Abstract
Objective:
Learning and memory impairments are prevalent among persons with multiple sclerosis (MS); however, such deficits are only weakly associated with MS disease severity (brain atrophy). The cognitive reserve hypothesis states that greater lifetime intellectual enrichment lessens the negative impact of brain disease on cognition, thereby helping to explain the incomplete relationship between brain disease and cognitive status in neurologic populations. The literature on cognitive reserve has focused mainly on Alzheimer disease. The current research examines whether greater intellectual enrichment lessens the negative effect of brain atrophy on learning and memory in patients with MS.
Methods:
Forty-four persons with MS completed neuropsychological measures of verbal learning and memory, and a vocabulary-based estimate of lifetime intellectual enrichment. Brain atrophy was estimated with third ventricle width measured from 3-T magnetization-prepared rapid gradient echo MRIs. Hierarchical regression was used to predict learning and memory with brain atrophy, intellectual enrichment, and the interaction between brain atrophy and intellectual enrichment.
Results:
Brain atrophy predicted worse learning and memory, and intellectual enrichment predicted better learning; however, these effects were moderated by interactions between brain atrophy and intellectual enrichment. Specifically, higher intellectual enrichment lessened the negative impact of brain atrophy on both learning and memory.
Conclusion:
These findings help to explain the incomplete relationship between multiple sclerosis disease severity and cognition, as the effect of disease on cognition is attenuated among patients with higher intellectual enrichment. As such, intellectual enrichment is supported as a protective factor against disease-related cognitive impairment in persons with multiple sclerosis.
GLOSSARY
- AD
= Alzheimer disease;
- ANOVA
= analysis of variance;
- MPRAGE
= magnetization-prepared rapid gradient echo;
- MS
= multiple sclerosis;
- SRT
= Selective Reminding Test;
- TVW
= third ventricle width;
- WASI
= Wechsler Abbreviated Scale of Intelligence.
Learning and memory impairments are prevalent among persons with multiple sclerosis (MS)1; however, estimates of MS disease severity (brain atrophy) account for only about 10% to 15% of the variance in verbal learning and memory.2,3 This incomplete relationship between brain disease and cognitive status is typical of neurologic diseases more generally (e.g., Alzheimer disease [AD]4), and has stimulated a search for factors that may moderate this relationship. One factor that has emerged is lifetime intellectual enrichment: neurologic patients with greater lifetime intellectual enrichment are at reduced risk for cognitive impairment/dementia.5 According to this cognitive reserve hypothesis, intellectual enrichment lessens the negative impact of brain disease on cognition. The current research examines whether higher intellectual enrichment lessens the negative impact of brain atrophy on learning and memory in persons with MS.
METHODS
Subject enrollment.
Subjects were 44 patients (39 women, 5 men) with MS,6 no exacerbation in the last 4 weeks, no current corticosteroid use, and no history of serious psychiatric illness, substance abuse, learning disability, or other neurologic condition (sample: age, 44.9 ± 6.9 years; education, 16.1 ± 2.3 years; disease duration, 10.5 ± 7.0 years; disease course, 34 relapsing-remitting, 7 secondary progressive, 3 primary progressive).
Standard protocol approvals, registrations, and patient consents.
Institutional review boards responsible for ethical standards at UMDNJ and the Kessler Foundation Research Center approved this study. Written informed consent was obtained from all subjects prior to participation.
Verbal learning and memory.
Verbal learning and memory were assessed with the open-trial Selective Reminding Test (SRT).7 Subjects were asked to learn a list of 10 words over 15 trials, or until a learning criterion of complete recall (all 10 words) on 2 consecutive trials was achieved. Verbal learning was defined as the total number of words recalled across the 15 trials. If the learning criterion was achieved, full credit was awarded for all subsequent trials up to trial 15. Verbal memory was defined as free recall of the word list after a 30-minute delay.
Brain atrophy.
Brain atrophy was estimated with third ventricle width (TVW), which has been identified as the best neuroanatomic predictor of learning and memory performance in patients with MS.2,3 Consistent with established procedures,2,3 TVW was defined as the distance in millimeters between the left and right boundaries of the third ventricle as imaged in the axial plane of high-resolution 3-dimensional images of the brain acquired from magnetization-prepared rapid gradient echo (MPRAGE) scans performed in a 3.0-T Siemens Allegra scanner. Detailed procedures are provided elsewhere,8 with high interrater and intrarater reliabilities (rs >0.96). Mean TVW was 4.9 ± 2.0 mm.
Intellectual enrichment.
Intellectual enrichment was estimated with the vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (WASI),9 which assesses depth and breadth of acquired vocabulary knowledge. Enriching life activities such as educational attainment10 and frequent reading11 are unique and significant contributors to vocabulary knowledge in adults, even when controlling for general intelligence. Research with elders has also examined the contribution of specific life experiences, such as education or occupational history5; however, the young age at MS onset raises the probability that such experiences are curtailed by the disease itself. Acquired vocabulary knowledge, however, may be viewed as a summary measure of benefit from many different sources of intellectual enrichment throughout one's life, even those outside the realm of formal education or occupation. Importantly, vocabulary knowledge is robust against decline in neurologic diseases, including MS,1,8 and is therefore a valid estimate of lifetime intellectual enrichment. Vocabulary knowledge was normal overall (mean T score = 53.9 ± 8.1), and was unrelated to brain atrophy (r = −0.02, p > 0.5). Raw scores were used for subsequent analyses.
Statistical analyses.
Preliminary analyses examined the relationship between demographic/disease variables (age, gender, disease duration, disease course) on the dependent measures of learning (SRT Total Learning) and memory (30-Minute Recall). Any variables significantly associated with dependent measures were controlled for in subsequent analyses. Hierarchical regressions were used to investigate the contribution of brain atrophy (step 1: TVW) and intellectual enrichment (step 2: WASI vocabulary) to verbal learning (SRT Total Learning) and memory (30-Minute Recall). We also investigated the interaction between brain atrophy and intellectual enrichment (step 3) to determine if the effect of brain atrophy on learning and memory is moderated by intellectual enrichment. Finally, after using median splits to divide our sample into subgroups of brain atrophy (lower, higher) and intellectual enrichment (lower, higher), we conducted a 2 (brain atrophy) × 2 (intellectual enrichment) × 15 (SRT learning trials) repeated-measures analysis of variance (ANOVA) to test whether intellectual enrichment moderates the effect of brain atrophy on learning efficiency (i.e., slope across trials).
RESULTS
Preliminary analyses.
There were no relationships between gender, disease duration, or disease course and verbal learning or memory (p > 0.10). Age was marginally related to learning (r = 0.29, p = 0.06) and memory (r = 0.30, p = 0.05).
Verbal learning: Regression.
The full regression accounted for 32% of the variance in verbal learning (F3,40 = 6.28, p = 0.001). There was a negative effect of brain atrophy on learning in step 1 (t = -2.23, p < 0.05, R2 Δ = 0.10) and a positive effect of intellectual enrichment in step 2 (t = 2.86, p < 0.01, R2 Δ = 0.15); however, these effects were moderated by a brain atrophy × intellectual enrichment interaction in step 3 (t = 2.08, p < 0.05, R2 Δ = 0.07). The negative impact of brain atrophy on learning was attenuated among patients with higher intellectual enrichment (figure 1A). Given the marginal relationship between age and learning, we repeated the regression analysis controlling for age in step 1, with essentially no change in the results (indeed, the interaction effect was slightly stronger: R2 Δ = 0.09).
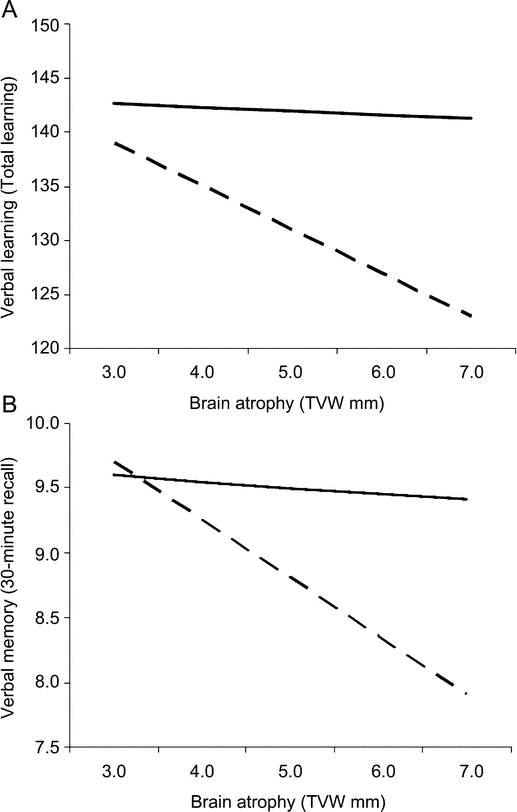
Figure 1 Interaction between intellectual enrichment and brain atrophy on learning and memory
Intellectual enrichment moderates the effect of brain atrophy on (A) verbal learning and (B) verbal memory. The dashed line represents subjects with vocabulary knowledge at the 25th percentile of our sample, which corresponds to approximately the 38th percentile of the WASI normative sample (T = 47). The solid line represents subjects with vocabulary knowledge at the 75th percentile of our sample, which corresponds to approximately the 86th percentile of the WASI normative sample (T = 61). TVW = third ventricle width; WASI = Wechsler Abbreviated Scale of Intelligence.
Verbal memory: Regression.
The full regression accounted for 25% of the variance in verbal memory (F3,40 = 4.42, p < 0.01). There was a negative effect of brain atrophy on memory in step 1 (t = -2.37, p < 0.05, R2 Δ = 0.12), but no effect of intellectual enrichment in step 2 (t = 1.37, p > 0.05, R2 Δ = 0.04). Most importantly, there was a brain atrophy × intellectual enrichment interaction in step 3 (t = 2.22, p < 0.05, R2 Δ = 0.09), such that the negative impact of brain atrophy on memory was attenuated among patients with higher intellectual enrichment (figure 1B). We repeated the regression analysis controlling for age in step 1, with essentially no change in the results (again, the interaction was slightly stronger, R2 Δ = 0.11).
Verbal learning across trials: ANOVA.
The sample was divided into intellectual enrichment subgroups (lower: n = 22, T = 47.0 ± 4.4; higher: n = 22, T = 60.8 ± 3.8) and brain atrophy subgroups (lower: n = 20, TVW = 3.3 ± 0.6; higher: n = 24, TVW = 6.3 ± 1.6). The 4 groups (2 × 2) were balanced for group membership [χ2(1) = 1.5, p > 0.10]. As expected, the 2 (brain atrophy) × 2 (intellectual enrichment) × 15 (SRT learning trial) repeated-measures ANOVA revealed a 3-way interaction (F14,560 = 1.98, p < 0.05, η2 = 0.05) such that higher brain atrophy was associated with inefficient learning among patients with lower intellectual enrichment, but not patients with higher intellectual enrichment (figure 2). These results were unchanged when reanalyzed with analysis of covariance controlling for age.
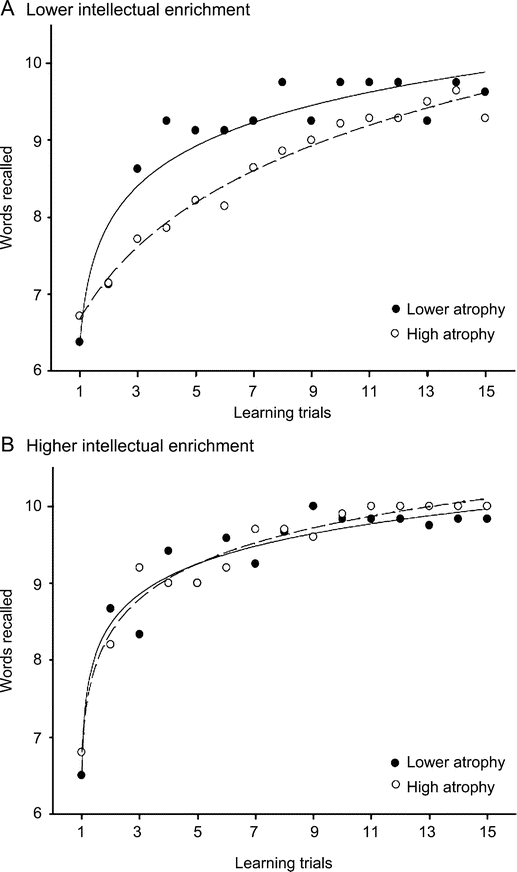
Figure 2 Interaction between intellectual enrichment and brain atrophy on learning efficiency
The negative impact of brain atrophy on learning efficiency is greater among patients with lower intellectual enrichment (A) relative to patients with higher enrichment (B). Subjects with lower and higher brain atrophy are presented in solid and dashed lines.
DISCUSSION
Higher lifetime intellectual enrichment lessens the negative impact of MS disease severity (brain atrophy) on learning and memory. This is consistent with recent functional MRI evidence that patients with MS with higher intellectual enrichment process information more efficiently,12 and therefore are less likely to have cognitive impairment12,13 and are protected from the negative effects of brain atrophy on cognitive efficiency (processing speed).8,12 These findings help to explain the incomplete relationship between MS disease severity and cognitive status,2,3 which likely varies with intellectual enrichment.8 Of note, the association of intellectual enrichment with cognition is greater among patients with more severe disease (interaction effect), suggesting that enrichment does not lead to universal gains in cognition per se; rather, enrichment reduces the negative effect of disease on cognition. When there is little or no disease, there is little or nothing to protect against. This explains the stronger correlation between intellectual enrichment and cognition in patients with MS relative to healthy controls.13
The current findings are consistent with research on cognitive reserve in AD. For instance, higher intellectual enrichment lessens the negative impact of AD neuropathology (neurotic plaques14 and fibrillar β-amyloid15) on cognitive status. Participation in cognitive leisure activities also uniquely protects elders from dementia.16 Although our measurement of acquired vocabulary knowledge in the current study may estimate the benefits of a cognitively stimulating lifestyle, additional research is needed to more directly investigate the unique contribution of specific cognitive activities in patients with MS (e.g., reading, hobbies).
The unpredictable nature of MS disease progression often complicates clinical predictions of future cognitive impairment; however, consideration of patients' lifetime intellectual enrichment may help identify persons at greater risk for future impairment. Patients with lower enrichment may benefit from early intervention cognitive rehabilitation programs to reduce the risk of future impairment. Of note, although we have used the term “lower” intellectual enrichment to describe half of our sample, such subjects actually possess “average” intellectual enrichment (mean T = 47.0). Persons with “below average” enrichment may be at greatest risk for disease-related cognitive impairment, although this needs to be investigated more directly in future research. In fact, one limitation of the current study is the unusually high educational attainment of our sample (16.1 ± 2.3 years). Also, given the relatively small number of subjects with MS disease courses other than relapsing-remitting, caution must be exercised when generalizing the results of this study to patients with progressive disease courses. Future research should determine whether newly diagnosed patients with MS can actively improve their cognitive reserve through intellectual enrichment after diagnosis.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. J.F. Sumowski.
ACKNOWLEDGMENT
The authors thank Rebecca DeLaurentis of Teachers College, Columbia University, and Hali Wood of the Kessler Foundation Research Center for assistance with data analysis.
DISCLOSURE
Dr. Sumowski receives research support from the NIH (NICHD HD060765 [PI]) and the National Multiple Sclerosis Society. Dr. Wylie receives research support from the NIH (NINDS R41 NS050007–03 [Co-I] and NIMH R01HD045798-Suppl [Co-I]), the Kessler Foundation Research Center, and the New Jersey Commission on Brain Injury Research. Dr. Chiaravalloti receives research support from NIH (NCMRR HD045798 [PI], NCMRR HD045798S [PI], NIDRR H133A070037 [PI], NIDRR H133G090078, and NIDRR H133P090009 [Project Director]). Dr. DeLuca serves as an Associate Editor of the Archives of Physical Medicine and Rehabilitation.
Address correspondence and reprint requests to Dr. James F. Sumowski, Neuropsychology & Neuroscience Laboratory, Kessler Foundation Research Center, 300 Executive Drive, Suite 10, West Orange, NJ 07052 jsumowski@kesslerfoundation.org
Editorial, page 1934
Study funding: Supported by the National Multiple Sclerosis Society (RG3330A1/3 to N.C.) and the NIH (HD045798 to N.C., HD060765 to J.F.S.).
Disclosure: Author disclosures are provided at the end of the article.
Received October 13, 2009. Accepted in final form February 3, 2010.
REFERENCES
- 1.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2.Benedict RH, Bruce JM, Dwyer MG, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 2006;63:1301–1306. [DOI] [PubMed] [Google Scholar]
- 3.Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 2004;61:226–230. [DOI] [PubMed] [Google Scholar]
- 4.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988;23:138–144. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 7.Chiaravalloti ND, Balzano J, Moore NB, et al. The Open-Trial Selective Reminding Test (OT-SRT) as a tool for the assessment of learning and memory. Clin Neuropsychol 2009;23:231–254. [DOI] [PubMed] [Google Scholar]
- 8.Sumowski JF, Chiaravalloti N, Wylie GR, et al. Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc 2009;15:606–612. [DOI] [PubMed] [Google Scholar]
- 9.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- 10.Staff RT, Murray AD, Deary IJ, et al. What provides cerebral reserve? Brain 2004;127:1191–1199. [DOI] [PubMed] [Google Scholar]
- 11.Stanovich KE, Cunningham AE. Where does knowledge come from? Specific associations between print exposure and information acquisition J Educ Psychol 1993;85:211–229. [Google Scholar]
- 12.Sumowski JF, Wylie GR, DeLuca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain 2010;133:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumowski JF, Chiaravalloti N, DeLuca J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol 2009;31:913–926. [DOI] [PubMed] [Google Scholar]
- 14.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003;60:1909–1915. [DOI] [PubMed] [Google Scholar]
- 15.Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11 labeled Pittsburgh compound B uptake. Arch Neurol 2008;65:1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 2001;57:2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]


