Abstract
Objectives:
Diagnostic challenges exist for differentiating HIV dementia from Alzheimer disease (AD) in older HIV-infected (HIV+) individuals. Similar abnormalities in brain amyloid-β42 (Αβ42) metabolism may be involved in HIV-associated neuropathology and AD. We evaluated the amyloid-binding agent 11C-Pittsburgh compound B (11C-PiB), a biomarker for Αβ42 deposition, in cognitively unimpaired HIV+ (n = 10) participants and matched community controls without dementia (n = 20).
Methods:
In this case-control study, all participants had an 11C-PiB scan within 2 years of concomitant CSF studies and neuropsychometric testing. Statistical differences between HIV+ and community controls for demographic and clinical values were assessed by χ2 tests. Participants were further divided into either low (<500 pg/mL) or normal (≥500 pg/mL) CSF Αβ42 groups with Student t tests performed to determine if regional differences in fibrillar amyloid plaque deposition varied with CSF Αβ42.
Results:
Regardless of CSF Αβ42 level, none of the HIV+ participants had fibrillar amyloid plaques as assessed by increased 11C-PiB mean cortical binding potential (MCBP) or binding potential within 4 cortical regions. In contrast, some community controls with low CSF Αβ42 (<500 pg/mL) had high 11C-PiB MCBP with elevated binding potentials (>0.18 arbitrary units) within cortical regions.
Conclusions:
Cognitively unimpaired HIV+ participants, even with low CSF Αβ42 (<500 pg/mL), do not have 11C-PiB parameters suggesting brain fibrillar amyloid deposition. The dissimilarity between unimpaired HIV+ and preclinical AD may reflect differences in Aβ42 production and/or formation of diffuse plaques. Future longitudinal studies of HIV+ participants with low CSF Aβ42 and normal 11C-PiB are required.
GLOSSARY
- Αβ42
= amyloid-β42;
- AD
= Alzheimer disease;
- ART
= antiretroviral therapy;
- CDR
= Clinical Dementia Rating;
- CHARTER
= CNS Highly Activated Retroviral Therapy Effects Research;
- GDS
= global deficit score;
- HAND
= HIV-associated neurocognitive disorder;
- LP
= lumbar puncture;
- MCBP
= mean cortical binding potential;
- PiB
= Pittsburgh compound B;
- ROI
= region of interest;
- WUSTL
= Washington University in St. Louis.

e–Pub ahead of print
HIV-associated neuroinflammation can occur despite virologic control with antiretroviral therapy (ART).1 The prevalence of HIV-infected (HIV+) participants >50 years old has risen as life expectancy increases with ART. If current trends continue, more than 50% of all HIV+ individuals will be >50 years old by 2015.2 Age is a risk factor for HIV-associated neurocognitive disorder (HAND) and Alzheimer disease (AD). As HIV+ participants age, clinicians face the challenge of differentiating individuals at risk for HAND from those with AD.
Genetic, biochemical, and animal models and autopsy studies have demonstrated a critical role for brain amyloid-β42 (Αβ42) aggregation in AD.3 Similar neuropathologic abnormalities occur with HIV. Postmortem HIV+ subjects have increased brain Aβ42 and tau deposition compared to age-matched community controls.4 Decreased CSF Αβ42 is observed in subjects with AD and some unimpaired community controls with fibrillar Aβ42.3 Subjects with HAND have CSF Aβ42 levels similar to participants with mild AD.1,5
Reduced CSF Αβ42 (<500 pg/mL) correlates with increased fibrillar amyloid deposition using the PET amyloid binding agent N-methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole (11C-PiB) in subjects with AD and unimpaired community controls with preclinical AD.3 It remains unknown if a similar relationship exists for HIV. We investigated if low CSF Αβ42 levels were predictive of increased 11C-PiB binding potentials in cognitively unimpaired HIV+ participants.
METHODS
Participants.
HIV+ participants (n = 10) (39–59 years of age) with confirmed serologic status were selected from the CNS Highly Activated Retroviral Therapy Effects Research (CHARTER) cohort at Washington University in St. Louis (WUSTL). Four participants with low CSF Αβ42 levels (<500 pg/mL) and 6 with normal CSF Αβ42 levels (≥500 pg/mL) were contacted. We selected community controls (n = 20) (44–63 years of age) of similar sex and education from memory and aging studies at the WUSTL Alzheimer's Disease Research Center (2 controls for every HIV+ subject). We received approval from the WUSTL ethical standards committee on human experimentation for experiments using human subjects. In this case-control study, written informed consent was obtained from all subjects participating in this study. The recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology criteria were followed whenever applicable.6
All participants had an 11C-PiB scan within 2 years of concomitant lumbar puncture (LP) and neuropsychometric testing. For HIV+ participants, cognition was assessed at the time of scan and approximately 2 years prior to LP. Cognition was evaluated in HIV+ subjects using the previously validated global deficit score (GDS) with impairment deemed significant if GDS ≥0.5.5 For community controls, impairment was assessed by the Clinical Dementia Rating (CDR) scale with impairment noted if CDR >0.3
CSF evaluation.
CSF collection used previously described methods.3 CSF Αβ42 was analyzed using a commercial enzyme-linked immunosorbent assay (Innogenetics, Ghent, Belgium). Samples were kept on ice with assays performed on aliquots after a single thaw.
Imaging.
Participants underwent 11C-PiB as previously described.7 Tracer was injected into the antecubital vein with a 60-minute 3-dimensional dynamic PET scan performed. Each subject had a T1-weighted anatomic scan with 11C-PiB images corrected for head motion and registered to this scan.3 The cerebellum was used as a reference as amyloid deposition has not been observed within this area in community controls.7 Logan graphical analyses were performed and 11C-PiB distribution volume calculated for the prefrontal, lateral temporal, precuneus, and gyrus rectus.11C-PiB binding potentials for each region of interest (ROI) and the mean cortical binding potential (MCBP) were calculated.7
Statistical analysis.
Statistical differences between HIV+ and community controls for demographic and clinical values were assessed by χ2 tests. Participants were divided into either low (<500 pg/mL) or normal (≥500 pg/mL) CSF Αβ42 groups using previously defined criterion with excellent sensitivity (100%) and good specificity (84%) for predicting subjects at risk for dementia.8 An analysis of variance with Bonferroni correction for multiple comparisons assessed if regional differences in fibrillar amyloid plaque deposition varied with CSF Αβ42.
RESULTS
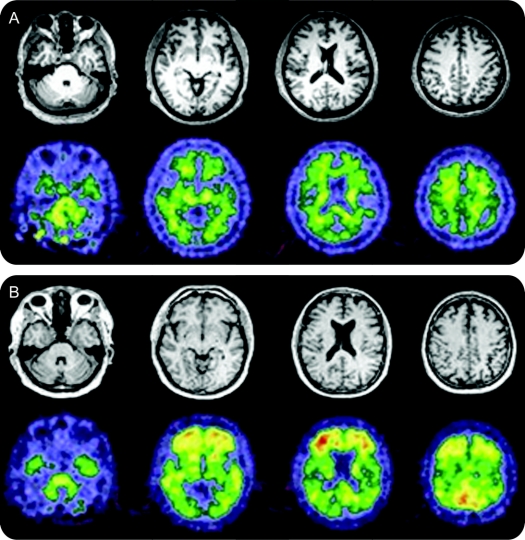
Demographic and clinical variables were similar (table). Neither group had significant cognitive impairment. HIV+ participants, even those with low CSF Αβ42 (<500 pg/mL), did not have increased fibrillar amyloid plaques using 11C-PiB (figure 1A). In contrast, community controls with low CSF Αβ42 had more fibrillar amyloid plaques (figure 1B). Several community controls had 11C-PiB measures indistinguishable from a typical AD pattern.7 These unimpaired community controls may have preclinical AD.8
Table Clinical and laboratory values for HIV-infected (HIV+) and community control participants
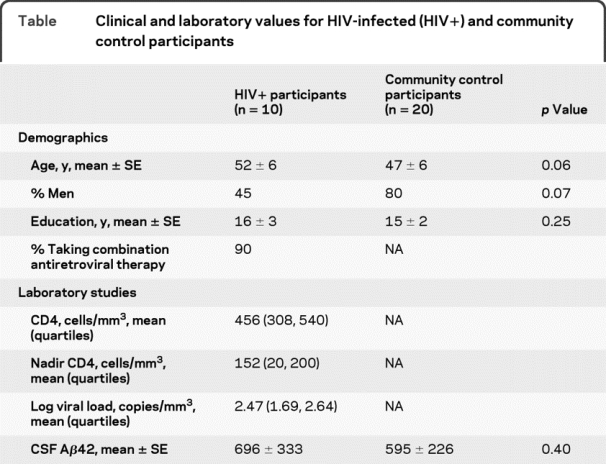
Figure 1 11C-PiB imaging for HIV+ participants and community controls
Representative structural MRI and amyloid binding agent N-methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole (11C-PiB) image from (A) an unimpaired HIV-infected (HIV+) participant with low CSF amyloid-β42 (Αβ42) (<500 pg/mL) and (B) an unimpaired community control with low CSF Αβ42 (<500 pg/mL). On visual inspection, greater binding potentials were seen for the community control compared to the HIV+ subject. The community control had values similar to a participant with Alzheimer dementia (AD) and may have preclinical disease.
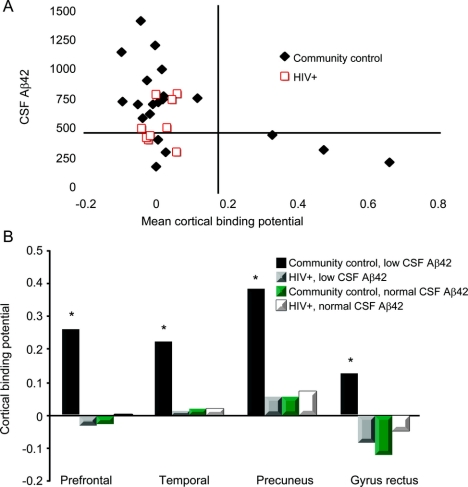
We assessed the relationship between fibrillar amyloid deposition using 11C-PiB and CSF Αβ42 for HIV+ participants and community controls. A 2 × 2 matrix was created using CSF (Αβ42 <500 pg/mL) and 11C-PiB MCBP (<0.18 arbitrary units) (figure 2A). All HIV+ participants were located in the left upper and lower quadrants. Community controls fell within 3 boxes: left upper and lower quadrants and right lower quadrant. Half of the community controls had low CSF Αβ42 and high 11C-PiB MCBP. A mismatch existed between CSF Αβ42 and 11C-PiB MCPB for HIV+ participants and community controls (left lower quadrant).
Figure 2 11C-PiB mean cortical binding potential (MCBP) for unimpaired HIV+ participants and community controls
(A) A 2 × 2 matrix is created using CSF Αβ42 (<500 pg/mL) and MCBP (>0.18 arbitrary units). All HIV+ participants had normal 11C-PiB (<0.18 arbitrary units) regardless of their CSF Αβ42. In contrast, half (3/6) of the community controls with reduced CSF Αβ42 (<500 pg/mL) had elevated MCBP (>0.18 arbitrary units). (B) Regional cortical binding potentials were determined for HIV+ participants and community controls with low (<500 pg/mL) and normal CSF Αβ42 (≥500 pg/mL). Community controls with low CSF Αβ42 (<500 pg/mL) had elevated binding potentials (>0.18 arbitrary units) compared to other groups (*p < 0.01).
Binding potentials were assessed within ROIs to determine degree of variation in fibrillar amyloid deposition. Binding potentials were elevated for community controls with low CSF Αβ42 compared to other groups within all areas. HIV+ participants including those with low CSF AB42 (n = 4) had binding potentials similar to community controls with normal CSF Αβ42 (figure 2B).
DISCUSSION
We observed that cognitively unimpaired HIV+ participants, even with low CSF Αβ42 (<500 pg/mL), did not have increased 11C-PiB that might indicate fibrillar brain amyloid deposition. However, community controls with a low CSF Αβ42 were more likely to have elevated 11C-PiB MCBP (>0.18 arbitrary units).3 Unimpaired community controls with increased 11C-PiB MCBP may have preclinical AD.8 Within a 2-year retrospective interval during which we followed the HIV+ participants, even those with low CSF Αβ42 had no significant changes in cognition (GDS = 0.18 at LP and GDS = 0.31 at subsequent 11C-PiB). Our findings suggest that 11C-PiB MCBP differs in cognitively unimpaired HIV+ individuals compared to community controls with low CSF Αβ42. In the setting of HIV, low CSF Αβ42 may not reliably predict fibrillar Aβ brain deposits as it does in preclinical AD.9 As the HIV+ population ages, this distinction could be diagnostically important. It remains necessary to understand whether fibrillar Aβ seen with increased 11C-PiB is present in patients with HAND. This would assist in differentiating HAND from AD. While APOE status was not determined for participants, future studies investigating the impact of genetic risk factors on 11C-PiB MCBP and CSF Αβ42 in HIV+ participants are required.2
Both 11C-PiB and CSF Αβ42 levels are biomarkers of brain amyloid deposition in patients with AD and antecedent measures of impairment in community controls with preclinical AD.8 A strong inverse correlation exists between these biomarkers. The lack of correlation between CSF Αβ42 and 11C-PiB MCBP in unimpaired HIV+ participants could result from decreased Aβ42 production, increased intraneuronal Aβ42 deposition leading to reduced extracellular concentrations, or more extracellular Aβ42 amyloid but in a diffuse, nonfibrillar Aβ form.4,9,10 In each instance, relatively normal 11C-PiB would occur. Future longitudinal examination, especially a larger sample of HIV+ participants with low CSF Aβ42 and normal 11C-PiB, are required to understand whether observed low CSF Aβ42 represents an aggregation of diffuse oligomeric forms (11C-PiB-negative) that eventually become substantial fibrillar (11C-PiB-positive) deposits,1,5 or simply the low normal end of CSF Aβ42 in HIV+ participants.9 Our findings reinforce the importance of understanding amyloid metabolism in HIV-associated neuropathology, while confirming that low CSF Αβ42 is not simply a manifestation of early fibrillar Aβ deposition in the brain.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Beau Ances and Dr. Chengjie Xiong.
ACKNOWLEDGMENT
The authors thank the participants of both the Memory and Aging Project and the CHARTER project at Washington University in St. Louis for their participation and Aarti Shah, MS, for processing community control CSF samples.
DISCLOSURE
Dr. Ances receives research support from the NIH (NIMH 1K23MH081786 [PI]), the Foundation for AIDS Research, and from the Dana Foundation. Dr. Teshome reports no disclosures. Mr. Christensen serves as Staff Scientist from the Washington University School of Medicine; and receives research support from the NIH (1R01DC00909501 [Staff Scientist], 5PS0NS00683341 [Staff Scientist], 5PS0NS00683340 [Staff Scientist], 5P01AG02627604 [Staff Scientist], 5P50AG00568125 [Staff Scientist] and 5U01AG03243802 [Staff Scientist]). Ms. Taylor reports no disclosures. Dr. Xiong serves as an Associate Editor of Biostatistics; and receives research support from the NIH (NIA K25 AG025189 [PI], NIA P01 AG26276-01 [Biostatistics Component Leader], NIA 5 P01 AG03991 [Biostatistics Core Director], NIA 5P50 AG05681 [Biostatistics Core Director], NIA U01 AG032438 [Biostatistics Core Director], and R01 AG029672 [Subcontract PI]), and from the Alzheimer Association. Ms. Aldea reports no disclosures. Dr. Fagan serves on a speakers' bureau for the Alzheimer's Association. Dr. Holtzman serves on scientific advisory boards for Satori Pharmaceuticals and EnVivo Pharmaceuticals; serves as an Associate Editor of Annals of Neurology, the Journal of Neuroscience, Neurobiology of Disease, and Experimental Neurology; may accrue revenue on pending US Patent 20080145941 (filed 6/18/08): Methods for Measuring the Metabolism of Neurally Derived Biomolecules in Vivo, pending US Patent 20090074775 (filed 3/19/09): Use of Anti-AB Antibody to Treat Traumatic Brain Injury, pending US Patent 20090035298 (filed 2/5/09): Methods to Treat Alzheimer's Disease or Other Amyloid Beta Accumulation Associated disorders; US Patent 7,195,761 (issued 3/27/07): Humanized antibodies that sequester abeta peptide, US Patent 7,015,044 (issued 3/21/06): Diagnostic for early stage Alzheimer's disease, US Patent 6,465,195 (issued 10/15/02): Predictive diagnostic for Alzheimer's disease; serves as a consultant to Merck Serono, Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Abbott, Comentis, Inc., Eisai Inc., and AstraZeneca; is cofounder of and receives board of directors compensation from C2N Diagnostics LLC; receives research support from AstraZeneca, Pfizer Inc., Eli Lilly and Company, Elan Corporation, Forest Laboratories, Inc., the NIH (NIA R37 AG13956 [PI], NINDS 1P30NS057105 [PI], NINDS P01-NS35902 [PI of project 3], NINDS P01-NS32636 [PI of project 3], NIA P01-AG026276 [Co-I], NIA R01-AG025824 [I], NINDS R01-NS034467 [I], NIA U01AG032438 [Co-I], NIA PO1-AG03991[PI of project 2]), Cure Alzheimer's Fund, and Fidelity Foundation; has received compensation from Washington University from license revenue received for licensing of patent application entitled “Methods for Measuring the Metabolism of Neurally Derived Biomolecules in Vivo” to C2N Diagnostics LLC; and may receive future royalty payments for Washington University licensing patent entitled “Methods for Measuring the Metabolism of Neurally Derived Biomolecules in Vivo” to C2N Diagnostics, LLC, and could receive future royalty payments from Washington University for licensing patent entitled “Humanized antibodies that sequester abeta peptide” US Patent 7,195,761 to Eli Lilly and Company. Dr. Morris serves on scientific advisory boards for AstraZeneca, Bristol-Myers Squibb, Genentech, Inc., Merck Serono, Novartis, Pfizer Inc., Schering-Plough Corp., Eli Lilly and Company, Wyeth, and Elan Corporation; serves on the editorial advisory board of Alzheimer's Disease and Associated Disorders; receives royalties from publishing Mild Cognitive Impairment and Early Alzheimer's Disease (John Wiley and Sons, 2008), Dementia (Clinical Publishing, 2007), Handbook of Dementing Illnesses, 2n Edition (Taylor & Francis, 2006), and for an editorial in Lancet Neurology (Elsevier, 2008); and receives research support from Elan Corporation, Wyeth, Eli Lilly and Company, Novartis, Pfizer Inc, Avid Radiopharmaceuticals, the NIH/ NIA (P50AG05681 [PI], P01AG03991 [PI], P01AG026276 [PI], U01AG032438 [PI], U01AG024904 [Neuropathology Core Leader], R01AG16335 [Consultant], and P50NS006833 [Investigator]), and from the Dana Foundation. Dr. Morris serves on scientific advisory boards for AstraZeneca, Bristol-Myers Squibb, Genentech, Inc., Merck Serono, Novartis, Pfizer Inc, Schering-Plough Corp., Eli Lilly and Company, Wyeth, and Elan Corporation; serves on the editorial advisory board of Alzheimer's Disease and Associated Disorders; receives publishing royalties from Mild Cognitive Impairment and Early Alzheimer's Disease (John Wiley and Sons, 2008), Dementia (Clinical Publishing, 2007), Handbook of Dementing Illnesses, 2n edition (Taylor & Francis, 2006), and for an editorial in Lancet Neurology (Elsevier, 2008); and receives research support from Elan Corporation, Wyeth, Eli Lilly and Company, Novartis, Pfizer Inc, Avid Radiopharmaceuticals, the NIH (NIA P50AG05681 [PI], P01AG03991 [PI], P01AG026276 [PI], U01AG032438 [PI], U01AG024904 [Neuropathology Core Leader], R01AG16335 [Consultant], and P50NS006833 [Investigator]), and from the Dana Foundation. Dr. Mintun serves as a consultant for Avid Radiopharmaceuticals, Inc. and receives research support from the NIH (1RC1AG036045-01 [PI], P30 NS048056-01 [PI], 2PO1 AG03991-26 [Director of Imaging Core], PO1 AG026276 [Co-I], P50 AG005681-22 [PI of Project 3], 1U01AG032438-02 [Director, Imaging Core], P50 NS006833 [Co-PI], R01 DC009095-03 [Co-I], P30 CA091842 [Co-I], UL1 RR024992 [Director, Imaging Unit], 1R01NS055963-01 [Co-I], and U54CA136398-02 [Director of the Human Imaging Core]). Dr. Clifford serves/has served on scientific advisory boards for Biogen Idec, Elan Corporation, Roche, Forest Laboratories, Inc., Genentech, Inc., GlaxoSmithKline, Millennium Pharmaceuticals, Inc., Schering-Plough Corp., Bristol-Meyers Squibb, and Genzyme Corporation; received speaker honorarium and funding for travel from GlaxoSmithKline; has received research support from Pfizer Inc, Schering-Plough Corp., Bavarian Nordic, NeurogesX, GlaxoSmithKline, Tibotec Therapeutics, Boehringer Ingelheim, and Gilead Sciences, Inc.; and receives research support from the NIH (UO1 NS32228 [PI], UO1 AI69495 [PI], NIMH 22005 CHARTER Project [Site PI], NIDA RO3 DA022137 [Co-I], NIMH MH058076 [Site PI], and R21 3857-53187 [PI]).
Address correspondence and reprint requests to Dr. Beau M. Ances, Department of Neurology, Washington University in St. Louis, Box 8111, 660 South Euclid Ave., St. Louis, MO 63110 bances@wustl.edu
Editorial, page 105
e-Pub ahead of print on June 9, 2010, at www.neurology.org.
Study funding: Supported by ADRC Pilot Grant (3255 ADRC 26) (B.M.A.), NIMH (1K23MH081786) (B.M.A.), Dana Foundation (DF10052) (B.M.A.), NIMH-22005 (CHARTER, D.B.C. and B.M.A.), NIH AG026276 (J.C.M.), Washington University Center for Translational Neuroscience 1P30NS057105 (D.M.H.), NIH P01-AG026276 (J.C.M.), and NIH PO1-AG03991 (J.C.M.).
Disclosure: Author disclosures are provided at the end of the article.
Received October 22, 2009. Accepted in final form February 12, 2010.
REFERENCES
- 1.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 2005;65:1490–1492. [DOI] [PubMed] [Google Scholar]
- 2.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004;63:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- 4.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 2006;111:529–538. [DOI] [PubMed] [Google Scholar]
- 5.Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology 2009;73:1982–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163–W194. [DOI] [PubMed] [Google Scholar]
- 7.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007;64:343–349. [DOI] [PubMed] [Google Scholar]
- 9.Cairns NJ, Ikonomovic MD, Benzinger T, et al. PiB-PET detection of cerebral Aβ may lag clinical, cognitive, and CSF markers of Alzheimer's disease: a case report. Arch Neurol 2009;66:1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005;19:407–411. [DOI] [PubMed] [Google Scholar]