken McKeown (South London and Maudsley NHS Foundation Trust), and Laura Southgate (Institute of Psychiatry, King's College London) for rating the CBT tapes and Izzy Taylor, Anna Silverman, and Lizzy Banwell (South London and Maudsley NHS Foundation Trust) for assistance with data entry.
DISCLOSURE
Prof. Goldstein serves on the Scientific Awards Panel for Epilepsy Action (UK); has received travel expenses and honoraria for speaking and educational activities not funded by industry; receives royalties from the publication of Clinical Neuropsychology (Wiley, 2004) and The Clinical Psychologist's Handbook of Epilepsy (Routledge, 1997); and receives research support from Department of Health/National Institute for Health Research (NIHR) UK, Epilepsy Research UK, the Institute of Social Psychiatry, the Wellcome Trust, and the Motor Neurone Disease Association UK. Prof. Chalder has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; serves as Editor of the Journal of Mental Health; receives royalties from the publication of Coping with Chronic Fatigue (Sheldon Press, 1995), Self Help for Chronic Fatigue Syndrome: A Guide for Young People (Blue Stallion Publication, 2002), and Overcoming Chronic Fatigue (Constable & Robinson, 2005); and receives research support from the Medical Research Council, the MS Society, the European Commission, National Institute for Health Research (NIHR), the Department of Health (DoH), and the DoH via the NIHR Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King's College London. C. Chigwedere receives research support from Trinity College, University of Dublin, and St Patrick's Hospital, Dublin. Dr. Khondoker receives research support from the Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King's College London. Dr. Moriarty receives research support from Epilepsy Research UK. Dr. Toone reports no disclosures. Dr. Mellers receives royalties from the publication of Lishman's Organic Psychiatry (4th edition): A Textbook of Neuropsychiatry (Wiley-Blackwell, 2009) and receives research support from Epilepsy Research UK.
Supplementary Material
Address correspondence and reprint requests to Prof. L.H. Goldstein, Department of Psychology, PO77, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, UK laura.goldstein@kcl.ac.uk
Supplemental data at www.neurology.org
Study funding: Supported by the Guy's and St. Thomas' Charitable Foundation.
Disclosure: Author disclosures are provided at the end of the article.
Received August 11, 2009. Accepted in final form March 16, 2010.
REFERENCES
- 1.World Health Organisation. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 3.Benbadis SR, Agrawal V, Tatum WO. How many patients with psychogenic nonepileptic seizures also have epilepsy? Neurology 2001;57:915–917. [DOI] [PubMed] [Google Scholar]
- 4.Mellers JDC. The approach to patients with ‘non-epileptic seizures.’ Postgrad Med J 2005;81:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuber M. Psychogenic nonepileptic seizures: answers and questions. Epilepsy Behav 2008;12:622–635. [DOI] [PubMed] [Google Scholar]
- 6.Baker GA, Brooks JL, Goodfellow L, Bodde N, Aldenkamp A. Treatments for non-epileptic attack disorder. Cochrane Database Syst Rev 2007;1:CD006370. [DOI] [PubMed]
- 7.Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clin Psychol Rev 2007;27:781–797. [DOI] [PubMed] [Google Scholar]
- 8.Kroenke K, Swindle R. Cognitive-behavioral therapy for somatization and symptom syndromes: a critical review of controlled clinical trials. Psychother Psychosom 2000;69:205–215. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med 2007;69:881–888. [DOI] [PubMed] [Google Scholar]
- 10.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev 2008;3:CD001027. [DOI] [PMC free article] [PubMed]
- 11.Goldstein LH, Deale AC, Mitchell-O'Malley SJ, Toone BK, Mellers JD. An evaluation of cognitive behavioral therapy as a treatment for dissociative seizures: a pilot study. Cogn Behav Neurol 2004;17:41–49. [DOI] [PubMed] [Google Scholar]
- 12.LaFrance JW, Miller IW, Ryan CE, et al. Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav 2009;14:591–596. [DOI] [PubMed] [Google Scholar]
- 13.Rusch MD, Morris GL, Allen L, Lathrop L. Psychological treatment of nonepileptic events. Epilepsy Behav 2001;2:277–283. [DOI] [PubMed] [Google Scholar]
- 14.Chalder T. Non-epileptic attacks: a cognitive behavioural approach in a single case approach with a four-year follow-up. Clin Psychol Psychother 1996;3:291–297. [Google Scholar]
- 15.Moher D, Schulz KF, Altman DG, CONSORT group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 2001;134:657–662. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LH, LaFrance Jr WC, Chigwedere C, Mellers JDC, Chalder T. Cognitive behavioral treatments. In: Schachter SC, LaFrance Jr WC, eds. Gates and Rowan's Non-Epileptic Seizures, 3rd ed. New York: Cambridge University Press; 2010:281–288. [Google Scholar]
- 17.Godfrey E, Chalder T, Ridsdale L, Seed P, Ogden J. Investigating the ‘active ingredients’ of cognitive behaviour therapy and counselling for patients with chronic fatigue in primary care: developing a new process measure to assess treatment fidelity and predict outcome. Br J Clin Psychol 2007;46:253–272. [DOI] [PubMed] [Google Scholar]
- 18.Marks IM. Behavioural Psychotherapy: Maudsley Pocket Book of Clinical Management. Bristol, UK: Wright; 1986. [Google Scholar]
- 19.Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461–464. [DOI] [PubMed] [Google Scholar]
- 20.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 21.Crawford JR, Henry JD, Crombie C, Taylor EP. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol 2001;40:429–434. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 23.Beecham J, Knapp M. Costing psychiatric interventions. In: Thornicroft G, ed. Measuring Mental Health Needs. London: Gaskell; 2001. [Google Scholar]
- 24.Cohen J. A power primer. Psychol Bull 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 25.Landau S, Everitt BS. A Handbook of Statistical Analyses using SPSS. Boca Raton, FL: Chapman & Hall/CRC; 2004. [Google Scholar]
- 26.Gates JR. Nonepileptic seizure. Epilepsy Behav 2002;3:28–33. [Google Scholar]
- 27.Goldstein LH, Mellers JD. Ictal symptoms of anxiety, avoidance behaviour, and dissociation in patients with dissociative seizures. J Neurol Neurosurg Psychiatry 2006;77:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuber M, Mitchell AJ, Howlett S, Elger CE. Measuring outcome in psychogenic nonepileptic seizures: how relevant is seizure remission? Epilepsia 2005;46:1788–1795. [DOI] [PubMed] [Google Scholar]
- 29.Reuber M, Pukrop R, Bauer J, Helmstaedter C, Tessendorf N, Elger CE. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol 2003;53:305–311. [DOI] [PubMed] [Google Scholar]
- 30.Oto M, Espie C, Pelosi A, Selkirk M, Duncan R. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry 2005;76:1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaFrance J, Rusch MD, Machan JT. What is “treatment as usual” for nonepileptic seizures? Epilepsy Behav 2008;12:388–394. [DOI] [PubMed] [Google Scholar]
- 32.Bowman ES. Psychopathology and outcome in pseudoseizures. In: Ettinger ABE, Kanner AME, eds. Psychiatric Issues in Epilepsy: A Practical Guide to Diagnosis and Treatment. Philadelphia: Lippincott Williams & Wilkins; 2001:355–377. [Google Scholar]
- 33.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.