Abstract
Objective:
To compare the annual change in MRI and CSF biomarkers in cognitively normal (CN), amnestic mild cognitive impairment (aMCI), and Alzheimer disease (AD). Comparisons were based on intergroup discrimination, correlation with concurrent cognitive/functional changes, relationships to APOE genotype, and sample sizes for clinical trials.
Methods:
We used data from the Alzheimer's Disease Neuroimaging Initiative study consisting of CN, aMCI, and AD cohorts with both baseline and 12-month follow-up CSF and MRI. The annual change in CSF (total-tau [t-tau], Aβ1-42) and MRI (change in ventricular volume) was obtained in 312 subjects (92 CN, 149 aMCI, 71 AD).
Results:
There was no significant average annual change in either CSF biomarker in any clinical group except t-tau in CN; moreover, the annual change did not differ by clinical group in pairwise comparisons. In contrast, annual increase in ventricular volume increased in the following order, AD > aMCI > CN, and differences were significant between all clinical groups in pairwise comparisons. Ventricular volume increase correlated with concurrent worsening on cognitive/functional indices in aMCI and AD whereas evidence of a similar correlation with change in CSF measures was unclear. The annual changes in MRI differed by APOE ε4 status overall and among aMCI while annual changes in CSF biomarkers did not. Estimated sample sizes for clinical trials are notably less for MRI than the CSF or clinical measures.
Conclusions:
Unlike the CSF biomarkers evaluated, changes in serial structural MRI are correlated with concurrent change on general cognitive and functional indices in impaired subjects, track with clinical disease stage, and are influenced by APOE genotype.
GLOSSARY
- AD
= Alzheimer disease;
- ADAS-Cog
= Alzheimer's Disease Assessment Scale–cognitive subscale;
- ADNI
= Alzheimer's Disease Neuroimaging Initiative;
- aMCI
= amnestic mild cognitive impairment;
- AUROC
= area under the receiver operator characteristic curve;
- BSI
= boundary shift integral;
- CDR-SB
= Clinical Dementia Rating–sum of boxes;
- CN
= cognitively normal;
- MMSE
= Mini-Mental State Examination;
- NFT
= neurofibrillary tangle;
- NT
= neuropil thread;
- PiB
= Pittsburgh compound B;
- t-tau
= total-tau.
The dominant pathologic findings in Alzheimer disease (AD) are Aβ-rich amyloid plaques, fibrillary tau deposits in neurofibrillary tangles (NFTs) and neuropil threads (NTs), as well as neuronal dysfunction and neurodegeneration. Accepted biomarker surrogates of the dominant pathologies in AD are Aβ1-42 and total-tau (t-tau) levels measured in CSF and atrophy seen on MRI. Low CSF Aβ1-42 levels reflect deposition of Aβ in amyloid plaques.1 Elevated CSF t-tau levels reflect abnormal tau accumulation in NFTs and NTs as well as active axonal and neuronal damage.1,2 Atrophy on MRI is the macroscopic manifestation of microscopic neurodegenerative changes and reflects the cumulative loss of neurons, synapses, and dendritic arborization.3 Cross-sectionally, biomarkers serve as in vivo indicators of disease stage. Longitudinal biomarker measures of change over time provide additive diagnostic and prognostic information about the rate of change in disease-related pathology and can serve as outcome measures in therapeutic trials.4
Although both MRI and CSF biomarkers have been studied extensively cross-sectionally and to a lesser extent longitudinally in small cohorts or single centers, few reports have compared longitudinal change on both CSF and MRI biomarkers in the same subjects examined serially in multicenter studies of large cohorts of cognitively normal (CN) individuals, subjects with amnestic-mild cognitive impairment (aMCI), and patients with AD. Thus, the aims of this study were 4-fold:
To measure the annual change in CSF Aβ1-42, CSF t-tau, and ventricular volume on MRI by clinical group and compare the annual change in biomarkers between clinical groups.
To assess the correlation between annual change in CSF and MRI measures and annual change on continuous measures of cognitive and functional performance: Clinical Dementia Rating–sum of boxes (CDR-SB), Mini-Mental State Examination (MMSE), and Alzheimer's Disease Assessment Scale–cognitive subscale (ADAS-Cog).
To evaluate the effect of APOE ε4 status on the annual change in the biomarkers.
To compare sample sizes needed in a hypothetical clinical trial.
METHODS
The data used in this study are from the Alzheimer's Disease Neuroimaging Initiative (ADNI), a longitudinal multisite observational study of CN, aMCI, and AD collected from 59 participating institutes.5 All 312 ADNI subjects with both baseline and 12-month follow-up CSF and usable MRI were considered in this study. The complete details of ADNI can be found at http://www.ADNI-info.org.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained for participation in these studies, as approved by the Institutional Review Board at each participating center.
Clinical and cognitive assessment.
We used MMSE,6 CDR-SB,7 and ADAS-Cog8 as overall indices of general cognitive performance and global functional status. In this study, we used the modified ADAS-Cog scores (ADAS-Cog-13) from ADNI, which has 2 additional components (delayed recall task and a number cancellation task). The clinical and cognitive assessments used were baseline clinical diagnosis of all 3 clinical groups and baseline, 6-month, and 12-month follow-up clinical/cognitive assessment scores.
CSF acquisition.
CSF was collected at each site, transferred into polypropylene transfer tubes followed by freezing on dry ice within 1 hour after collection, and shipped overnight to the ADNI Biomarker Core Laboratory at the University of Pennsylvania Medical Center on dry ice. When samples are received in the laboratory, they are thawed and aliquots are stored in bar-coded polypropylene vials at −80°C. A standardized protocol was implemented to quantify biomarker concentrations in each of the CSF ADNI baseline aliquots using a multiplex xMAP Luminex platform (Luminex Corp., Austin, TX) with Innogenetics (INNO-BIA AlzBio3, Ghent, Belgium; for research use only reagents) immunoassay kit-based reagents, which was validated in references 9 and 10. Quality control values obtained during the analyses of ADNI baseline CSF aliquots were interday reproducibilities (%CV) for an AD CSF pool and a routine clinic patient CSF pool of 4.5% and 6.4% for t-tau and 3.3% and 6.9% for Aβ1-42; r2 values for comparison of retested samples were 0.98 and 0.90. The CSF measurements obtained at baseline and 12 months were used.
MRI.
ADNI collects 1.5-T MRI examinations from all subjects and only these scans were used for this study. The acquired morphometric T1-weighted magnetization-prepared rapid gradient echos5 were corrected for gradient nonlinearity and intensity inhomogeneity and checked for geometric fidelity using the phantom scan acquired with each subject.5
MRI processing steps were performed by a research technician who was blinded to all clinical information. Brain atrophy was assessed by measuring ventricular expansion rates using the boundary shift integral (BSI) technique11 on spatially registered 3-dimensional image sets. The ventricular atrophy rate was derived by creating a binary ventricular mask for each subject that selectively extracted ventricular change from the BSI. Quality control testing in our laboratory shows that the intraclass correlation coefficient for test–retest reproducibility of ventricle rate measurements from short interval serial MRI scans with this method is 0.91.12 We chose ventricular BSI for measuring longitudinal change in MRI because it gives the best performance among the various longitudinal MRI measures, as evaluated in reference 12. If we required a cross-sectional measure at baseline with strong association with disease severity, we would have used hippocampal volumes. The MRI measurements obtained at baseline and 12 months was used. Although MRI scans were acquired at 0, 6, and 12 months, MRI change was measured without reference to the 6-month scans to put MRI on the same footing with CSF, which was sampled at only 0 and 12 months.
Statistical methods.
Baseline patient characteristics and cognitive, CSF, and MRI measurements and annual change in these measurements were summarized as median (interquartile range) for continuous measures and count (%) for categorical measures. Annual change in cognitive measures (CDR-SB, MMSE, ADAS-Cog) was defined by fitting a linear slope of time with each measure within individual subjects using the data available from the baseline and 6- and 12-month cognitive assessments. Annual change in CSF and MRI measures was defined as difference in the 12-month and baseline values divided by time between measurements.
Whether annual change was significantly different from zero was evaluated using Wilcoxon signed-rank tests. The relationship between annual change in cognition and annual percent change in CSF and MRI measures within each clinical diagnosis group was assessed using Spearman correlations. Area under the receiver operator characteristic curve (AUROC) and corresponding pairwise Wilcoxon rank-sum test p values were used to assess the differences in annual change in ventricular volume, Aβ1-42, and t-tau across clinical group (CN vs aMCI, CN vs AD, aMCI vs AD) and across APOE genotype (ε4-positive vs ε4-negative) within all subjects and within each diagnosis group separately.
To compare the suitability of the cognitive, MRI, and CSF change measures as surrogate endpoints for a clinical trial, we estimated the sample sizes needed to detect a 25% slowing of the rate of change in annualized change with 80% power assuming a 2-sided, 2-sample t test and an α level of 0.05. The sample size estimates in MCI and AD were not adjusted for the rate of change in normal aging (i.e., by subtracting the rate in CN from MCI and AD). The estimates were based on the observed means and standard deviations.
RESULTS
Patient characteristics.
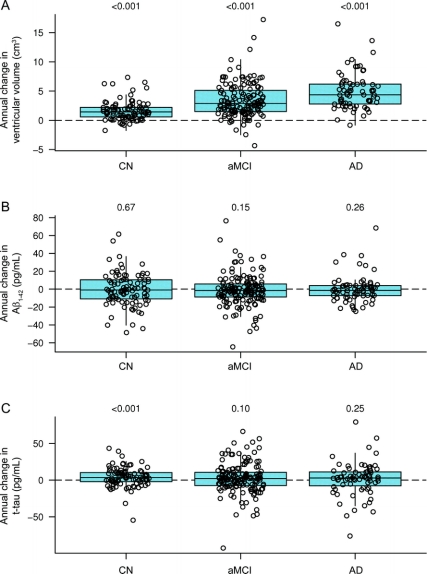
The demographics, clinical summary, and biomarker summary of all subjects with MRI and CSF data at baseline and 1-year follow-up are presented in table 1. There were no significant differences in age, gender, or education between the groups except that patients with AD were less educated than patients with MCI (p = 0.04) and there was a higher proportion of women in the CN than the MCI group (p = 0.03). As expected, the proportions of ε4 carriers were ordered as CN < MCI < AD. The median annual increase in ventricular volume was greater than 0 for CN, aMCI, and AD (p < 0.001) and the increase in ventricular volume was ordered by clinical group AD > aMCI > CN. The median annual change in t-tau was greater than 0 in CN (p < 0.001) and there was a trend of increasing t-tau in aMCI (p = 0.10) but not in AD (p = 0.25). The median annual change in Aβ1-42 was not different than 0 for CN, aMCI, or AD (p ≥ 0.15 for all). Box plots of annual change of MRI and CSF biomarker distributions by group are shown in figure 1.
Table 1 Descriptive characteristics of subjects
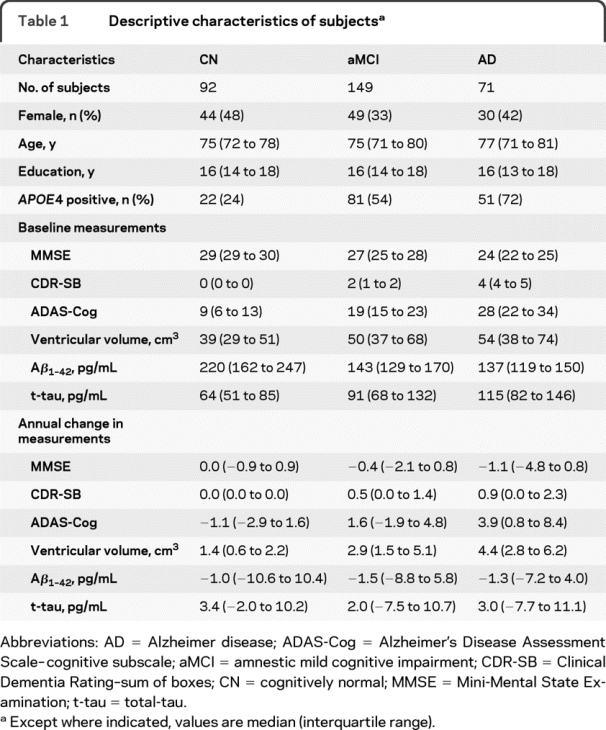
Figure 1 Box plots of annual change in ventricular volume (cm3), Aβ1–42 (pg/mL), and t-tau (pg/mL) by clinical diagnosis
Individual points have been randomly shifted along the x-axis to reduce overlap. Boxes represent the 25th, 50th, and 75th percentiles of the data. Whiskers represent the range of the non-outlier data estimated using Tukey method. The horizontal line indicates the reference of annual change of zero. p Value indicates the results from the test of whether the annual change was different from zero. AD = Alzheimer disease; aMCI = amnestic mild cognitive impairment; CN = cognitively normal; t-tau = total-tau.
Comparing annual change across clinical groups.
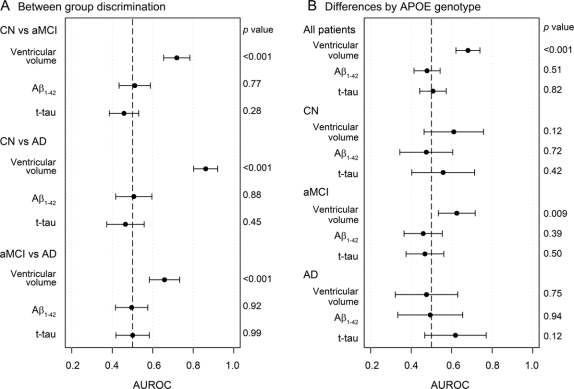
Annual change in CSF and MRI biomarkers was compared across clinical groups and the AUROC and p values for the pairwise discrimination between groups are presented in the left panel of figure 2. Annual change in ventricular volume separated all clinical groups (p < 0.001) and annual change in CSF Aβ1-42 and t-tau did not separate any of the clinical groups (p > 0.05).
Figure 2 Area under the receiver operating characteristic curve (AUROC) with 95% confidence intervals for (A) between-group discrimination and (B) between-APOE genotype discrimination within clinical groups based on annual change in ventricular volume, Aβ1-42, and t-tau
A reference line has been drawn at 0.50, which indicates no discriminative power for the measure. p Values along the right side of each panel are from Wilcoxon signed-rank tests. AD = Alzheimer disease; aMCI = amnestic mild cognitive impairment; CN = cognitively normal; t-tau = total-tau.
Correlations between annual change in CSF and MRI and change on continuous measures of cognitive and functional performance.
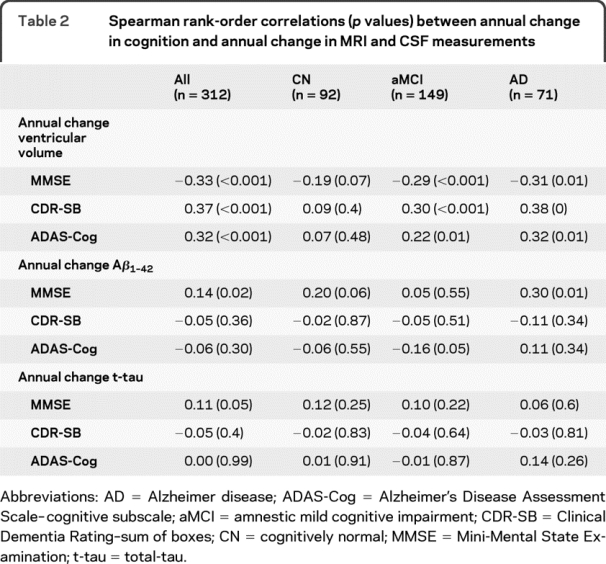
Spearman rank order correlations between annual change in cognitive/functional measures and annual change in MRI/CSF biomarker are shown in table 2. Figure e-1 (on the Neurology® Web site at www.neurology.org) illustrates the scatterplot of annual change in MRI/CSF biomarkers vs annual change in cognitive/functional measures. In each of the clinical diagnostic groups, there was no significant correlation between the annual change in CSF biomarkers and annual decline in cognitive and functional scores in any of the groups except decrease in MMSE correlated with decrease in CSF Aβ1-42 in AD (p = 0.01), increase in ADAS-Cog correlated with decrease in CSF Aβ1-42 in MCI (p = 0.05), and decrease in MMSE correlated with decrease in CSF Aβ1-42 in CN (p = 0.06). Overall, within each clinical group, the evidence of association between changes in CSF biomarkers with concurrent change in cognition was not clear. However, decrease in ventricular volume measured by MRI uniformly correlated with change in ADAS-Cog, CDR-SB, and MMSE in aMCI and AD groups (p ≤ 0.01), suggesting that the change in cognitive status in aMCI and AD is more tightly coupled with changes in structural MRI.
Table 2 Spearman rank-order correlations (p values) between annual change in cognition and annual change in MRI and CSF measurements
Effect of APOE ε4 status on annual change in biomarkers.
The effect of APOE ε4 status on the annual change in biomarkers was evaluated overall and within each clinical group and the results are presented in figure 2. There was no significant difference in the annual change of CSF biomarkers in all subjects combined and within each of the clinical groups when ε4 carriers were compared to noncarriers. The annual increase in ventricular volume was greater in ε4 carriers than noncarriers in aMCI subjects alone (p = 0.009) and when all subjects were combined (p < 0.001). This latter finding was probably driven by aMCI and CN subjects since there was no significant difference in the increase of the ventricular volume in APOE ε4 carriers and noncarriers among patients with AD.
Sample size calculations.
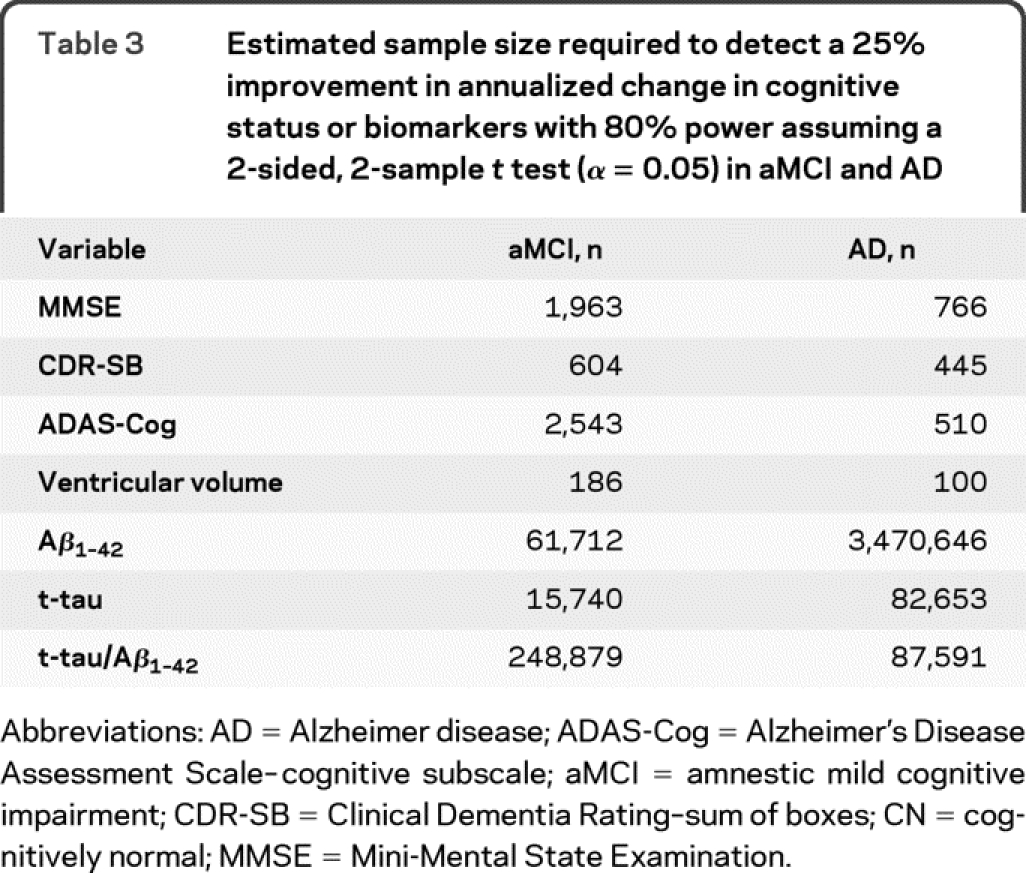
The estimated sample size needed to detect a 25% improvement in annualized change in cognitive status or biomarkers with 80% power assuming a 2-sided, 2-sample t test (α = 0.05) in aMCI and AD are shown in table 3. The estimated sample size required to detect a difference in the CSF biomarkers and the standard clinical assessment metrics was generally large. Conversely, MRI required 100 subjects with AD and 186 subjects with MCI per group to detect a 25% slowing of the rate of change.
Table 3 Estimated sample size required to detect a 25% improvement in annualized change in cognitive status or biomarkers with 80% power assuming a 2-sided, 2-sample t test (α = 0.05) in aMCI and AD
DISCUSSION
Aβ amyloid deposition is increasingly recognized to be an early pathologic event that occurs prior to clinical symptoms.13,14 In addition, amyloid burden at autopsy does not correlate with disease duration.15 These findings have been interpreted to indicate that Aβ amyloid deposition itself is not directly responsible for clinical symptoms, but rather initiates a pathologic cascade that later results in clinical symptoms.16,17 Both CSF Aβ1-42 and Pittsburgh compound B (PiB)-PET scans are used as in vivo indicators of Aβ amyloid deposition with nearly complete concordance between positive PiB-PET scans and low CSF Aβ1-42.18 The serial CSF Aβ1-42 results found in this article are consistent with earlier CSF Aβ1-42 and PiB-PET studies that found little or no increase in amyloid deposition over time as measured by CSF Aβ1-4219,20 or PiB16,21 in subjects (e.g., aMCI and AD) whose cognition declined significantly over the same time period. In an earlier cross-sectional study22 in this same cohort, baseline CSF Aβ1-42 measurements also showed poor correlation with continuous measures of cognitive and functional performance within each clinical group. Our longitudinal data indicating that CSF Aβ1-42 does not change appreciably with worsening cognition is therefore in agreement with most literature on this subject.
Increased CSF t-tau is a marker of neuronal injury and it correlates well with the severity of NFTs and NTs at autopsy in subjects with AD.1,23 Given the good cross-sectional correlation between NFT/NT pathology and clinical disease severity and duration of clinical AD, one might expect change in t-tau to correlate with change in clinical measures and t-tau levels to increase over time in subjects with aMCI and AD, with little increase over time in CN. In contrast, we found evidence of increasing t-tau only in CN (p < 0.001), a trend toward increasing t-tau in aMCI (p = 0.10), and no evidence of increasing t-tau in AD (p = 0.25). Moreover, there was no correlation between change in cognition and change in t-tau levels within each clinical group, including aMCI and AD. These results seem counterintuitive. T-tau levels are clearly greater at baseline in aMCI and AD than in CN, and therefore at some point in time, t-tau had to increase measurably with time in these subjects. One possible explanation for the relative stability of t-tau over time in aMCI and AD might be that increases in t-tau reflect a pathologic process (release of tau from injured neurons) that reaches a ceiling when subjects who will ultimately develop clinical symptoms are in the CN and very early MCI phases of the disease. Note that ADNI subjects with MCI were selected to be late in the MCI phase on the basis of impaired performance on delayed memory recall. Alternatively, t-tau levels might have increased over time in measurable amounts had the observation period been longer than 12 months; however, our results are consistent with some longitudinal t-tau studies that have shown stable t-tau measures over extended periods of time up to 3 years.20,24 A final possibility is that t-tau did increase in our subjects with aMCI and subjects with AD but the measurement precision was inadequate to detect the increases that were biologically present. This seems unlikely, however, given the analytical performance of the xMAP immunoassay system as described in Methods.10
Atrophy on structural MRI correlates with Braak NFT stage and NFT load25,26 but the most proximate histologic correlate is neurodegenerative shrinkage of the brain; i.e., loss of neurons and synapses.3,27 Our results of good correlations between ventricular enlargement and cognitive worsening in aMCI and AD is consistent with the MRI literature, which is nearly unanimous in indicating close correlation between cognitive decline and volume loss on MRI.12,28–30 There was no consistent relationship in our CN subjects since CN on an average do not have disease-related decline on measures of general cognition. The results are also consistent with 2 recent studies that investigated similar questions using ADNI MRI data.31,32
Our findings on rates of brain atrophy and APOE ε4 status are also consistent with some studies in the literature.33,34 The most logical explanation for greater rates of atrophy in aMCI ε4 carriers is simply that carriers more likely have prodromal AD (and thus higher rates of atrophy), while noncarriers are less likely to have prodromal AD and include persons with nonprogressive conditions. This is supported by our recent study which showed higher amyloid load in APOE ε4 CN and MCI carriers compared to noncarriers at the same level of cognitive performance35 and the fact that APOE ε4 status is predictive of time to conversion from aMCI to AD in this cohort (p = 0.04).
Implications of these data for estimating sample sizes for clinical trials are that if the treatment effect is calculated in a traditional way where the treatment modifies the rate of change that occurs naturally in the disease course then CSF biomarkers would be ineffective. However, if the treatment effect were to reverse the effect of the disease course, i.e., Aβ1-42 increase and t-tau decrease due to treatment, then CSF might become an effective biomarker. The sample size estimates in this study for longitudinal MRI are comparable to those in the existing literature for MRI.36–39 It should be noted that while CSF and MRI biomarkers have not yet been validated as surrogate endpoints for regulatory purposes and therefore cannot be used as the primary indicators of efficacy, the impact of interventions on these biomarkers may still be useful in capturing pharmacodynamic effect. Also, the observations made in this study, while generalizable to populations with similar characteristics, may not generalize to subject populations that significantly differ from ADNI on major demographic variables.
Biomarkers for measuring disease progression.
The 3 disease markers examined in this article reflect different aspects of AD pathology. A recent dynamic model of biomarkers16,40 proposed that there is an ordered onset of biomarker abnormalities beginning with CSF Aβ1-42 (amyloid deposition) followed by CSF t-tau (neuronal dysfunction) and last MRI (neurodegeneration) with the main underlying substrate of cognitive impairment being neurodegeneration. While longer periods of follow-up with longitudinal biomarker measurements are required, the results of our study with 12-month change measurements are in agreement with this model. We suggest that the lack of change over time in CSF Aβ1-42 and t-tau in subjects with aMCI and subjects with AD may be because both of these biomarkers become abnormal prior to appearance of clinical symptoms. In particular, we suggest that CSF Aβ1-42 becomes abnormal while subjects are still cognitively intact, and that both CSF Aβ1-42 and t-tau have reached a ceiling by the time subjects are in late MCI phase (i.e., representing the ADNI MCI cohort) of the disease and beyond. MRI, in contrast, becomes abnormal later in the disease progression than either CSF Aβ1-42 or tau, but retains a close relationship with clinical symptoms later into disease progression. We suggest that both MRI and CSF biomarkers are needed to fully characterize the different aspects of disease-related pathology. Our results (specifically the sample size estimates) support the use of longitudinal MRI measurements as an outcome measure for detecting highly relevant neurodegenerative changes throughout the clinically evident phases of the disease.
AUTHOR CONTRIBUTIONS
Study concept and design: P.V., C.R.J.; analysis and interpretation of the study: S.D.W., H.J.W., P.V., C.R.J., D.S.K., J.Q.T.; drafting of the manuscript: P.V., C.R.J.; critical revision of the manuscript for intellectual content: H.J.W., S.D.W., D.S.K., J.Q.T., L.M.S., M.A.B., P.S.A., M.W.W., R.C.P. Statistical analysis was conducted by Heather J. Wiste and Stephen D. Weigand.
ACKNOWLEDGMENT
The authors thank Maria S. Shiung for running the boundary shift integral software on all the scans.
DISCLOSURE
Dr. Vemuri receives support from the Robert H. Smith Family Foundation Research Fellowship. Ms. Wiste and Mr. Weigand report no disclosures. Dr. Knopman serves as an Associate Editor for Neurology; has served on data safety monitoring boards for sanofi-aventis, GlaxoSmith Kline, and Eli Lilly and Company; is an investigator in clinical trials sponsored by Elan Corporation, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH (R01-AG023195 [PI], R01-AG11378 [Co-I], P50 AG16574 [Co-I], U01 AG 06786 [Co-I], and R01 HL70825 [Co-I]). Dr. Trojanowski has received funding for travel and honoraria from Takeda Pharmaceutical Company Ltd. and to attend numerous conferences not funded by industry; serves as an Associate Editor of Alzheimer's & Dementia; may accrue revenue on patents re: Modified Avidin-Biotin Technique, Method of Stabilizing Microtubules to Treat Alzheimer's Disease, Method of Detecting Abnormally Phosphorylated Tau, Method of Screening for Alzheimer's Disease or Disease Associated with the Accumulation of Paired Helical Filaments, Compositions and Methods for Producing and Using Homogeneous Neuronal Cell Transplants, Rat Comprising Straight Filaments in Its Brain, Compositions and Methods for Producing and Using Homogeneous Neuronal Cell Transplants to Treat Neurodegenerative Disorders and Brain and Spinal Cord Injuries, Diagnostic Methods for Alzheimer's Disease by Detection of Multiple MRNAs, Methods and Compositions for Determining Lipid Peroxidation Levels in Oxidant Stress Syndromes and Diseases, Compositions and Methods for Producing and Using Homogenous Neuronal Cell Transplants, Method of Identifying, Diagnosing and Treating Alpha-synuclein Positive Neurodegenerative Disorders, Mutation-specific Functional Impairments in Distinct Tau Isoforms of Hereditary Frontotemporal Dementia and Parkinsonism Linked to Chromosome-17: Genotype Predicts Phenotype, Microtubule Stabilizing Therapies for Neurodegenerative Disorders; and Treatment of Alzheimer's and Related Diseases with an Antibody; and receives research support from the NIH (NIA P01 AG 09215-20 [PI], NIA P30 AG 10124-18 [PI], NIA PO1 AG 17586-10 [Project 4 Leader], NIA 1PO1 AG-19724-07 [Core C Leader], NIA 1 U01 AG 024904-05 [Co-PI Biomarker Core Laboratory], NINDS P50 NS053488-02 [PI], NIA UO1 AG029213-01 [Co-I]; RC2NS069368 [PI], RC1AG035427 [PI], and NIA P30AG036468 [PI]), and from the Marian S. Ware Alzheimer Program. Dr. Shaw has received funding for travel and speaker honoraria from Pfizer Inc; serves on the editorial board of Therapeutic Drug Monitoring; may potentially receive revenue for patent pending (application number 10/192,193): O-methylated rapamycin derivatives for alleviation and inhibition of lymphoproliferative disorders, licensed by the University of Pennsylvania to Novartis; receives royalties from publication of Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring (Wolters Kluwer/Lippincott Williams & Wilkins, 2005); receives research support from the NIH (AG024904 [Co-PI Biomarker Core Laboratory]); and receives board of directors' compensation and holds stock options in Saladax Biomedical. Dr. Bernstein serves as an Associate Editor of Medical Physics and on the editorial board of Magnetic Resonance in Medicine; may accrue revenue on patents re: Under-sampled 3D MRI using a shells k-space sampling trajectory, Motion correction of magnetic resonance images, MRI RF power monitor, and Method of performing magnetic resonance angiography using two-dimensional imaging and de-rated gradients; receives royalties from the publication of Handbook of MRI Pulse Sequences (Elsevier's Academic Press, 2004), Thinking About Equations: A Practical Guide for Developing Mathematical Intuition in the Physical Sciences and Engineering (John Wiley and Sons, 2009); and receives research support from Pfizer Inc and from the NIH (NIA AG24904-01 [Co-I]). Dr. Aisen serves on a scientific advisory board for NeuroPhage; serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Neurochem Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, and Medivation, Inc.; receives research support from Pfizer Inc, Baxter International Inc., Neuro-Hitech, Abbott, Martek, and the NIH (NIA U01-AG10483 [PI], NIA U01-AG024904 [Coordinating Center Director], NIA R01-AG030048 [PI], and R01-AG16381 [Co-I]); and has received stock options from Medivation, Inc. and NeuroPhage. Dr. Weiner serves on scientific advisory boards for Bayer Schering Pharma, Eli Lilly and Company, CoMentis, Inc., Neurochem Inc, Eisai Inc., Avid Radiopharmaceuticals Inc., Aegis Therapies, Genentech, Inc., Allergan, Inc., Lippincott Williams & Wilkins, Bristol-Myers Squibb, Forest Laboratories, Inc., Pfizer Inc, McKinsey & Company, Mitsubishi Tanabe Pharma Corporation, and Novartis; has received funding for travel from Nestlé and Kenes International and to attend conferences not funded by industry; serves on the editorial board of Alzheimer's & Dementia; has received honoraria from the Rotman Research Institute and BOLT International; serves as a consultant for Elan Corporation; receives research support from Merck & Co., Radiopharmaceuticals Inc., the NIH (U01AG024904 [PI], P41 RR023953 [PI], R01 AG10897 [PI], P01AG19724 [Co-I], P50AG23501 [Co-I], R24 RR021992 [Co-I], R01 NS031966 [Co-I], and P01AG012435 [Co-I]), the US Department of Defense (DAMD17-01-1-0764 [PI]), the Veterans Administration (MIRECC VISN 21 [Core PI]), and from the State of California; and holds stock in Synarc and Elan Corporation. Dr. Petersen serves on scientific advisory boards for Elan Corporation, Wyeth, and GE Healthcare; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA (U01 AG 06786 [PI], P50 AG 16574 [PI], U01 AG 024904 [Subcontract PI], and R01 AG11378 [Co-I]). Dr. Jack serves as a consultant for Eli Lilly and Company and Elan Corporation; is an investigator in clinical trials sponsored by Baxter International Inc., Pfizer Inc, the NIH/NIA (AG11378 [PI], P50-AG16574 [Co-I], and U01 AG024904–01 [Co-I]), and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock in GE Healthcare.
Supplementary Material
Address correspondence and reprint requests to Dr. Clifford R. Jack, Jr., Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905 jack.clifford@mayo.edu
Supplemental data at www.neurology.org
Data used in the preparation of this article were obtained from the Alzheimer's disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete list of ADNI investigators is available in appendix e-1 at www.neurology.org.
Study funding: Supported by NIH grant AG11378; a Robert H. Smith Family Foundation Research Fellowship; the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation, USA; and Opus building NIH grant C06 RR018898. The Foundation for the National Institutes of Health (www.fnih.org) coordinates the private sector participation of the $60 million ADNI public-private partnership that was begun by the National Institute on Aging (NIA) and supported by the National Institutes of Health. To date, more than $27 million has been provided to the Foundation for NIH by Abbott, AstraZeneca AB, Bayer Schering PharmaAG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly and Co., Merck & Co., Inc., Novartis AG, Pfizer Inc., F. Hoffmann-LaRoche, Schering-Plough, Synarc Inc., and Wyeth, as well as nonprofit partners the Alzheimer's Association and the Institute for the Study of Aging.
Disclosure: Author disclosures are provided at the end of the article.
Received November 21, 2009. Accepted in final form March 10, 2010.
REFERENCES
- 1.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009;66:382–389. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 1995;26:231–245. [DOI] [PubMed] [Google Scholar]
- 3.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience 2000;95:721–725. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 7.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 8.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 9.Vanderstichele H, De Meyer G, Shapiro F, et al. Biomarkers for Early Diagnosis of Alzheimer's Disease. Hauppauge, NY: Nova Science Publishers; 2008. [Google Scholar]
- 10.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging 1997;16:623–629. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 2004;62:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 2009;65:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009;66:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004;62:925–931. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009;132:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain 2008;132:1310–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- 19.Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett 2003;339:99–102. [DOI] [PubMed] [Google Scholar]
- 20.Andersson C, Blennow K, Almkvist O, et al. Increasing CSF phospho-tau levels during cognitive decline and progression to dementia. Neurobiol Aging 2008;29:1466–1473. [DOI] [PubMed] [Google Scholar]
- 21.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain 2006;129:2856–2866. [DOI] [PubMed] [Google Scholar]
- 22.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology 2009;73:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell TW, Mufson EJ, Schneider JA, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer's disease. Ann Neurol 2002;51:182–189. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland T, Wolozin B, Galasko D, et al. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol Psychiatry 1999;46:750–755. [DOI] [PubMed] [Google Scholar]
- 25.Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 2002;58:1476–1482. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002;58:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarow C, Vinters HV, Ellis WG, et al. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol 2005;57:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet 2004;363:392–394. [DOI] [PubMed] [Google Scholar]
- 29.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci USA 2002;99:4703–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Leon MJ, Convit A, George AE, et al. In vivo structural studies of the hippocampus in normal aging and in incipient Alzheimer's disease. Ann NY Acad Sci 1996;777:1–13. [DOI] [PubMed] [Google Scholar]
- 31.Nestor SM, Rupsingh R, Borrie M, et al. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain 2008;131:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans MC, Barnes J, Nielsen C, et al. Volume changes in Alzheimer's disease and mild cognitive impairment: cognitive associations. Eur Radiol 2010;20:674–682. [DOI] [PubMed] [Google Scholar]
- 33.van de Pol LA, van der Flier WM, Korf ES, Fox NC, Barkhof F, Scheltens P. Baseline predictors of rates of hippocampal atrophy in mild cognitive impairment. Neurology 2007;69:1491–1497. [DOI] [PubMed] [Google Scholar]
- 34.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology 2005;64:1520–1524. [DOI] [PubMed] [Google Scholar]
- 35.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol 2010;67:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR Jr, Slomkowski M, Gracon S, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology 2003;60:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schott JM, Frost C, Whitwell JL, et al. Combining short interval MRI in Alzheimer's disease: implications for therapeutic trials. J Neurol 2006;253:1147–1153. [DOI] [PubMed] [Google Scholar]
- 38.Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol 2000;57:339–344. [DOI] [PubMed] [Google Scholar]
- 39.Hua X, Leow AD, Lee S, et al. 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage 2008;41:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.