Abstract
Background:
Anticholinergic properties of certain medications often go unrecognized, and are frequently used by the elderly population. Few studies have yet defined the long-term impact of these medications on the incidence of cognitive impairment.
Methods:
We report a 6-year longitudinal, observational study, evaluating 1,652 community-dwelling African American subjects over the age of 70 years who were enrolled in the Indianapolis-Ibadan Dementia Project between 2001 and 2007 and who had normal cognitive function at baseline. The exposure group included those who reported the baseline use of possible or definite anticholinergics as determined by the Anticholinergic Cognitive Burden scale. Our main outcome measure was the incidence of cognitive impairment, defined as either dementia or cognitive impairment not dementia, or poor performance on a dementia screening instrument during the follow-up period.
Results:
At baseline, 53% of the population used a possible anticholinergic, and 11% used a definite anticholinergic. After adjusting for age, gender, educational level, and baseline cognitive performance, the number of definite anticholinergics was associated with an increased risk of cognitive impairment (odds ratio [OR] 1.46, 95% confidence interval [CI] 1.07–1.99; p = 0.02), whereas the number of possible anticholinergics at baseline did not increase the risk (OR 0.96, 95% CI 0.85–1.09; p = 0.55). The risk of cognitive impairment among definite anticholinergic users was increased if they were not carriers of the APOE ε4 allele (OR 1.77, 95% CI 1.03–3.05; p = 0.04).
Conclusions:
Limiting the clinical use of definite anticholinergics may reduce the incidence of cognitive impairment among African Americans.
GLOSSARY
- ACB
= Anticholinergic Cognitive Burden scale;
- CERAD
= Consortium to Establish a Registry for Alzheimer's Disease;
- CI
= confidence interval;
- CIND
= cognitive impairment no dementia;
- CSI-D
= Community Screening Interview for Dementia;
- DSM-III-R
= Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised;
- IIDP
= Indianapolis Ibadan Dementia Project;
- OR
= odds ratio.
Over the past 2 decades, numerous epidemiologic studies have identified a spectrum of modifiable risk factors for dementia, such as lack of physical, cognitive, or social activities, and the presence of diabetes, hypertension, or hyperlipidemia.1 Although the older adult population uses multiple chronic medications, few studies have evaluated the exposure to medications as possible modifiable risk factors for Alzheimer disease (AD) and other dementing illnesses.2–5
The muscarinic cholinergic receptor, M1, has been associated with cognitive performance in rodent models, with direct antagonism of this receptor resulting in declining cognitive function.6–8 It has been suggested that the progression of Alzheimer-type pathology may be amplified with M1 blockade.9 Data also suggest that enhancing cholinergic transmission through the M1 receptor may reduce the deposits of Aβ peptides.10,11 Thus, the potential to advance the progression of AD through the chronic exposure of anticholinergic medications warrants further evaluation.
With the established link between anticholinergics and the risk of acute cognitive impairment,12 we examined data from an ongoing observational epidemiologic study of community-dwelling, African American older adults. We hypothesized that the use of medications with both possible and definite anticholinergic activity would increase the risk of cognitive impairment and that such a risk would be cumulative and modified by the presence of APOE ε4 carrier status.
METHODS
Study participants.
Participants included in this analysis were those evaluated in the 2001 wave of the Indianapolis Ibadan Dementia Project (IIDP). The IIDP is a prospective community-based comparative epidemiologic study that enrolled only African Americans living in Indianapolis, Indiana, and Yoruba from Ibadan, Nigeria. The aim of the ongoing study is to describe the rates and risk factors for age-associated dementia between the 2 populations.13 This analysis is limited to African Americans in Indianapolis. The original study began in 1992 and enrolled community-dwelling African Americans over the age of 65 years. In 2001, the IIDP enrolled additional community-dwelling participants randomly selected from Medicare records, self-identified as African American, and were at least 70 years of age.14 This study included participants remaining from the original cohort in 1992 and those enrolled at the 2001 evaluation wave.
Study design.
Enrolled participants were evaluated using a 2-stage study design at each data collection point. Cognitive performance evaluations were collected at baseline, defined as the 2001 evaluation for this analysis, and at 3 and 6 years after the baseline evaluation. The screening stage included an in-home interview with the Community Screening Interview for Dementia (CSI-D). The CSI-D evaluates multiple cognitive domains (language, memory, attention, and calculation, among others) and includes a standardized interview of physical and social function from a caregiver informant or relative if available.15,16 Based upon the initial cognitive screening, participants were classified as good, intermediate, or poor performers based upon CSI-D cutoffs. The CSI-D performance groups were determined by combining the cognitive score and the informant interview score in a discriminant function score. For cases in which there was not an informant, cognitive scores alone were used to determine the performance group.
The second stage utilized a full diagnostic clinical assessment, which was offered to all participants in the poor performance group on the CSI-D screening instrument, 75% of those with an intermediate performance, and 2% of those identified as good performers. The comprehensive cognitive and clinical assessment included 1) a neuropsychological battery adapted from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD)17; 2) a standardized neurologic and physical examination and functional status review (The Clinician Home-Based Interview to assess Function)18; and 3) a structured interview with a close relative adapted from the Cambridge Examination for Mental Disorders of the Elderly informant interview.19 Following the second stage of evaluation, participants were diagnosed into 1 of 3 mutually exclusive major cognitive diagnostic categories: normal cognitive function, dementia, or cognitive impairment no dementia (CIND). Diagnosis was made in a consensus diagnostic conference of clinicians reviewing the comprehensive clinical assessment and available medical records. Clinicians were blinded to CSI-D scores and screening performance group. Dementia was diagnosed with both International Classification of Diseases, 10th Revision,20 and DSM-III-R21 criteria. CIND was diagnosed if 1) there was an informant report of a clinically significant decline in cognitive function, 2) evidence of significant cognitive decline on physician examination, or 3) impaired CERAD test performance and no or only minimal impairments in activities of daily living.17
Standard protocol approvals, registrations, and participant consents.
The study was approved by the Indiana University–Purdue University–Indianapolis Institutional Review Board. All participants enrolled provided informed consent.
Study outcome.
Participants identified as having dementia or CIND or who were in the poor screening performance group at baseline were excluded from this analysis. The study outcome was incident cognitive impairment, defined as a diagnosis of dementia or CIND as determined by the comprehensive clinical assessment, or poor performance on the cognitive screening. Those who were in the good or intermediate performance groups on screening at all participating waves were considered to be cognitively unimpaired.
Medication.
Medication data were collected by examining all prescription and over-the-counter bottles/containers participants had in their homes and reported using at the time of the in-home interview. Medication use was collected at baseline and at each follow-up evaluation. Medications were identified to have either possible or definite anticholinergic properties based on the Anticholinergic Cognitive Burden scale (ACB).3 Drugs with possible anticholinergic effects were defined as those with serum anticholinergic activity or in vitro affinity to muscarinic receptors but with no known clinically relevant negative cognitive effects (score of 1 on the ACB). Drugs with established and clinically relevant cognitive anticholinergic effects were considered as definite anticholinergics (score of 2 or 3 on the ACB).3,22 Using the ACB scale, a pharmacist reviewed all medications used by the study population and categorized anticholinergics into possible or definite anticholinergics.
A secondary analysis evaluated anticholinergic drug use over time. Participants included in the analysis were classified into 3 groups—continuous use, intermittent use, and nonuse—according to their use of anticholinergics at each evaluation wave up to their endpoint. Continuous use was defined as the use of anticholinergics during all participating waves. Intermittent use was defined as the use of anticholinergics during at least 1 participating wave but not all waves. Nonusers were those who did not use anticholinergics at any wave.
Blood samples and APOE genotyping.
Blood samples for genotype assessments were collected in 10-mL (ethylenediaminetetraacetic acid) Vacutainer tubes during the screening phase in 2001. The samples were centrifuged and red blood cells, buffy coat, and plasma were separated. DNA was extracted from the buffy coat using standard protocols. HhaI digestion of amplified products was used to determine APOE genotype.23 Informed consent was obtained prior to blood collection.
Other covariates.
Information collected during the baseline evaluation was included in the analyses. Covariates included age, sex, years of education, alcohol and smoking history, family history of dementia, and a number of medical conditions ascertained by self and informant reports: cancer, diabetes, heart disease, Parkinson disease, brain injury, stroke, and depression. Additionally, history of hypertension was ascertained by self or informant report, or use of antihypertensive medications.
Statistical analysis.
t Tests for continuous variables and χ2 tests for categorical variables were used when comparing the demographic characteristics of the groups with and without incident cognitive impairment and also those receiving or not receiving definite anticholinergic medications. The associations between the outcome (incident cognitive impairment) and various measures of exposure (use of anticholinergic medications) were analyzed using logistic regression models that were adjusted for gender, years of education, age at baseline, and baseline cognitive score. Since participants in the entire cohort were evaluated at predetermined evaluation times (3 and 6 years past baseline), logistic regression models were shown to approximate survival models with interval censored data.24 In addition to the main covariates, inclusion of other comorbidities was determined by their univariate association with the outcome using logistic regression significant at the α = 0.10 level. Medical history covariates were included in the final logistic regression model if the association with the outcome and exposure was significant at the α = 0.05 level in the multivariate model. Odds ratios (OR), 95% confidence intervals (CI), and p values are reported from the final models. Additionally, the various logistic models were also stratified by the presence of APOE ε4 alleles as a risk factor for AD.25–27 Comparisons were made between those lost to follow-up and participants included in the analysis using t tests for continuous variables and χ2 tests for categorical variables. The statistical software SAS version 9.1 was used for the analysis.28
RESULTS
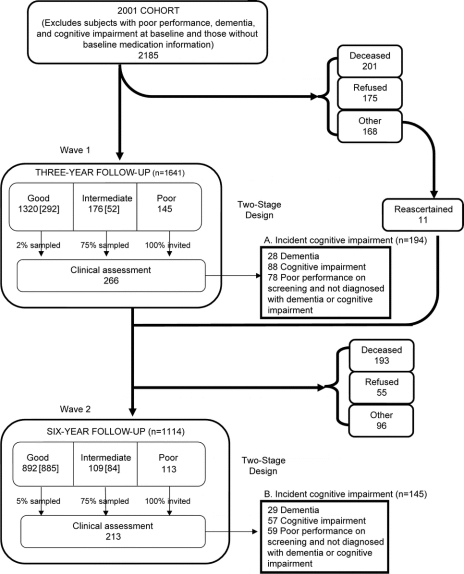
The study identified 2,185 study participants who were cognitively intact and had baseline medication information at the 2001 evaluation. The figure describes the flow of participants through each wave of the study. At the first follow-up evaluation (2004), 544 participants were lost to follow-up for various reasons including death and participant refusal. However, 11 participants from this group later rejoined the study and were included in the second follow-up evaluation in 2007. Therefore, this study includes 1,652 participants who had at least 1 follow-up evaluation, with 339 developing incident cognitive impairment during the 6-year study period (194 identified in the first follow-up evaluation and 145 in the second follow-up). The remaining 1,313 participants had preserved cognition.
Figure Description of study sample
Number of participants from each group who comprise the preserved cognition cohort (292 + 52 + 885 + 84 = 1,313). A + B = number of participants who comprise the incident cognitive impairment cohort (194 + 145 = 339).
The population demographics, education, and medication use are described in table 1. This community-dwelling, African American population in Indianapolis had a mean age of 81.8 ± 5.3 years and included over two-thirds women (69.1%). At baseline, this population used a mean of 4.9 ± 3.3 medications daily, with 53.3% reporting the use of at least 1 medication with possible anticholinergic properties, and 10.8% using at least 1 medication with definite anticholinergic properties (table 1). Table 2 describes baseline demographic and comorbidity information of the study participants stratified by the exposure of definite anticholinergic medications.
Table 1 Baseline characteristics of the study participants (n = 1,652)
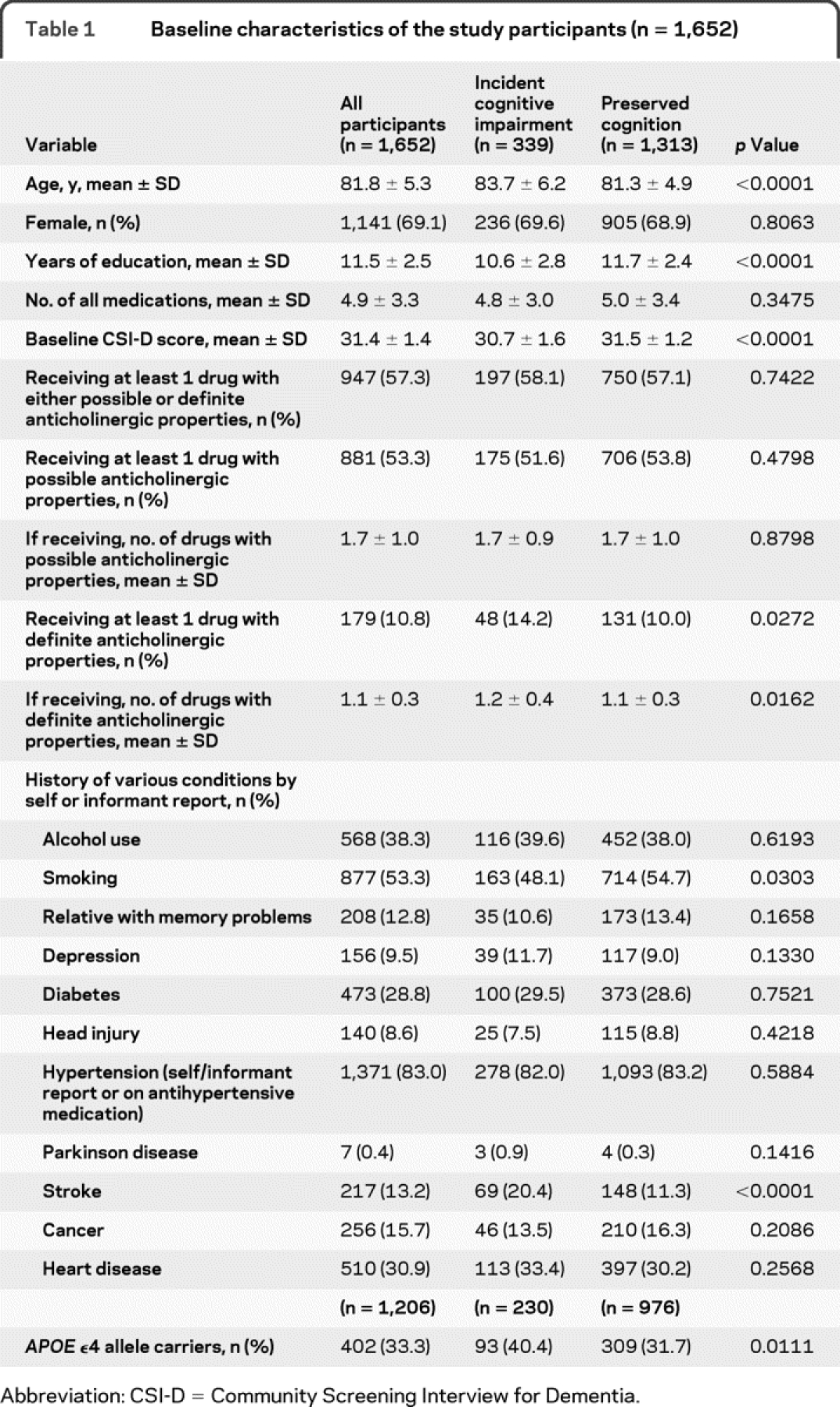
Table 2 Population characteristics by baseline exposure to definite anticholinergic medications
The majority of anticholinergic medications used by this population were classified as possible anticholinergics. The most commonly used possible anticholinergics in this population are frequently prescribed for cardiovascular disease. The most frequently used definite anticholinergic medications were oxybutynin and meclizine, although the general frequency was low.
Table 3 describes the association between the use of anticholinergic medications and incident cognitive impairment. Model 1 examined the use of possible anticholinergic medications with the likelihood of incident cognitive impairment. After adjusting for age, gender, education, and baseline CSI-D score, the OR of developing incident cognitive impairment in those using medications with possible anticholinergic properties was 0.87 (95% CI 0.67–1.13) when compared with those who did not use any type of anticholinergic medications. There was no significant association between the number of possible anticholinergic medications used per participant and incident cognitive impairment (model 2).
Table 3 Use of anticholinergic medications at baseline and risk of incident cognitive impairment
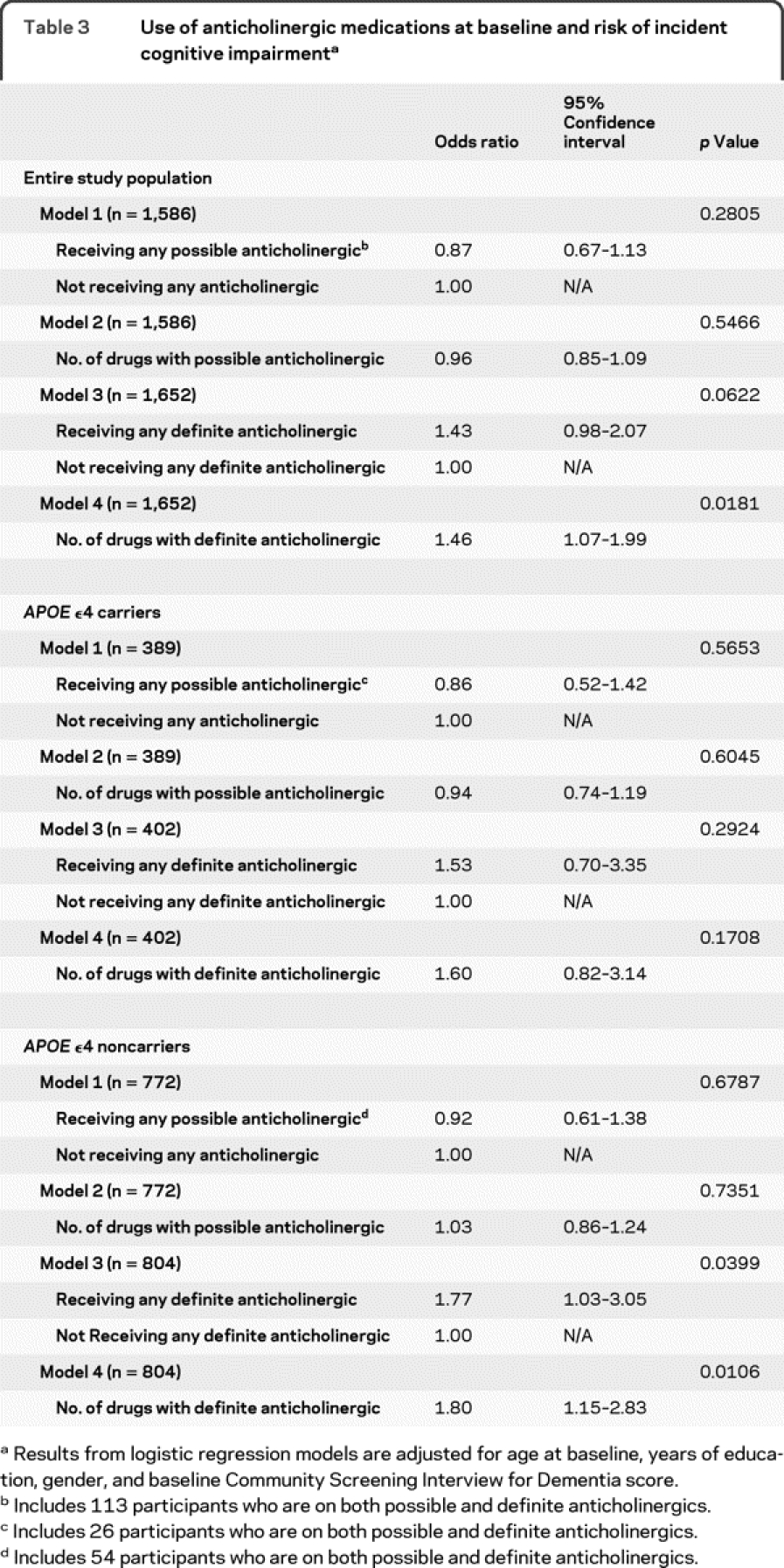
Models 3 and 4 examine the association between the use of definite anticholinergic medications and incidence of cognitive impairment. When compared with those not using definite anticholinergics, the use of definite anticholinergics was associated with the development of cognitive impairment, resulting in an OR of 1.43 (95% CI 0.98–2.07). The number of definite anticholinergics used per study participant was evaluated as the exposure parameter in model 4, and resulted in an OR of 1.46 (95% CI 1.07–1.99). We evaluated a list of comorbidities as potential confounders and found that only a history of stroke had a significant influence on both the outcome and exposure. Adjusting for stroke, in addition to the baseline characteristics of age, sex, education, and baseline cognitive function score, revealed an OR for model 4 for the number of definite anticholinergic medications of 1.40 (95% CI 1.02–1.92; p = 0.0397). We also evaluated the cumulative use of anticholinergic medications, measured by adding the sum of ACB scores for any anticholinergic medication. The comparison of the total ACB score with the incidence of cognitive impairment revealed an OR of 1.04 (95% CI 0.96–1.12; p = 0.3188).
APOE ε4carrier status was available from 1,206 participants included in the study. Stratification by APOE ε4 carrier status (table 3) indicates a trend of definite anticholinergic exposure in increasing the risk of cognitive impairment in the group identified as carriers of the APOE ε4 allele, though statistical significance is not retained. Interestingly, for those participants without the APOE ε4 allele, the risk of cognitive impairment with the use of definite anticholinergic medications becomes stronger (OR 1.77, 95% CI 1.03–3.05; p = 0.04).
Logistic regression models were used for further subgroup analysis of the cognitive effect of anticholinergic medications on the separate outcomes of dementia. We compared the use of anticholinergics between those who met criteria for dementia (n = 57) with those who were cognitively normal (n = 1,313). The OR for developing incident dementia was 1.08 (95% CI 0.47–2.49; p = 0.8517) adjusting for age at baseline, years of education, gender, and baseline cognitive score.
We next evaluated the use of definite anticholinergic medications over time (baseline and 3- and 6-year evaluations). Due to missing medication data during at least 1 follow-up visit, 1,578 participants were available for this analysis. Of these, 72 participants were continuous users and an additional 251 participants were intermittent users during the 6-year study period. The OR for developing incident cognitive impairment in those exposed to continuous use of definite anticholinergics was 1.40 (95% CI 0.77–2.54; p = 0.2651) compared to nonusers after adjusting for age at baseline, gender, education, and baseline CSI-D score. The OR for developing incident cognitive impairment in participants who were intermittent users of definite anticholinergics was 1.63 (95% CI 1.17–2.28; p = 0.0042) when compared to nonusers.
To ensure our results were not biased by those lost to follow-up, we compared demographic and medical conditions between the 1,652 study participants included in the analysis and the 533 participants who were lost to follow-up after the baseline assessment. Those lost to follow-up were older, had less education, had worse cognitive scores, were more likely male, and more often had a history of depression, Parkinson disease, alcohol use, or smoking (p < 0.05). However, the lost to follow-up group did not differ significantly from those with follow-up evaluations in the proportions of participants using possible anticholinergics (56.8% vs 53.3%, p = 0.1563) or in the proportions of participants using definite anticholinergics (11.8% vs 10.8%, p = 0.5288). Thus it is possible that the poorer cognitive function observed at baseline in the lost to follow-up group was the result of comorbid conditions and not due to the greater burden of anticholinergic use.
DISCUSSION
Results from this study in African Americans with normal cognitive function at baseline do not support the hypothesis that anticholinergic medications increase the risk of dementia when compared to those not using these medications. Rather, our results suggest that definite anticholinergics alone may increase the risk of less severe forms of cognitive impairment. Our study adds to the existing evidence evaluating anticholinergic exposure and cognitive impairment. First, in-home assessments of medication use allowed for accurate cross-sectional collection of medications a participant may have in the home, including the use of over-the-counter, herbal, and supplement products. Secondly, the study design included validated cognitive screening and diagnostic tools by trained specialists to confirm an accurate diagnosis of cognitive function. Most previous studies evaluating a similar association utilized global measures of performance instead of expert clinical judgment necessary for diagnostic evaluation.
Our study results confirm the suggestion that anticholinergics adversely affect cognitive abilities, as suggested by many previous investigations.29–32 Interestingly, based on the lack of association with dementia, our results suggest that the effect of anticholinergics on cognitive function may only influence the development of less severe forms of cognitive impairment. In contrast to recent work by Carriére et al.,33 our results do not show an association between long-term anticholinergic use and incident dementia. Differences in population, burden of comorbid disease, and medications included in the evaluation may offer explanations for this discrepancy. Our sample size is insufficient to definitively determine the reversibility of the association of anticholinergics and cognitive impairment and should be pursued in future research.
Many of the medications considered possibly anticholinergic are used for cardiovascular diseases, such as hypertension34 and congestive heart failure,35 which have been suggested to correlate with cognitive impairment. Previously, the use of antihypertensives was shown to reduce the risk of cognitive impairment.36 The reduction in risk of incident cognitive impairment with possible anticholinergics may explain the lack of cumulative effect of anticholinergics seen in our population. This result contrasts with published work by Han et al.36 that supports a cumulative effect of anticholinergic exposure on cognitive performance. We found that the cumulative effect only exists in those using definite anticholinergic medications.
Our study included medications contained in the study participants' home at the initial in-home evaluation, and does not evaluate adherence or dose of each medication between follow-up periods. Although our medication data collection resulted in an accurate list of medications to which the study participants were exposed, a more comprehensive method of medication utilization, including dispensing records, may better define the impact of long-term exposure to anticholinergic medications. Secondly, our sample size may have been insufficient to evaluate continuous use of definite anticholinergic medications on cognitive function. Although the OR for continuous use of definite anticholinergics was similar to that of the baseline analysis, a larger cohort of consistent users of definite anticholinergic medications is required to confirm this association. Additionally, this study may be limited by indication bias, in that participants with cognitive impairment may have been more likely to be exposed to anticholinergic medications due to the presence of cognitive impairment. Finally, we did not have information on changes in anticholinergic medication use after the baseline assessment and the reasons for any change in use. Therefore our results may be confounded by medication indication.
The use of definite anticholinergic medications should be considered when evaluating cognitive performance. The risks of these medications should be factored into prospective clinical decision-making when considering the potential for medication-related benefits and harms in each participant. With a growing prevalence of cognitive impairment in the older adult population, prescribers should be aware of the impact anticholinergics have on the development of cognitive and executive dysfunction.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Kathleen A. Lane and Dr. Sujuan Gao.
DISCLOSURE
Dr. Campbell, Dr. Boustani, and Ms. Lane report no disclosures. Dr. Gao receives research support from the NIH (RO1 AG09956 [Co-I] and P30 AG010133-16 [Data Core Leader]). Dr. Hendrie and Dr. Khan report no disclosures. Dr. Murrell may accrue revenue on patents re: Method for screening for Alzheimer's disease and Transgenic mouse expressing APP770-V717F; has received royalties payments from Elan Corporation for a patent re: Transgenic mouse expressing APP770-V717F; and receives research support from the NIH (NIA 1RC2AG036650-01 [Co-PI], NIA PHS P30 AG10133 [Co-I], NIA R01 AG09956 [Co-I], NIA PHS 3 U24 AG21886 [Co-I], NIA R01 AG025688 [Co-I], and NINR U01 NR004508 [Co-I]). Dr. Unverzagt serves on the editorial board of the Journal of the International Neuropsychological Society and Neuropsychology; receives research support from Eli Lilly and Company, Posit Science Inc., and the NIH (R01 AG026096 [PI], U01 NR004508 [PI], R01 AG09956 [Co-I], P30 AG10133 [Co-I], U01 NS041588 [Co-I], ITRAC [PI], R01 AG19181 [Co-I], and RSGPB04-089-01-PBP, ACS [Co-I]); and holds stock in Eli Lilly & Company. Dr. Hake receives royalties from publications in Up-To-Date; received a speaker honorarium from Eisai Inc.; serves on speakers' bureaus for Accera, Inc., Eisai Inc./Pfizer Inc, Forest Laboratories, Inc., Ortho-McNeil-Janssen Pharmaceuticals, Inc., and Novartis; and receives research support from Medivation Inc., Eisai Inc., Elan Corporations/Ortho-McNeil-Janssen Pharmaceuticals, Inc., and from the NIH (NIA 2 R01 AG009956-15 [Sub-I], NIA R01 AG026096 [Sub-I], NIA AG23038-01A1 [Sub-I], NCIRE U01 AG024904 TPA 37ADNI, [Sub-I], NIA U24 AG026395 [Sub-I], and P30-AG10133 [Sub-I]). Ms. Smith-Gamble reports no disclosures. Dr. Hall receives research support from the NIH (NIA 5RO1AG009956- 18 [PI]).
Address correspondence and reprint requests to Dr. Noll L. Campbell, Wishard Health Services, 1001 West 10th Street, Indianapolis, IN 46202-2872 noll.campbell@wishard.edu
Study funding: The original study was supported by NIH grant RO1 AGO9956 and the Alzheimer's Disease and Related Disorders Association.
Disclosure: Author disclosures are provided at the end of the article.
Received November 4, 2009. Accepted in final form March 18, 2010.
REFERENCES
- 1.Boustani M, Ham RJ. Alzheimer disease and other dementias. In: Ham RJ, Sloane PD, Warshaw GA, Bernard MA, Flaherty E, eds. Primary Care Geriatrics: A Case-Based Approach, 5th ed, ch 16. Philadelphia: Mosby; 2007:219–236. [Google Scholar]
- 2.Hanlon JT, Schamder KE, Boult C, et al. Use of inappropriate prescription drugs by older people. J Am Geriatr Soc 2002;50:26–34. [DOI] [PubMed] [Google Scholar]
- 3.Boustani MA, Campbell NL, Munger S, Maidment I, Fox GC. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008;4:311–320. [Google Scholar]
- 4.Boustani M, Hall KS, Lane KA, et al. The association between cognition and histamine-2 receptor antagonists in African Americans. J Am Geriatr Soc 2007;55:1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahin RL, Pecha M, Welmerink DB, et al, for the Ginkgo Evaluation of Memory Study Investigators. Concomitant use of prescription drugs and dietary supplements in ambulatory elderly people. J Am Geriatr Soc 2009;57:1197–1205. [DOI] [PubMed] [Google Scholar]
- 6.Aigner TG, Mishkin M. The effects of physostigmine and scopolamine on recognition memory in monkeys. Behav Neural Biol 1986;45:81–87. [DOI] [PubMed] [Google Scholar]
- 7.Fibiger HC, Damsma G, Day JC. Behavioral pharmacology and biochemistry of central cholinergic neurotransmission. Adv Exp Med Biol 1991;295:399–414. [DOI] [PubMed] [Google Scholar]
- 8.Miller EK, Desimone R. Scopolamine affects short-term memory but not inferior temporal neurons. NeuroReport 1993;4:81–84. [DOI] [PubMed] [Google Scholar]
- 9.Haroutunian V, Greig N, Pei XF, et al. Pharmacological modulation of Alzheimer's beta-amyloid precursor protein levels in the CSF of rats with forebrain cholinergic system lesions. Brain Res Mol Brain Res 1997;46:161–168. [DOI] [PubMed] [Google Scholar]
- 10.Haring R, Fisher A, Marciano D, et al. Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to β-amyloid protein secretion. J Neurochem 1998;71:2094–2103. [DOI] [PubMed] [Google Scholar]
- 11.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein α-secretases. J Neurosci Res 2003;74:342–352. [DOI] [PubMed] [Google Scholar]
- 12.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging 2009;4:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African-Americans. Am J Psychiatry 1995;152:1485–1492. [DOI] [PubMed] [Google Scholar]
- 14.Szwast S, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology 2007;69:1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community based study of dementias: methods and performance of the survey instrument Indianapolis, USA, and Ibadan, Nigeria. Int J Meth Psychiatr Res 1996;6:129–142. [Google Scholar]
- 16.Hall KS, Hendrie HC. CSI“D” and culture fair cognitive testing. In: Copeland J, Abou-Saleh M, Blazer D, eds. Principles and Practices of Geriatric Psychiatry, 2nd ed. Sussex, England: John Wiley & Sons; 2002. [Google Scholar]
- 17.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry of Alzheimer's disease (CERAD): clinical and neuropsychological assessment of Alzheimer's disease. Psychopharmacol Bull 1988;24:641–652. [PubMed] [Google Scholar]
- 18.Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project: report of Critical Evaluation Study Committee. Alzheimers Dement 2006;2:12–32. [DOI] [PubMed] [Google Scholar]
- 19.Hendrie HC, Hall KS, Brittain HM, et al. The CAMDEX: a standardized instrument for the diagnosis of mental disorder in the elderly: a replication with a US sample. J Am Geriatr Soc 1988;36:402–408. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. ICD-10: The International Statistical Classification of Diseases and Related Health Problems: 1 and 2. Washington, DC: American Psychiatric Association; 1992:V.3. [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 22.Richardson K, Fox C, Maidment I, et al, and the MRC-CFAS. Current anticholinergic medication use and cognitive impairment in the older population. Poster presented at the International Conference on Alzheimer's Disease; July 2009; Vienna, Austria. [Google Scholar]
- 23.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990;31:545–548. [PubMed] [Google Scholar]
- 24.Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. JASA 1988;83:414–425. [Google Scholar]
- 25.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late-onset families. Science 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 26.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 1993;342:697–699. [DOI] [PubMed] [Google Scholar]
- 27.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 1993;43:1467–1472. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute Inc. SAS/STAT User's Guide, version 9.1. Cary, NC: SAS Institute; 2009. [Google Scholar]
- 29.Tune LE, Egeli S. Acetylcholine and delirium. Dement Geriatr Cogn Dis 1999;10:342–344. [DOI] [PubMed] [Google Scholar]
- 30.Broks P, Preston GC, Traub M, et al. Modeling dementia: effects of scopolamine on memory and attention. Neuropsychologia 1988;26:685–700. [DOI] [PubMed] [Google Scholar]
- 31.Beatty WW, Butters N, Janowsky DS. Patterns of memory failure after scopolamine treatment: implications for cholinergic hypotheses of dementia. Behav Neural Biol 1986;45:196–211. [DOI] [PubMed] [Google Scholar]
- 32.Itil T, Fink M. Anticholinergic drug-induced delirium: experimental modification, quantitative EEG, and behavioral correlations. J Nerv Ment Dis 1966;143:492–507. [PubMed] [Google Scholar]
- 33.Carriére I, Fourrier-Reglat A, Dartigues J-F, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population. Arch Intern Med 2009;169:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray MD, Lane KA, Gao S, et al. Preservation of cognitive function with antihypertensive medications: a longitudinal analysis of a community-based sample of African Americans. Arch Intern Med 2002;162:2090–2096. [DOI] [PubMed] [Google Scholar]
- 35.Cacciatore F, Abete P, Ferrara N, et al. Congestive heart failure and cognitive impairment in an older population: Osservatorio Geriatrico Campano Study Group. J Am Geriatr Soc 1998;46:1343–1348. [DOI] [PubMed] [Google Scholar]
- 36.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc 2008;56:2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]