Abstract
Context. There is limited data comparing results of fine needle aspiration biopsies (FNABs) to histological diagnosis in children. Design. FNABs were performed in 707 children and cytological results were compared to histology in 165 cases. The usefulness of immunostaining with anti-TPO monoclonal antibodies (MoAb47) on FNAB samples was examined in 54 operated patients. Results. Among unsatisfactory, benign, suspicious, and malignant FNAB, the histological diagnoses were benign in 12/12 (100%), 69/70 (98.5%), 40/50 (80.0%), and 0/33 (0%), respectively. After surgery, malignancy was established in 44/165 (26.6%) cases. The sensitivity, specificity, and positive and negative predictive values were 95.4%, 55.8%, 61.7%, and 95% with standard FNAB; and 100%, 75%, 73.3, and 100% with MoAb47. Among suspicious FNAB, positive MoAb47 staining was a reliable marker for exclusion of malignancy. Conclusion. Benign and malignant FNAB accurately predict histological diagnosis. In suspicious FNAB, MoAb47 immunostaining may be a useful adjunct to standard cytology.
1. Introduction
The prevalence of thyroid nodules in adults is well established through large population-based studies, with a reported range of 3.2% to 8% [1]. In children, thyroid nodules are less frequently detected than in adults and sources quote a prevalence of 1.5% [2]. However, the rate of malignancy in operated pediatric thyroid nodules is higher than in adults, varying from 9.2% to 50% [3–7]. As a consequence, there is increased anxiety for malignancy in childhood thyroid nodules and a more aggressive treatment approach has been suggested for any nodule discovered in childhood.
Fine-needle aspiration biopsy (FNAB) is currently considered the most effective technique for morphological diagnosis of thyroid nodules. Traditionally, FNAB results are divided into four categories: inadequate or nondiagnostic, benign, indeterminate (otherwise described as suspicious for neoplasm), and malignant [8]. High diagnostic accuracy of FNAB was reported in cases of benign and malignant cytology. However, the suspicious FNABs are problematic with 10% to 60% of these lesions proven to be malignant after histological examination. In recent recommendation of Papanicolaou Society of Cytopathology, suspicious FNABs were subcategorized into three groups: indeterminate, follicular neoplasms, and suspect of carcinoma [9]. In a large study including 4703 FNABs, the rates of malignancy in operated indeterminate, follicular neoplasms, and suspect of carcinoma FNAB were 13.5%, 32.2%, and 64.7%, respectively [10].
A number of new techniques have been investigated in order to improve the accuracy of FNAB. These techniques attempt to take advantages of differences in genetic, molecular, and biochemical signatures between benign and malignant thyroid lesions to identify reliable malignancy markers. Some markers, such as thyroid peroxidase, dipeptidyl-aminopeptidase IV (DPPIV), and Hector Battifora mesothelial antigen-1 (HBMA-1) were shown to be strongly correlated with thyroid cancer progression and are considered as candidate for cytological practice [11, 12].
Thyroid peroxidase (TPO) is an integral thyroid enzyme that is imperative for normal thyroid function and is considered as one of the major differentiated gene products of thyroid follicular cells. TPO immunodetection with monoclonal antibodies (MoAb47) in thyroid tissue showed positive immunoreactivity in normal thyroid cells and cells from thyroid adenomas but negative immunostaining was found in the majority of carcinoma [13]. In FNAB samples, MoAb47 immunostaining improved the accuracy of standard FNAB, especially in diagnosis of follicular thyroid lesions [14, 15].
The potential importance of malignancy markers in the diagnosis of pediatric thyroid nodules was suggested by previous studies [5]. Taking into consideration that pediatric thyroid cancer is prone to rapid progression, the consequence of false-negative FNAB is of great concern. On the other hand, aggressive therapeutic approach based on false-positive FNAB can result in unnecessary surgery.
The present study was undertaken to review the diagnostic accuracy of traditional cytology in a large cohort of pediatric patients and to determine the utility of MoAb47 immunostaining for diagnoses of pediatric thyroid nodules.
2. Material and Methods
2.1. Patients
The protocol for this study was approved by the ethical and clinical research committee of Center for Endocrine Surgery Kiev, Ukraine. From January 1999 through December 2008, thyroidal FNABs were performed at Department of Cytology, Center for Endocrine Surgery Kiev, Ukraine in 17,809 patients including 707 (0.4%) children with ages ranged from 6 to 17 years old. Forty six patients that underwent FNAB (6.5%) were born before 1986, implicating possible irradiation after the Chernobyl accident.
2.2. Fine Needle Aspiration Biopsies
FNABs were done with or without ultrasound guidance. Thyroid nodules that were easily palpable were aspirated by a board-certified cytopathologist and nonpalpable thyroid nodules were aspirated under ultrasound guidance by a radiologist.
All aspirations were performed with 23- to 25-gauge needles attached to a 10- or 20-mL syringe with or without a syringe holder. Air-dried smears were immediately examined by a cytologist for the presence of epithelial cells and samples were considered as adequate if it contained 8 to 10 clusters of at least 6 to 8 well-preserved and well-visualized follicular cells. After fixation in methanol, aspirates were stained using Giemsa method.
The results of FNAB were classified as (1) unsatisfactory (impossible to perform cytological interpretation), (2) benign (nonneoplastic and autoimmune thyroiditis), (3) suspicious (cellular adenomatoid nodule, “follicular lesions” and cases with atypical cytology), and (4) malignant (thyroid carcinoma or other malignancy).
2.3. Immunostaining
Immunocytochemistry with MoAb 47 was performed on air-dried FNA samples within 24 hours after biopsy. After neutralization of endogenous peroxidase in a phosphate-buffered saline (PBS) solution containing 0.1% hydrogen peroxide for 5 minutes, slides were incubated overnight at 4°C with anti-TPO monoclonal antibody (MoAb 47 clone; Dako, Glostrup, Denmark). Immunostaining was performed with the Vectastain Universal Quik kit (Vector Lab, Burlingame CA) according to the manufacturer's instructions.
The percentage of positive cells was evaluated as previously described [12]. The reaction with MoAb47 was considered as positive when more than 80% of epithelial cells demonstrated dark-brown granular staining of the cytoplasm with a halo around the nuclei. Cells exhibiting an abnormal staining pattern, which include low intensity with no perinuclear ring, were not considered as positive. Positive controls were performed on smears from operated benign nodules. Negative controls were performed by omitting the primary antibody.
2.4. Pathology
One hundred sixty-five children were surgically treated in the Department of Surgery and thyroid tissue samples were available for comparison of cytological examination with histological analysis. Histological diagnoses were established according to the WHO classification of thyroid tumors and included 121 benign and 44 malignant thyroid lesions.
2.5. Statistical Analysis
SPSS software (version 13.0, 2004 SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The sensitivity (Se), specificity (Sp), positive predictive values (PPVs), and negative (NPV) were calculated as following: Se = true positive/(true positive + false negative); Sp = true negative/(true negative + false positive); PPV = true positive/(true positive + false positive); and NPV = true negative/(true negative + false negative).
3. Results
3.1. Demographic Data and Distribution of Cytological Diagnoses in Children with Thyroid Nodules
In total, 707 FNAB from 559 female and 148 male patients (female/male 3.7 : 1) were examined. The age of patients ranged from 5 to 17 years. There were 52 prepubertal patients (age 5–10 years) including 33 female and 19 male (female/male 1.7 : 1); and 655 pubertal patients (age 11–17 years) including 526 female and 129 male (female/male 4.0 : 1). The number of patients that underwent thyroid FNAB in function of their age is presented in Table 1, showing a distinct rise during puberty and a disproportionate increase in females.
Table 1.
Number of pediatric patients that underwent thyroid FNAB.
| Age | Number of patients | |||
|---|---|---|---|---|
| Female | Male | Total | ||
| 4 | 2 | 1 | 3 | |
| 5 | 0 | 1 | 1 | |
| 6 | 0 | 4 | 4 | |
| 7 | 3 | 3 | 6 | |
| 8 | 4 | 0 | 4 | |
| 9 | 7 | 4 | 11 | |
| 10 | 17 | 6 | 23 | |
| 11 | 16 | 7 | 23 | |
| 12 | 20 | 11 | 31 | |
| 13 | 42 | 9 | 51 | |
| 14 | 74 | 20 | 94 | |
| 15 | 126 | 27 | 153 | |
| 16 | 139 | 24 | 163 | |
| 17 | 109 | 31 | 140 | |
| Total | 559 | 148 | 707 | |
The total distributions of initial cytological diagnoses in the four diagnostic categories were as follows: 66 unsatisfactory (9.3%), 510 benign (72.1%), 86 suspicious (12.2%), and 45 malignant (6.4%). The cytological diagnoses, classified by ages and gender groups are shown in Table 2.
Table 2.
Cytological diagnoses in prepubertal and pubertal children.
| Age | Cytology | |||
|---|---|---|---|---|
| Non adequate (66) | Benign (510) | Suspicious (86) | Malignant (45) | |
| Gender | ||||
| prepubertal | ||||
| Female (33) | 4 | 22 | 2 | 5 |
| Male (19) | 3 | 8 | 6 | 2 |
| Pubertal | ||||
| Female (526) | 43 | 385 | 67 | 31 |
| Male (129) | 16 | 95 | 11 | 7 |
3.2. Comparison of Cytological and Histological Diagnoses
One hundred sixty-five patients with preoperative FNAB underwent partial or total thyroidectomy based on clinical data and FNAB results. Most of patients with benign FNAB were referred to surgery because of large nodule size (average 34.4 mm). The average nodule size in patients with a benign FNAB was significantly higher compared to patients with suspicious FNAB (23.0 mm) or malignant FNAB (27.2 mm). On histology there were 121 benign and 44 malignant diagnoses. The distribution of cytological diagnoses in function of histology is shown in Table 3.
Table 3.
Distribution of cytological diagnoses in function of histology.
| Histology | Cytology | |||
|---|---|---|---|---|
| Non adequate (12) | Benign (70) | Suspicious (50) | Malignant (33) | |
| Benign (121) | ||||
| Cyst | 8 | 0 | 0 | 0 |
| MNG | 0 | 23 | 8 | 0 |
| FA | 4 | 42 | 21 | 0 |
| AA | 0 | 1 | 8 | 0 |
| AID | 0 | 3 | 3 | 0 |
| Malignant (44) | ||||
| PTC | 0 | 0 | 4 | 25 |
| FVPC | 0 | 1 | 5 | 4 |
| FTC | 0 | 0 | 1 | 2 |
| MTC | 0 | 0 | 0 | 2 |
In patients with unsatisfactory FNAB, histological examination frequently reveals the presence of cysts. In patients with benign FNAB, 69/70 (98.5%) were diagnosed as benign on histology. The most frequent diagnoses were multinodular goiter (MNG) and follicular adenomas (FA). The discrepancy between cytological and histological diagnoses in this category was in a solitary case in which a follicular variant of papillary thyroid cancer (FVPTC) was misdiagnosed as a benign thyroid lesion. In patients with suspicious FNAB, the final histological analysis revealed malignancy in 10/50 (20%). Tumors with uncertain malignant potential such as adenomas with limited nuclear features of papillary cancer (AA) were diagnosed in 8/50 (16%). In 32/50 (64%) histology revealed benign thyroid lesions (MNG, FA, and autoimmune thyroid diseases (AID).
3.3. Accuracy of FNAB
To determine the accuracy of FNAB, we analyzed only adequate samples (153 cases). The sensitivity, specificity, and predictive values were determined using two approaches: considering suspicious FNAB as either “negatives” or “positives” for malignancy.
When only malignant FNABs were considered as “positive” and suspicious FNABs were considered as “negative”, there were 33 true positive, 11 false negative, 109 true negative, and 0 false positive cases. The sensitivity and specificity were 75.0% and 100%, respectively. When malignant and suspicious FNABs were considered as “positive”, there were 43 true positive, 1 false negative, 69 true negative, and 40 false positive cases. The sensitivity and specificity were 97.7% and 63.3%, respectively.
3.4. TPO Immunocytochemistry with MoAb47 on FNAB
In the present study we assessed the utility of TPO immunocytochemistry with MoAb47 in the diagnosis of pediatric thyroid nodules. The preoperative MoAb47 immunostaining was performed in 54 patients that consequently underwent surgery. Histological examination showed the presence of benign and malignant thyroid lesions in 32 and 22 cases, respectively. Twenty four of 33 patients with benign histology demonstrated high level of MoAb47 staining (more than 80% cells are MoAb47 positive), but all malignant lesions showed low level of staining.
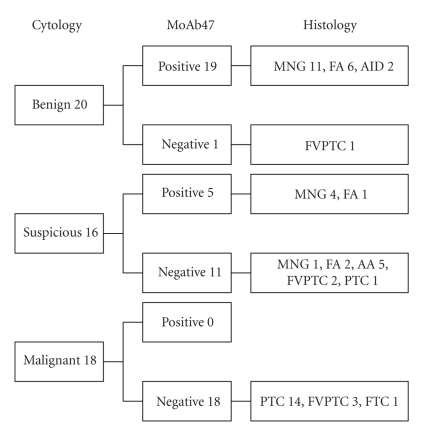
The results of standard cytology and MoAb47 immunostaining in function of histology are summarized in Figure 1. In benign FNAB, MoAb47 immunostaining was positive in 19/20 cases, but negative in 1 case that was determined to be an FVPC by histology. In patients with suspicious FNAB, MoAb47 immunostaining was positive in 7 lesions with benign histology (MNG and FA), but decreased in cancers and in tumors with limited nuclear features of papillary thyroid cancers. All malignant FNABs were characterized by decreased level of MoAb47 immunostaining. Statistical analysis of results with standard cytology and MoAb47 immunostaining is presented in Table 4.
Figure 1.
Comparison of standard cytological diagnoses to MoAb47 and histology.
Table 4.
Diagnostic accuracy of standard cytology and MoAb47 immunostaining.
| Standard cytology | MoAb47 | |
|---|---|---|
| Sensitivity | 95.4% | 100% |
| Specificity | 55.8% | 75% |
| PPV | 61.7% | 73.3% |
| NPV | 95% | 100% |
4. Discussion
Fine-needle aspiration biopsy of the thyroid gland is minimally invasive and highly accurate in the diagnosis of thyroid nodules in adults and in the pediatric population [8, 16]. The usefulness of this technique has been documented in numerous studies comparing thousands of FNAB with final histology in the adult population [10, 17, 18]. However, there is limited data on FNAB followed by surgery in pediatric thyroid nodules.
This retrospective study represents the largest number of FNAB samples in pediatric patients with histological confirmation and the first study to report on the use of MoAb47 as a diagnostic tool for pediatric FNAB.
The cohort of patients in our study were evaluated by FNAB after a thyroid nodule was discovered on routine physical exam and represents less than 0.5% of all patients that underwent FNAB in the Center for Endocrine Surgery in Kiev. While the geographic location suggests exposure to ionizing radiation from the Chernobyl accident, 92.5% of the patients in this study were born after this event, reducing the likelihood of this effect.
Results from the demographic data demonstrated a marked increase in the prevalence of thyroid nodules in children of pubertal age, with a disproportionate increase in female patients. These data suggest that hormones associated with puberty may promote the development of thyroid nodules in adolescence. Previous studies have demonstrated an association of estrogen receptor expression and serum IGF-1 level with development of thyroid nodules [19–23]. However, additional studies are needed to determine if a causal relationship exists.
The distribution of cytological diagnoses in the present study was similar to previously reported pediatric FNAB data [8, 16]. More than 50% of patients that were operated at the Center for Endocrine Surgery had suspicious or malignant FNAB, therefore confirming the role of FNAB as a screening method for selection of patients for surgery.
Comparison of FNAB results to final histology showed that benign and malignant FNABs have a high negative and positive predictive value, respectively. However, suspicious FNAB diagnoses were the major source of false-negative as well as false-positive results. These data are consistent with previously reported findings [3–7, 10, 18, 24] and showed that suspicious FNABs are a major diagnostic pitfall in making therapeutic decisions for pediatric patients.
Histological analysis of operated suspicious FNAB in our series revealed that this group includes malignant tumors (20%), tumors with uncertain malignant potential or atypical adenomas (16%) and purely benign lesions (64%). In other reported series, the rate of malignancy in thyroidectomy specimens of patients with an initial cytologic diagnosis of “suspicious FNAB” varies from 15% to 38% in adults and from 10% to 35% in children [3–7, 10, 18, 24].
These findings reflect the diagnostic dilemma surrounding follicular thyroid lesions. It was demonstrated that FNAB followed by standard staining cannot distinguish benign from malignant follicular tumors such as FTCs and follicular variant of PTC [25, 26].
Malignancy markers have shown promise as an adjunct in establishing the diagnosis of cancer on FNAB. Thyroid peroxidase immunostaining using MoAb47 is one of the first malignancy markers reported to have potential utility to distinguish benign and malignant lesions on thyroid FNAB samples [27]. MoAb47 has been shown to have a sensitivity of 98% and specificity of 80% [28, 29] in the diagnosis of thyroid nodules in adults.
In this study we examined the usefulness of MoAb47 immunostaining in a series of 54 pediatric FNABs. Loss of immunoreactivity with MoAb47 was observed in all malignant thyroid lesions and in tumors with uncertain malignant potential. In contrast, a high level of staining with MoAb47 was detected on FNAB from most histologically benign thyroid lesions. MoAb47 immunostaining in suspicious FNAB was helpful in identifying histologically benign thyroid lesions. These results are consistent with previously reported data demonstrating that MoAb47 immunostaining enhances sensitivity and specificity of standard cytology.
In summary, among children and adolescents who underwent FNAB, thyroid cytology had a sensitivity and specificity similar to those reported in the adult population. Most suspicious FNABs in pediatric nodules were benign on final histology. Our data suggests that MoAb47 immunostaining may be a useful adjunct in establishing cytological diagnoses, especially in cases of suspicious FNAB.
Acknowledgment
The authors thank Dr. Catherine De Micco, Mediterranean University for her support of this project.
References
- 1.Wartofsky LaVN D. Thyroid Cancer: A Comprehensive Guide to Clinical Management. 2nd edition. Totowa, NJ, USA: Humana Press; 2005. [Google Scholar]
- 2.Dinauer C, Francis GL. Thyroid Cancer in Children. Endocrinology and Metabolism Clinics of North America. 2007;36(3):779–806. doi: 10.1016/j.ecl.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Amrikachi M, Ponder TB, Wheeler TM, Smith D, Ramzy I. Thyroid fine-needle aspiration biopsy in children and adolescents: experience with 218 aspirates. Diagnostic Cytopathology. 2005;32(4):189–192. doi: 10.1002/dc.20197. [DOI] [PubMed] [Google Scholar]
- 4.Arda IS, Yildirim S, Demirhan B, Firat S. Fine needle aspiration biopsy of thyroid nodules. Archives of Disease in Childhood. 2001;85(4):313–317. doi: 10.1136/adc.85.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocrine-Related Cancer. 2006;13(2):427–453. doi: 10.1677/erc.1.00882. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WM. Management of thyroid nodules in children and adolescents. Hormones. 2007;6(3):194–199. [PubMed] [Google Scholar]
- 7.Hosler GA, Clark I, Zakowski MF, Westra WH, Ali SZ. Cytopathologic analysis of thyroid lesions in the pediatric population. Diagnostic Cytopathology. 2006;34(2):101–105. doi: 10.1002/dc.20388. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 9.Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. CytoJournal. 2008;5:p. 6. doi: 10.1186/1742-6413-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111(5):306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 11.Bartolazzi A, Orlandi F, Saggiorato E, et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. The Lancet Oncology. 2008;9(6):543–549. doi: 10.1016/S1470-2045(08)70132-3. [DOI] [PubMed] [Google Scholar]
- 12.de Micco C, Savchenko V, Giorgi R, Sebag F, Henry J-F. Utility of malignancy markers in fine-needle aspiration cytology of thyroid nodules: comparison of Hector Battifora mesothelial antigen-1, thyroid peroxidase and dipeptidyl aminopeptidase IV. British Journal of Cancer. 2008;98(4):818–823. doi: 10.1038/sj.bjc.6604194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Micco C, Ruf J, Chrestian M-A, Gros N, Henry J-F, Carayon P. Immunohistochemical study of thyroid peroxidase in normal, hyperplastic, and neoplastic human thyroid tissues. Cancer. 1991;67(12):3036–3041. doi: 10.1002/1097-0142(19910615)67:12<3036::aid-cncr2820671218>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.De Micco C, Vassko V, Henry J-F. The value of thyroid peroxidase immunohistochemistry for preoperative fine-needle aspiration diagnosis of the follicular variant of papillary thyroid cancer. Surgery. 1999;126(6):1200–1204. doi: 10.1067/msy.2099.101428. [DOI] [PubMed] [Google Scholar]
- 15.De Micco C, Vasko V, Garcia S, et al. Fine-needle aspiration of thyroid follicular neoplasm: diagnostic use of thyroid peroxidase immunocytochemistry with monoclonal antibody 47. Surgery. 1994;116(6):1031–1035. [PubMed] [Google Scholar]
- 16.Cooper DS, Doherty GM, Haugen BR, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 17.Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. Value of repeated fine needle aspirations of the thyroid: an analysis of over ten thousand FNAs. Thyroid. 2007;17(11):1061–1066. doi: 10.1089/thy.2007.0159. [DOI] [PubMed] [Google Scholar]
- 18.Sangalli G, Serio G, Zampatti C, Bellotti M, Lomuscio G. Fine needle aspiration cytology of the thyroid: a comparison of 5469 cytological and final histological diagnoses. Cytopathology. 2006;17(5):245–250. doi: 10.1111/j.1365-2303.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen N, Laurberg P, Perrild H, Bülow I, Ovesen L, Jørgensen T. Risk factors for goiter and thyroid nodules. Thyroid. 2002;12(10):879–888. doi: 10.1089/105072502761016502. [DOI] [PubMed] [Google Scholar]
- 20.Tavangar SM, Monajemzadeh M, Larijani B, Haghpanah V. Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singapore Medical Journal. 2007;48(8):744–747. [PubMed] [Google Scholar]
- 21.Völzke H, Friedrich N, Schipf S, et al. Association between serum insulin-like growth factor-I levels and thyroid disorders in a population-based study. Journal of Clinical Endocrinology and Metabolism. 2007;92(10):4039–4045. doi: 10.1210/jc.2007-0816. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Q, Chen GG, Vlantis AC, Tse GM, van Hasselt CA. The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. Journal of Pathology. 2008;214(4):425–433. doi: 10.1002/path.2297. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor—ERK pathway. Cell Proliferation. 2007;40(6):921–935. doi: 10.1111/j.1365-2184.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones. 2007;6(3):200–209. [PubMed] [Google Scholar]
- 25.Banks ND, Kowalski J, Tsai H-L, et al. A diagnostic predictor model for indeterminate or suspicious thyroid FNA samples. Thyroid. 2008;18(9):933–941. doi: 10.1089/thy.2008.0108. [DOI] [PubMed] [Google Scholar]
- 26.Kato MA, Fahey TJ., III Molecular markers in thyroid cancer diagnostics. Surgical Clinics of North America. 2009;89(5):1139–1155. doi: 10.1016/j.suc.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 27.de Micco C, Zoro P, Henry J-F. Malignancy markers in the cytological diagnosis of thyroid nodules. Thyroid peroxidase. Annales de Pathologie. 1994;14(6):378–383. [PubMed] [Google Scholar]
- 28.Christensen L, Blichert-Toft M, Brandt M, et al. Thyroperoxidase (TPO) immunostaining of the solitary cold thyroid nodule. Clinical Endocrinology. 2000;53(2):161–169. doi: 10.1046/j.1365-2265.2000.01035.x. [DOI] [PubMed] [Google Scholar]
- 29.Faroux MJ, Theobald S, Pluot M, Patey M, Menzies D. Evaluation of the monoclonal antibody antithyroperoxidase MoAb47 in the diagnostic decision of cold thyroid nodules by fine-needle aspiration. Pathology Research and Practice. 1997;193(10):705–712. doi: 10.1016/S0344-0338(97)80030-1. [DOI] [PubMed] [Google Scholar]