Abstract
Prion disorders are infectious, neurodegenerative diseases that affect humans and animals. Susceptibility to some prion diseases such as kuru or the new variant of Creutzfeldt-Jakob disease in humans and scrapie in sheep and goats is influenced by polymorphisms of the coding region of the prion protein gene, while other prion disorders such as fatal familial insomnia, familial Creutzfeldt-Jakob disease, or Gerstmann-Straussler-Scheinker disease in humans have an underlying inherited genetic basis. Several prion strains have been demonstrated experimentally in rodents and sheep. The progression and pathogenesis of disease is influenced by both genetic differences in the prion protein and prion strain. Some prion diseases only affect the central nervous system whereas others involve the peripheral organs prior to neuroinvasion. Many experiments undertaken in different species and using different prion strains have postulated common pathways of neuroinvasion. It is suggested that prions access the autonomic nerves innervating peripheral organs and tissues to finally reach the central nervous system. We review here published data supporting this view and additional data suggesting that neuroinvasion may concurrently or independently involve the blood vascular system.
1. Background
Other than by direct invasion following traumatic and iatrogenic incidents, infectious agents can gain access to the brain by two mechanisms: using peripheral nerves as physical conduits (neural neuroinvasion) or via the blood (haematogenous neuroinvasion). Documented examples of the first include viruses, such as lyssaviruses or herpesviruses, and some bacterial infections such as listeriosis. Examples of the second include lentiviruses or flaviviruses and meningitides and thromboembolic encephalitides of bacterial aetiology.
The precise nature of the infectious agent of the transmissible spongiform encephalopathies (TSEs) or prion diseases is still to be determined, although the most widely accepted theory (the “protein-only” hypothesis) is that they are caused by an infectious proteinaceous agent. This agent, usually termed “prion” and often designated as PrPsc, hypothetically originates as a result of an aberrant misfolding process of the cellular, normal host prion protein (PrPc). TSEs are a group of neurodegenerative disorders that can affect animals and humans and include, amongst others, scrapie in sheep and goats, bovine spongiform encephalopathy (BSE), chronic wasting disease (CWD) in cervids, transmissible mink encephalopathy (TME), and Creutzfeldt-Jakob disease (CJD) and its variant form (vCJD) in humans. Common to other protein misfolding neurological disorders, like Alzheimer's disease, TSE-affected individuals progressively accumulate disease-associated abnormal forms of PrPc, generically designated PrPd, in the brain and, in many instances, in other tissues. Regardless of the precise nature of the infectious agent, and although TSEs can be experimentally or iatrogenically reproduced by a variety of routes, it is believed that most natural infections are acquired by the alimentary route, so that the first barrier that the infectious agent encounters is the gastrointestinal epithelium. Once this barrier has been crossed, TSE agents could theoretically reach the brain by the same two pathways as any other infectious agent.
In this review we will present the findings that have led to the prevailing hypothesis of neuroinvasion in animal TSEs, that is, the neural route. We will also discuss whether such findings and recent data are compatible with an alternative or complementary pathway, that is, the haematogenous route. Emphasis will be laid on data from experimental TSE models in rodent species and sheep, although findings arising from natural infections in several species will also be considered.
One precaution to be taken when interpreting studies on pathogenesis is to consider carefully the techniques employed. In the case of TSEs, some of those studies, particularly early ones, were carried out by bioassay of selected tissues in laboratory animal species. While this approach is the only one that can demonstrate actual infectivity, it cannot determine which tissue structure or cell-type is infected because of the difficulties in fine dissection procedures and the risk of cross-contamination between tissues at necropsy. More recent and often comprehensive studies use PrPd as a surrogate marker of infection, albeit the correlation between the two is not always precise. The disease-specific form of the prion protein can be detected in the brain and viscera of TSE affected animals by a number of techniques, some of which, like ELISA or Western blot, employ extraction in detergents and incomplete enzymatic degradation to reveal protease-resistant PrP (PrPres). However, these techniques still lack definition of the precise structures harbouring the surrogate marker because sample dissection is done on fresh or frozen tissue. PrPd detected by immunohistochemistry (IHC) circumvents issues of potential contamination and can be precisely ascribed to specific tissues, structures, and cells, and even to the intra- or extracellular compartments. Moreover, IHC is able to detect diverse morphological types of PrPd which, depending on the protocol used, can include both protease-resistant and protease-sensitive forms. By using a panel of PrP antibodies, the integrity of the protein (full length or truncated, and their location) can also be determined.
2. Neural Neuroinvasion: The Prevailing View
2.1. Spread of Infectivity along Nerves
A number of studies have provided evidence in support of anterograde and retrograde transport of prion infectivity along peripheral nerves, and of transynaptic spread of infectivity within the central nervous system (CNS). The most easily interpretable paradigms arose from experiments based on cranial and peripheral nerve challenges. Thus, intraocular injection results in spread of infectivity along retinal ganglion axons and once within the brain follows transynaptic dissemination through the visual pathways [1, 2]. Similarly, following intralingual inoculation, infectivity ascends rapidly and retrogradely via the XII cranial nerve reaching the hypoglossal nucleus within two weeks [3]. Curiously, evidence of transport along the olfactory nerves following intranasal exposure is still lacking [4, 5], and only the most recent experiments done by Bessen et al. [6] showed that only one hamster clinically affected out of five had traces of PrPd concomitantly within the olfactory sensory epithelium and in the glomerular layer of the olfactory bulbs. This result suggests that some transport of infectivity by olfactory nerves is possible although it is unclear if it would occur anterogradely from the CNS or retrogradely from the olfactory epithelium.
Estimates of the speed of axonal transport of prions vary from 0.5–3 mm/day along peripheral or cranial nerves, or within the central nervous system [7, 8]. Although these estimates face the significant confounding factor of the rate at which infectivity or PrPd is amplified or accumulated in the reporter cell or tissue, the data suggest that rates of spread of infectivity do not precisely correspond with either slow or fast axonal transport mechanisms. Nevertheless, neurotropic viruses also show a similar broad range of transport velocities; herpesviruses, for example, travel at rates of fast axonal transport [9], while the calculated velocity of rabies virus may be as low as 12 mm/day [10]. It is difficult therefore to conclude how prion infectivity may spread along neuronal processes simply from crude estimates of transport velocity.
The numerous intranerve transmission studies provide conclusive evidence that infectivity can be transported into the CNS along or within axons. There is also evidence that infection may also be transported out of the CNS along axons. Experimental rodent infections with the agents of scrapie and TME have shown that infection can spread from the thoracic segments of the spinal cord to their corresponding spinal [7] and to the sciatic [8] nerves. Similarly, intracerebral challenge of deer with cattle BSE results in PrPd in the enteric nervous system (ENS) in the absence of lymphoid tissue involvement and only after widespread PrPd accumulation in the brain [11]; such findings are most probably due to anterograde transport of infectivity from the CNS along nerve fibres.
2.2. Involvement of Lymphoid Tissues and Peripheral Nerves in TSEs
As early as 1967, studies of mouse scrapie after sub-cutaneous injection suggested that lymphoreticular system (LRS) tissues of hamsters such as the spleen and lymph nodes acquired infectivity prior to the brain [12]. In contrast, Kimberlin and Walker [13] did not find a similar role for the spleen when a different route was used. In those studies, intragastric administration of the scrapie strain 139A into mice concluded that agent replication in the Peyer's patches (PPs) occurred as fast as in the cervical lymph nodes, and earlier than in the spleen and CNS. Furthermore, splenectomy in those animals had no effect on incubation periods, in contrast to previous observations obtained after intraperitoneal inoculation with the same scrapie strain [14, 15]. These results, obtained in murine models, formed the pillars of the current neuroinvasion dogma, which suggests that after intraperitoneal infection neuroinvasion is initiated from the spleen and involves the sympathetic nervous system [14, 15], whereas neuroinvasion after intragastric infection is initiated directly from the intestines (via the PPs) and involves the enteric nervous system (ENS; [13]).
Several time course studies were initiated to investigate the spread of infectivity from the LRS to the CNS, [14, 16, 17]. Such studies reported a common neuroinvasion pathway for the intraperitoneal, intravenous (tail vein), and subcutaneous routes (scruff of the neck and foot pad), suggesting that neuroinvasion occurred through the spleen (and presumably visceral lymph nodes), along autonomic nerves such as the splanchnic nerves to the mid-thoracic spinal cord, from where it would disseminated gradually to the rest of the CNS (brain and lumbar spinal cord). Although direct evidence for the initial spleen-thoracic spinal cord pathway was lacking because of difficulties in sampling small autonomic nerves and ganglia in mice, indirect evidence based on detection of infectivity in the stromal fraction of the spleen, in which nerve endings are abundant, was provided [18]. At the same time, centrifugal spread from the spinal cord and brain to peripheral nerves was demonstrated [7, 19].
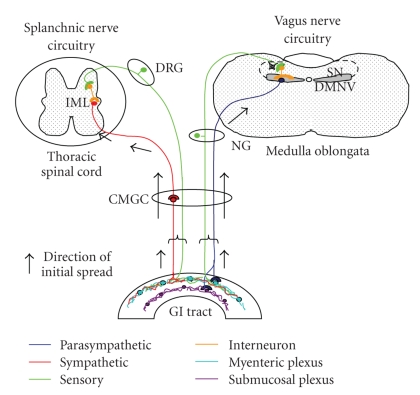
Working with orally or intraperitoneally inoculated hamsters, Baldauf et al. [20] provided strong evidence for an alternative route of access of the 263 K strain to the brain that bypassed the spinal cord. It was proposed that the fibres of the vagus nerve would be the most likely direct pathway, although other cranial nerves and a blood- or even a cerebrospinal fluid- (CSF-) borne access were not ruled out. Similar to previous data obtained by Western blot analysis [21], spleens of infected hamsters did not consistently show PrPres thus questioning mandatory lymphoid tissue replication prior to neuroinvasion. Further evidence from experimental scrapie rodent models [22–26] confirmed the early involvement of the enteric and abdominal ganglia not only by doing infectivity assays but also by detecting the PrPd by IHC or paraffin-embedded tissue blot. Spatio-temporal studies on PrPd deposition revealed that the dorsal motor nucleus of the vagus nerve (DMNV) and the intermediolateral columns (IMLC) of the thoracic spinal cord were the first two CNS target sites to accumulate PrPd. These authors suggested that the infectious agent reached the CNS retrogradely from the ENS by two different circuits: the splanchnic nerves circuit and the vagus nerve circuit as illustrated in Figure 1. The splanchnic circuit involved the cranial mesenteric and celiac ganglia and the IMLC followed by the dorsal root ganglia. The vagus nerve circuit involved the DMNV by passing the nodose ganglion. Subsequent experiments demonstrated the presence of infectivity in the vagus nerve and the cranial mesenteric ganglia by hamster bioassays [22].
Figure 1.
Schematic representation of the most generally accepted hypothesis of the routes of neuroinvasion in TSEs. Permission was obtained for reproducing this figure originally published in [22]. DRG, dorsal root ganglia; NG, nodose ganglion; CMGC, celiac and mesenteric ganglion complex; GI tract, gastrointestinal tract.
Using 263 K infection of hamsters, Diringer [27] described a low level of “viraemia” lasting for at least 40 days, with detectable amounts of infectivity in the CNS, purportedly in capillary endothelial cells, but no sustained replication elsewhere. Experiments using the same hamster scrapie model discouraged consideration of haematogenous dissemination of infectivity by describing replication in the spleen and thoracic spinal cord earlier than in the brain [14, 28]. Evidence for a high and persistent “viraemia” was only available for some specific TSE models, such as the K.Fu strain of CJD in mice [29]. Similar to experiments in mice, the topographical PrPd distribution using the hamster model of 263 K did not reveal any evidence for haematogenous spread of infection to the brain [22]. Consequently, neuroinvasion in most studied rodent TSE models was thought to be crucially dependent on a non-blood-related compartment connecting the lymphoid tissue and the CNS, that is, the peripheral nervous system (PNS).
2.3. Support to Neural Neuroinvasion Gained from Transgenic and Mutant Rodent Models
An important contribution towards determining neuroinvasion pathways has been the use of transgenic, knockout, or mutant mice. From the early 1990s, such approaches have emphasised the role of key immune cell components in the up-take and replication of infectivity in the periphery and their contribution in transporting infectivity to the CNS [26]. In this respect, Klein et al. [30, 31] found no effect on susceptibility to disease or on spleen infectivity when mice deficient in T-lymphocytes were challenged with scrapie. In contrast, neuroinvasion was impaired in mice that were immunodeficient only in B-lymphocytes. Further investigations using scrapie-infected TNFα−/− mice lacking mature follicular dendritic cells (FDCs) but not B lymphocytes [32, 33] concluded that accumulation of infectivity and PrPres/PrPd in spleen and subsequent neuroinvasion were dependent upon mature FDCs. Parallel research towards investigating the role of macrophages in TSE pathogenesis concluded that macrophages were involved in the clearance of infectivity [34]. Complementary studies using transgenic mice overexpressing PrPc highlighted the importance of determining PrP interacting molecules or receptors which might be crucial for the normal function of FDCs and the interaction with B cells [26]. Although extensive research has focused on FDCs as cells for prion replication, current research highlights the role of dendritic cells, B-cells and macrophages in the transfer of infectivity from the gut to lymphoid organs [35, 36].
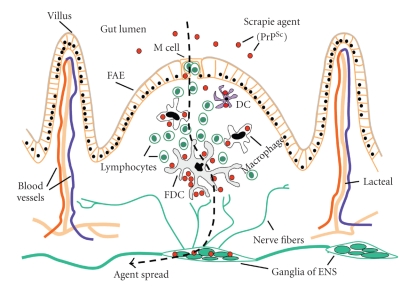
It is the interpolations between data obtained from all different animal models that have led to the most accepted view on absorption and transport of the infectious agent to the autonomic nervous system after oral exposure to prions. Figure 2 provides a diagrammatic representation of the possible ways by which the ENS can become infected after oral/intragastric exposure. Many studies have focused on the role of M cells [25], dendritic cells [37], and possibly enteroendocrine cells as important candidates for the up-take or absorption of infectivity from the gastrointestinal tract. The infectious agent would then be transported to the lymphoid follicles of the PPs, where it would replicate in a B-cell and FDC-dependent manner. Subsequently, infectivity would reach nerve endings in and around the follicles and retrograde transport of the infectious agent would occur from the nerve endings towards the neuronal somata of the submucosal and myenteric plexuses. Alternatively, the agent could be transported from the gut lumen through the absorptive epithelium of the villi and could infect nerve endings of the lamina propria before being transported to the enteric plexuses [39].
Figure 2.
Diagrammatic representation of the possible pathways of absorption of TSE agents from the gut lumen and their transport to the ENS. Permission was obtained for reproducing this figure originally published in [26].
Further support for the role of enteric neurons in the disease is their high PrPc mRNA levels [40]. In parallel, several rodent models have been produced in order to further support the idea that sympathetic nerves are responsible for transporting infectivity from the spleen to the spinal cord. Apparently this transfer of infectivity, which is facilitated in highly innervated spleens (as reviewed by [41]), occurs in a PrPc-dependent fashion [42] and its rate is limited by the splenic sympathetic innervation [43]. The fact that mice with hyperinnervated spleens showed shorter incubation periods and higher infectivity titres and PrPd accumulation [43, 44] supports this statement, whereas the fact that denervated mice still develop scrapie [41] and, similarly, the failure of splenectomy to prevent disease implies that alternative routes participate in the neuroinvasion process and that spleen innervation might not be a prerequisite. In this respect, a major role of parasympathetic nerve fibers like the vagus nerve has been suspected for decades. However, similar evidence supporting the role of the vagus nerve in the pathogenesis of prion diseases is lacking as data from vagotomised rodent models is not available.
2.4. Neuroinvasion in Experimental Sheep Models
The first studies on sheep scrapie pathogenesis date back to the late 70s, at which period bioassay in rodents was the only tool available to test for the presence of infectivity in non-CNS tissues. Using such tools Hadlow et al. [45, 46] demonstrated that scrapie infectivity replicates in the PPs and other secondary lymphoid tissues before reaching the CNS. Interestingly, blood was postulated as the vehicle of infection for those peripheral LRS tissues, even though no infectivity was detected in blood from naturally scrapie-infected sheep at the time. Later, sheep experiments traced infectivity by PrPd or PrPres detection, [47–51] implicating, in addition to the PPs and other LRS tissues, the PNS, and the ENS in the spread of infectivity to the CNS.
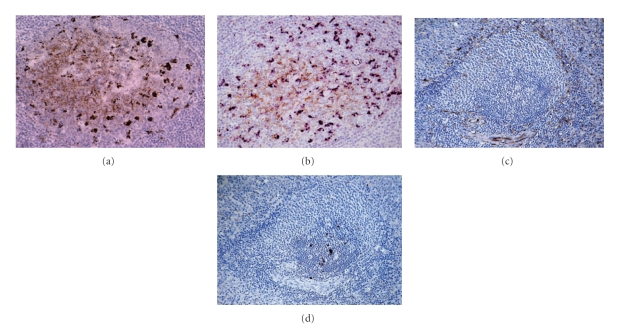
Cell types supporting infectivity and PrPd accumulation are generally found throughout all lymphoid tissues, with the exception of the thymus, and usually also in neurons of the ENS [49, 53–55]. PrPd accumulation in LRS tissues is found in association with mature FDCs of the light zone and tingible body macrophages (TBMs) of the light and dark zones of secondary follicles (Figure 3). Ultrastructural studies confirmed that PrPd within the germinal centre of follicles locates mainly in association with the plasma membrane of FDCs and lysosomes of TBMs [56]. At early stages of infection, PrPd has been associated with dendritic cells or macrophages in the dome of the PPs in addition to cells of lymphoid tissues [53] or in the lacteals [38, 39]. The demonstration of initial PrPd accumulation in the gut-associated lymphoid tissue (GALT) and tonsil [49, 53] of sheep with preclinical natural scrapie strongly supported the hypothesis of infectivity crossing the mucosal barrier of the gastrointestinal tract by M-cell transcytosis followed by dendritic cell/macrophage transport, as illustrated in Figure 2.
Figure 3.
Scrapie infection in lymphoreticular tissues in ARQ/ARQ Suffolk sheep. (a) PrPd accumulation is associated with both FDC and macrophages in clinically affected sheep as shown by single IHC for PrP antibody R145. Peyer's patches of the distal ileum. (b) PrPd within TBMs is N terminally truncated whereas PrPd associated with FDC is not [52]. TBM and FDC can be distinguished in tissue sections by using N or C terminal PrP antibodies. FDCs (brown), labelled by the N terminal antibody FH11, are in light zone whereas TBMs (purple), labelled by the C terminal antibody R145, are both in dark and light zones of secondary lymphoid follicles: double IHC for FH11 (brown, DAB substrate) and R145 (purple, VIP substrate). In spleen, nerve endings located in the marginal zone ((c), IHC for PgP9.5) do not colocalised with PrPd deposition ((d), IHC for R145). (a)–(d) x20.
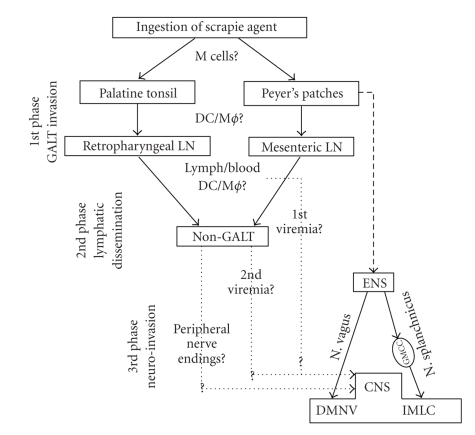
In a series of experiments, van Keulen and colleagues described the temporal spread of PrPd accumulation, in the GALT, non-GALT LRS, ENS, PNS, and CNS. They postulated that scrapie pathogenesis occurred in three different phases (Figure 4). In the first phase, after oral exposure to infection, the scrapie agent would be taken up by M cells followed by trancytosis to dendritic cells or macrophages, which would transport infection towards the secondary lymphoid follicles of the PPs and then drained to regional lymph nodes. A second phase of lymphatic dissemination would take place once infectivity is circulating the lymph (and blood) and therefore non-GALT LRS tissues would become involved. The fact that most data available on LRS examinations in sheep scrapie models describe infectivity of all lymphoid tissues at more or less the same time is highly suggestive of blood being an early contributing factor to dissemination of infection. Finally, neuroinvasion would arise in a third phase as a result of infection of nerve endings in the gut, GALT, and non-GALT LRS tissues and its retrograde spread through sympathetic and parasympathetic pathways.
Figure 4.
Pathogenesis of sheep scrapie. Permission was obtained for reproducing this figure originally published in [53]. For abbreviations see text.
Several sheep studies, encompassing sheep of different PrP genotypes, agree on the ENS being the first neural tissue in which PrPd can be demonstrated [49, 51, 57, 58]. In the latter study, the authors identified the duodenum and ileum as the initial ENS sites to accumulate PrPd and suggested subsequent spread towards the upper and lower gastrointestinal tract [51]. Infection of the different plexuses of the ENS could arise from various origins ([59]; reviewed by [60]): (i) fine nerve fibres underneath the villous epithelium could bring infection to the submucosal plexus; (ii) nerve endings at follicles of the PPs could also take infection to the submucosal plexus, (iii) indirect contact by means of lymph or active transport between the PPs and the ENS. In other studies, Heggebø et al. [59, 61] demonstrated PrPd accumulation in the marginal zone of the spleen, which is a highly innervated area. Therefore, as suggested for rodent models, retrograde transport from sympathetic nerve fibres connecting the spleen with the spinal cord could support neuroinvasion in sheep scrapie. Interpretation from all these studies postulated that entry to the DMNV was either by a retrograde spread of PrPd from the ENS along efferent parasympathetic fibres of the vagus nerve or by anterograde spread from other nuclei such as the nucleus of the tractus solitarii. The fact that neurons of the trigeminal and nodose ganglia accumulate PrPd later than the brain [51] would suggest that these ganglia become affected as a result of descending transport.
The interval between infection and first PrPd detection in peripheral tissues of naturally exposed sheep will depend on age of exposure and dose but experimental data show that strain and genotype also markedly influence rates of PrPd accumulation. Sheep of ARQ/ARQ genotype naturally infected with scrapie or experimentally infected with BSE [54, 55] accumulate PrPd in LRS tissues more slowly than do naturally or experimentally exposed VRQ/VRQ sheep [49, 53]. It is not clear whether these effects are solely strain or genotype effects or a combination of both.
Comprehensive pathogenetic studies have been performed on sheep infected with BSE, including infectivity assays [62] and tissue distribution of PrPd deposition by immunohistochemistry [55, 60, 62, 63]. Despite spatio-temporal differences reported between them, these studies complement each other as all had in common the type of inoculum, dose, and host PRNP genotype. Bellworthy et al. [62] demonstrated BSE infectivity in a wide range of tissues of ARQ/ARQ sheep including the PPs as early as 4 months postinfection, the spleen at 10 m after dosing, and other LRS tissues, CNS, and liver at 16 m postinoculation. In common with rodent studies, bioassay detecting infectivity was more sensitive than IHC in detecting presence of PrPd. Resistance to oral BSE infection was observed in sheep of ARQ/ARR and ARR/ARR PRNP genotypes, which can be overcome if the intracerebral route is used [64], although the resulting magnitude of PrPd accumulation is lower than in ARQ/ARQ sheep inoculated by the same route [63], and LRS dissemination is only observed in sheep of the last genotype [65]. Importantly, no influence of the genotype or of the route of inoculation was observed on the signature of the BSE agent in terms of intracellular truncation of BSE PrPd and proteinase-K cleavage site of BSE PrPres. These experiments suggest that the pathogenesis of experimental sheep BSE can differ depending on the route of inoculation and the host PRNP genotype.
Van Keulen et al. [60] showed the progressive accumulation of PrPd in 11 sheep orally dosed with cattle BSE and killed at 6 months postinfection and thereafter at 2-3-month interval. As with natural scrapie in VRQ/VRQ sheep, BSE-infected ARQ/ARQ Texel sheep developed clinical disease after peripheral accumulation of PrPd within the lymphoid tissues and autonomic nervous system. The first LRS tissues to accumulate PrPd were the GALT, followed by the GALT-draining lymph nodes and the spleen, and at a later stage, the non-GALT lymph nodes. Similar data were found in an earlier study albeit with some subtle differences. Jeffrey et al. [54] described first involvement of the retropharyngeal lymph nodes prior to the PPs in Romney sheep at 4 months post infection. Furthermore, PrPd deposition in CNS with no apparent LRS or PNS involvement up to 22 months postinoculation was observed in 40% (2/5) of clinically sick sheep [55]. In the Texel sheep, by 9 month postinoculation, PrPd was present in ENS neurons followed by the coeliac-mesenteric ganglion and the first two target sites in the CNS: the IMLC in the T7-L1 segments of the spinal cord and the DMNV in the brain. By 12-13 months dissemination to all non-GALT tissues had taken place. Colocalization of PrPd and CD68-positive macrophages was apparent in the marginal zone of spleen, suggesting a possible active trapping and phagocytosis of PrPd from circulating blood with potential for neuroinvasion as this is a compartment highly innervated by sympathetic autonomic fibres (Figure 3(c)). From 12-13 months postinoculation onwards spread within the ENS and IMLC affected all gastrointestinal tissues examined and all thoracic and lumbar spinal cord levels, respectively. It was not until 19 months postinoculation that sheep showed clinical signs of disease and PrPd deposition in the cerebral cortex.
2.5. Neuroinvasion in Other Natural TSEs
The mechanism of neuroinvasion in cattle affected with BSE remains uncertain. Although PrPd accumulation [66] and infectivity [66–70] has been reported in the GALT and ENS of cows orally challenged with exceedingly high doses of BSE, in natural BSE cases there is no known involvement of any viscera or the PNS. A recent report of two animals that were part of a sequential kill experiment after oral exposure to a high dose of BSE agent concluded that BSE infectivity is retrogradely transported from the gastrointestinal tract to the CNS by two pathways [71]: (i) via the coeliac and mesenteric ganglion complex, through the splanchnic nerves towards the thoracolumbar spinal cord, and (ii) via the vagus nerve. Such conclusions arise from finding PrPd accumulation within the DMNV, coeliac and caudal mesenteric ganglia, and spinal cord segments but only in one of the reported cows. In this same experiment, authors anticipated that the presence of PrPd in the CNS prior to the dorsal root ganglia and possibly other peripheral nerves, that is, sciatic nerve, could be due to a secondary retrograde spread from the periphery to the CNS. Although the authors suggested a neural rather than lymphoreticular neuroinvasion-based pathway, importantly, none of the two cows had ENS involvement or showed PrPd in the vagus nerve even though several sections were examined.
The pathogenesis of CWD in cervids seems to vary between elk and deer [72]. Lymphoreticular dissemination occurs in preclinically affected deer but neuroinvasion has been reported in elk with no LRS involvement (reviewed by [73]). It is suspected that such differences might be partially defined by the species and the PRNP genotype. Accumulation of PrPd in the PNS has been demonstrated in mule deer with CWD [74], suggesting a similar pathogenesis to that reported for small ruminants with scrapie.
The mechanisms of neuroinvasion in transmissible mink encephalopathy (TME) have been extensively investigated in hamster models. Early studies described two biologically distinct strains in hamsters infected with TME, the hyper (HY) and the drowsy (DY) strains [75]. Both strains cause neuropathology in the CNS but only the HY disseminates within the LRS compartment. In a large number of experiments and using a variety of routes, Bartz et al. [3, 8, 76] have demonstrated that LRS involvement is not required for prion neuroinvasion, and that it occurs via peripheral or cranial nerves. Recent investigations [6] conclude that neuroinvasion is dependent on LRS involvement for specific routes of entry such as the nasal cavity but not for others such as the tongue (oral cavity). These different routes of ascending infection result in different CNS targeting [76].
For obvious reasons, the pathogenesis of CJD in the natural host is the least studied amongst prion diseases. The pathogenesis of sCJD differs from that of vCJD. Accumulation of PrPd is widespread in neural and lymphoreticular tissues in vCJD, whereas it is mostly restricted to the CNS in sCJD [77]. Consequently, tonsil and spleen biopsies are optimal tissues for preclinical vCJD diagnosis. Deposition of PrPd in the posterior roots of spinal cord with absence of ENS involvement in sCJD patients is thought to be due to centrifugal spread of infectivity [78]. Interestingly, some sCJD patients with prolonged clinical periods displayed detectable PrPres in skeletal-muscle and spleen homogenates by Western blot analysis [79]. Unfortunately, no IHC examination was performed in those positive samples. Thus PrPres deposition found in muscles could have been associated with peripheral nerves, muscle spindles, or myocytes and in spleen may have been associated with peripheral nerves or lymphoid follicles.
2.6. Caveats in the Prevailing View of Neural Neuroinvasion
Although the onset of PrPd accumulation in the LRS generally occurs significantly before that in the CNS, the consistency and time of appearance of such depositions varies depending on age, PRNP genotype, TSE strain, or source and route of inoculation. Thus, it is known that lymphoid follicles of the GALT undergo involution with age, and this is believed to have an effect on susceptibility to infection after oral exposure. In the case of TSEs, however, it has been shown that such physiological involution may be modulated by the infection itself, so that it does not seem to occur in the GALT that accumulates PrPd [66, 80, 81]. As far as the PRNP genotype is concerned, some earlier studies [82, 83] described lack of, or minimal, involvement of LRS tissues in sheep of the VRQ/ARR and VRQ/ARQ PRNP genotypes naturally infected with scrapie. Equally, a small proportion of scrapie-infected goats (4/72) were found to accumulate PrPd only in the brain and not in any of 10 LRS tissues examined; all those four animals carried the methionine allele at codon 142 [84]. Examples of the effect of the TSE agent strain or source include natural BSE [69], sporadic CJD [85], experimental CH1641 infection of sheep [38, 39], and Nor-98 [86], in all of which PrPd accumulates in the CNS without prior replication in LRS tissues. Therefore, to explain the neural neuroinvasion pathway, infection of the ENS and other nerve terminals of the autonomic nervous system would have to occur without previous LRS amplification.
Replication of infectivity in the GALT and non-GALT LRS tissues may often be demonstrated early in the disease process. However, nerve endings in such tissues may not necessarily become infected or if infected may not result in retrograde transport to the CNS. Peripheral nerve terminals can be found in the PPs of the intestine and could pick up infectivity by contact with PrPd-containing TBMs and FDCs. However, while a rich network of nerves is present in the T-cell areas of the PPs—in which there is no PrPd accumulation—, nerves are very rarely found in the secondary follicles [59, 80, 87, 88], where FDCs, the principle cells which amplify infection in viscera, are located. Similarly, nerves cannot readily be detected in secondary follicles of other LRS tissues, particularly lymph nodes, and it is therefore difficult to see how infectivity could be transferred from the LRS to the PNS. Moreover, morphological analysis in confocal microscopy concluded that neuroimmune connections in PPs, spleens, and mesenteric lymph nodes from preclinical scrapie infected mice are established between dendritic cells in T-cell areas and peripheral nerves rather than arising from FDC in B-cell areas [36]. Although ovine noradrenergic fibres are relatively close to FDCs in the spleen [89, 90], no direct contact has been established, and patterns of immunolabelling for PrPd resembling nerve-like structures have not been reported. Innervation of lymphoid tissues is affected by the age-related involution process [91], so that a general increase in nerve density of the intestine during early phases of life may contribute to an increased susceptibility of young animals to oral infection [80].
Following subcutaneous challenge, scrapie infectivity may reach the local lymph nodes via the afferent drainage and leave via efferent drainage to reach the blood. Recent studies done in sheep indicate that infectivity reaches the contra-lateral lymph nodes and lymph nodes distant to the site of inoculation almost as quickly as the ipsi-lateral nodes, suggesting that all of them were exposed more or less simultaneously (Chianini et al. unpublished observations). Similarly, naturally and orally challenged sheep with scrapie generally show early and widespread involvement of all LRS, including those that drain the head or limbs and are not anatomically linked to the alimentary system. The speed with which all lymph nodes become infected is not consistent with one lymph node or a group of lymph nodes being initially infected and subsequently amplifying infectivity for spread to other nodes.
The transcytosis and absorption of PrPd within a scrapie brain homogenate placed within the gut lumen was followed across the gut mucosa of sheep using IHC. PrPd within the homogenate was rapidly translocated (within 15 minutes) to mucosal and submucosal lymphatics [39]. From such a location infectivity would rapidly reach the vascular system. In this experiment no evidence to support transport of the homogenate across the dome and thus directly into PPs could be found; yet this model produced accelerated infection of PPs resulting in PrPd accumulation within 30 days, compared with more than 8 months for the corresponding natural disease. Thus, another possibility would be that germinal centres of LRS tissues are most exposed to infectivity via blood.
A recent study conducted on 67 preclinically infected sheep exposed to natural or experimental scrapie or BSE by various routes (oral, subcutaneous, intracerebral, or intravenous) showed that, regardless of the route, initial PrPd accumulation consistently occurred in the DMNV followed by the hypothalamus [92]. These findings are difficult to reconcile with a unique neuroinvasion ENS/PNS axis pathway. An alternative explanation would be that infection, which is present in blood, reaches the vagus nerve from its terminals in any organ or tissue compartment. Head et al. [93] have published the topographical brain distribution of amyloid plaques and lesion profiles of a linked human blood donor (orally infected) and blood recipient (intravenously infected). An almost identical severity and distribution of plaques and vacuoles were found both in donor and recipient thus supporting a common CNS portal of entry regardless of the route of exposure.
3. Haematogenous Neuroinvasion: The Alternative Hypothesis
3.1. Evidence for Infectivity in the Blood
Despite the fact that intravenous infection systematically resulted in shorter incubation periods than the intraperitoneal and subcutaneous routes, Kimberlin and Walker [16] were unable to find evidence of blood infectivity by bioassay. However, after further studies with the same 139A scrapie strain, a short “viraemic” phase immediately after injection was observed, leading Millson et al. [94] to conclude that the haematogenous spread of the agent was the likely means by which infection spread to the spleen and lymph nodes. Subsequent rodent blood bioassay experiments showed that a “viraemic” or “prionaemic” phase was evident early in the incubation period [27]. In the case of infection taking place by the oral route, it has been proposed that prionaemia could take place in two phases: an early one immediately after cell-mediated or cell-free uptake of infection from the gut lumen into the lymphatic system, and a second phase after amplification in LRS tissues [92].
An important series of experiments directed towards establishing the risk of transmission from blood products have provided the most comprehensive data on the presence of infectivity in blood. Infectivity demonstrated in the blood of BSE-infected sheep [92, 95, 96, 99], of natural scrapie cases [95, 100, 101], and of CWD-infected deer [102] agrees with data suggesting that vCJD can be transmitted by blood transfusion from donors incubating the disease. Descriptions of five transfusion-related vCJD cases have confirmed transmission of infection by blood-derived products [103–107]. Moreover, sheep experiments have shown that scrapie and BSE may be transmitted with blood collected early in the incubation period, even before any other evidence of infection is apparent [92, 95, 96], although the likelihood of transmission increased together with the incubation period in the blood donors. This would imply that infectivity in blood is an early and permanent (maybe even progressive) event rather than a transient one, in contradiction with the results on PrPres detection in the blood of hamsters by protein misfolding cyclic amplification [108].
3.2. Evidence That Infectivity in Blood May Establish Tissue Infection
Haematogenous dissemination of infection may have relevance for more than one aspect of TSE pathogenesis. Experiments with blood transfusions in sheep demonstrated how the route, oral (BSE donors) versus intravenous (BSE recipients) can affect peripheral pathogenesis (Figure 5). Although a similar scenario might be predicted for vCJD, it remains to be established if spleens of vCJD patients infected after receiving blood fractions contain higher PrPd magnitudes than those from patients infected by consumption of BSE-contaminated products. Accumulation of PrPd can be demonstrated in placenta [109], mammary gland [110], salivary glands [111], and kidney [96, 97, 112] of TSE-affected sheep. In the latter, with both scrapie [112] and BSE (S. Sisó, personal observations) infections, PrPd can be detected in mesenchymal cells between collecting ducts and loops of Henle in the renal pelvis in the absence of inflammation, suggesting that mesenchymal cells become infected from blood after filtration in the glomeruli or by extravasation from the vasa recta. Also in sheep, PrPd in the mammary gland [110, 113] and infectivity in milk [113, 114] also most probably derive from the blood, as it does in scrapie-affected goats, in which accumulation of PrPd in the mammary gland only occurred in animals with widespread LRS involvement regardless of the involvement or otherwise of neural tissues [115]. In the gut loop experiments described previously [39], in which PrPd was detected rapidly in lymphatics, PPs of the intestine became infected at an early disease stage: as no evidence was obtained for the inoculum being transported across the dome to the PPs, the most likely source of scrapie infection of FDCs is thought to be from blood.
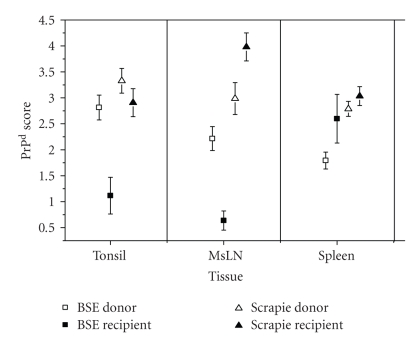
Figure 5.
Magnitude of PrPd accumulation in LRS tissues of sheep as part of the blood transfusion experiment [95]. BSE donor sheep showed the highest levels of PrPd accumulation in palatine tonsil and mesenteric lymph node (MsLN) but the lowest in the spleen. In contrast, the spleen in BSE-transfused sheep showed significant higher magnitudes of PrPd. Graphic representation including scrapie data has been modified from [96, 97].
3.3. Evidence That Infectivity in Blood May Establish Brain Infection
In the brains of BSE-infected sheep, the distribution of PrPd was found to be similar irrespective of the route of inoculation being intravascular, intracerebral, or alimentary [63], suggesting common patterns of neuroinvasion and CNS spread. The findings of vascular amyloid PrPd deposition in the basement membrane of endothelial cells of the hypothalamus [51] were an early indication that infectivity could reach the brain from the blood. However, such vascular PrPd plaques are a relatively rare finding in TSEs [116], which would not justify haematogenous neuroinvasion being a frequent event or having an important role.
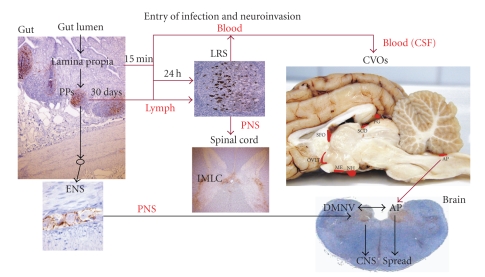
It is generally accepted that access of infectious agents to the brain via the blood is hampered by a defensive mechanism: the so-called blood-brain barrier. However, there are specific structures in the brain, the circumventricular organs (CVOs) which, having fenestrated capillaries, are more permissive than other brain areas to the passage of large molecules and provide a two-way communication between CNS and the rest of the body. Recent studies have shown that PrPd accumulation in the CVOs of sheep was an early and consistent event that was not affected by the route of challenge or TSE strain, suggesting that CNS entry of infectivity can occur through these structures [92]. No evidence has been obtained that arrival of PrPd in the CVOs is cell-mediated, as white blood cells were not observed in these organs at preclinical or clinical stage of disease; it is therefore possible that TSE agents are present in plasma as cell-free, soluble molecules. This hypothesis would be reinforced by the above-mentioned findings of PrPd in kidneys of scrapie-affected sheep [96, 97, 112], which suggest that PrPd arrives in the renal papillary interstitium by filtration or extravasation of soluble molecules. Similarly, CVOs could initially up-take soluble infectivity present in plasma (or in CSF) and act as receptors, supporters, and transporters of infectivity. The variability in severity of PrPd accumulation observed between examined CVOs may suggest different participation of these organs in terms of acquiring and amplifying infectivity. Hypothetically, the sensory CVOs may be more susceptible to initial infection, and the secretory CVOs in releasing it to extracranial structures; that is, infectivity present in the pituitary gland may be transported into the adrenal gland.
Once infection is established in the sensory CVOs further spread to neighbouring neural structures through efferent connections could occur (Figure 6). Thus, for example, PrPd in the hypothalamic paraventricular nucleus and in the bed nucleus of the stria terminalis could result from spread from the subfornical organ and from the organum vasculosum of the lamina terminalis rather than from the DMNV. The involvement of the DMNV and the area postrema is often simultaneous [98, 115] making it difficult to determine if the mechanism of entry of infection at this level of the brain is through the vagus nerve or from the blood, or from both. If the access was from blood through the area postrema, or through CVOs in general, it is possible that neurons of these organs would be infected and would start to accumulate PrPd as soon as any circulating infectivity in blood reaches them. However, it might also be possible that neurons of neighbouring structures, such as the DMNV or the hypothalamus, to which CVOs are connected, amplify infectivity or accumulate PrPd more rapidly than CVO neurones, resulting in an earlier detection in those structures.
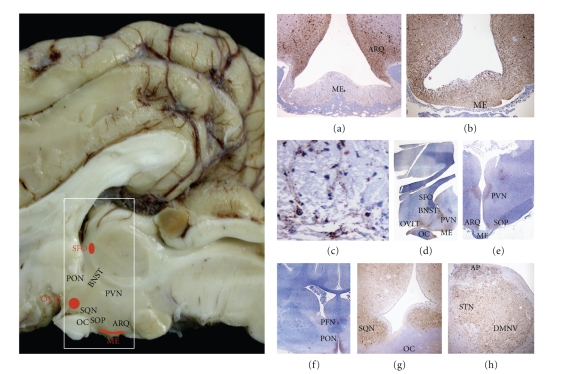
Figure 6.
CVO involvement may contribute to the spread of infection into the brain parenchyma. Preclinically affected TSE sheep show mild early PrPd accumulation in the median eminence (ME) (a; x4), or severe deposition in later stages (b; x4). Higher magnification is needed to detect mild PrPd accumulation in the ME (c; x60). In later stages, preclinical sheep do show accumulation of PrPd in those brain areas with established connections with the CVOs. A sagittal section of the diencephalon (d; x1) has been produced from the area compressed in the white rectangle in the macroscopic sagittal view of the brain highlighting some of the neural structures. Thus, the involvement of the ME correlates with PrPd accumulation in the arcuate nucleus (ARQ) (a,b,e), and that of the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO) correlates with PrPd in several nuclei: preoptic (PON) (f, x1), suprachiasmatic (SQN) (g; x4), supraoptic (SOP) (e, x1), paraventricular (PVN) (d, e), bed nucleus of the stria terminalis (BNST) (d) and perifornical (PFN) (f). Such correlations are difficult in the vagal complex because of the widespread severe PrPd accumulation (h).
Until recently, the CVOs had received no or very little attention in any human or animal TSE. McBride et al. [22] reported absence of PrPd immunodeposits in the area postrema of hamsters experimentally inoculated with scrapie by the oral route but investigations did not address the involvement of other CVOs. Similarly, no CVOs were studied in mule deer with chronic wasting disease, in which the hypothalamus was reported to accumulate PrPd before the medulla but after the DMNV [117]. We routinely examine the CVOs in current experiments and have retrospectively examined formalin-fixed brains from earlier studies. So far, we have found PrPd deposition in CVOs from clinically affected animals of different species infected with several strains (Figure 7), such as in mice intracerebrally infected with ME7, 87V, 79A, 22A, and 301V strains, and deer [11] and cattle orally infected with cattle BSE, (Sisó et al. unpublished observations) and in goats with natural scrapie [115].
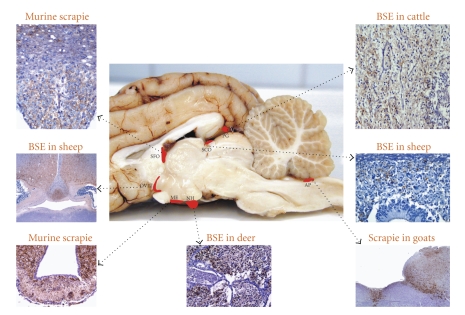
Figure 7.
PrPd accumulation within CVOs in clinically affected animals with different TSE strains. AP, area postrema; PG, pineal gland; ME, median eminence; SCO, subcommisural organ; OVLT, organum vasculosum of lamina terminalis; SFO, subfornical organ; NH, neurophypophysis.
A further support for a blood-borne neuroinvasion pathway was provided by the study of the topographical distribution of PrPd in the brain of 27 sheep that had been intracerebrally challenged with BSE and presented porencephalic lesions resulting from the traumatic injury [98]. The key findings (Figure 8) were as follows: (i) same PrPd topography, involving bilaterally the DMNV and the hypothalamus, as for infections by other routes; (ii) involvement of CVOs; (iii) focal accumulation of PrPd at the porencephalic lesions, but extending locally to neighbouring ipsilateral cerebrocortical areas in some cases; and (iv) absence of PrPd in such lesions in cases that did not accumulate PrPd anywhere else in the brain. It was therefore suggested that, rather than a local replication of the infectious agent at the site of injection, the inoculum would be completely reabsorbed into the CSF and recirculated in the blood gaining access back to the brain through the CVOs and through the porencephalic lesions, where fenestrated neocapillaries were also abundant as part of the repair tissue process.
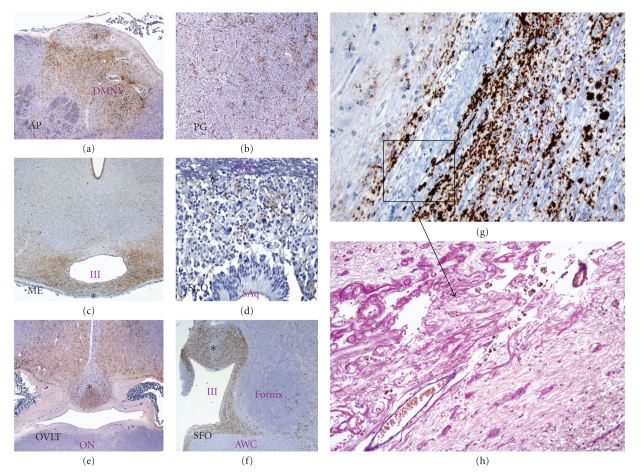
Figure 8.
PrPd accumulation in CVOs and in the porencephalic lesion. Sheep CVOs highlighted with an asterix in pictures (a–f) of low magnification showed consistent PrPd deposition after intracerebral inoculation with BSE. Note that the porencephalic lesion which is surrounded by PrPd accumulations (g; x10) has abundant proliferation of newly formed vessels as revealed by a Van-Gieson staining (h; x20). CVOs: AP, area postrema; PG, pineal gland; ME, median eminence; SCO, subcommisural organ; OVLT, organum vasculosum of the lamina terminalis; SFO, subfornical organ. Brain structures: DMNV, dorsal nucleus of the vagus nerve; IV, fourth ventricle; Saq, Silvios' acqueduct; ON, optic chiasm; III, third ventricle; AWC, anterior white commisure. The figure is modified from (Sisó et al. [98]).
The haematogenous route, therefore, can represent a parallel or alternative pathway of neuroinvasion to ascending infection via the ENS and autonomic nervous system by accessing the CVOs and encephalic lesions of traumatic or vascular origin. Figure 9 represents schematically the possible pathways of neuroinvasion to be considered in TSEs.
Figure 9.
Schematic view of the neuroinvasion process involving the roles of the blood and the CVOs. For abbreviations see text and Figure 7 legend.
4. Conclusions
The most widely accepted neuroinvasion mechanism in prion diseases is thought to involve amplification of infectivity within lymphoid tissues and subsequent retrograde spread of infection along autonomic nervous system nerves until it reaches the brain. However, the peripheral pathogenesis of prion diseases can vary depending on several factors. In most TSE cases, infection of the lymphoid system appears to facilitate neuroinvasion, increasing attack rates and shortening incubation periods, although in some scrapie cases and in most or all of natural BSE cases and in sCJD, the lymphoid system does not seem to be involved. In view of the fact that, regardless of the route of infection and other factors that affect the peripheral pathogenesis (agent, PRNP genotype, etc.), the initial accumulation of PrPd in the brain and its spread appears to follow the same pattern, it is unlikely that neuroinvasion occurs solely by a mechanism involving retrograde transport of infectivity along peripheral nerves, at least those of the ENS/PNS axis, as the prevailing view suggests.
Alternatively, the observation that PrPd accumulates in peripheral organs, such as the kidney or the mammary gland, as does in the LRS tissues themselves, clearly shows a vascular dispersion of infectivity, which is consistent with an haematogenous route of neuroinvasion. Additional support for this alternative pathway derives from the early and systematic detection of PrPd in the CVOs which, lacking a blood-brain barrier, could act as portals of entry of infection.
Whether the haematogenous neuroinvasion pathway is coincidental with a neural pathway, and the relative importance of them for different TSEs, remains to be determined.
References
- 1.Fraser H. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature. 1982;295(5845):149–150. doi: 10.1038/295149a0. [DOI] [PubMed] [Google Scholar]
- 2.Scott JR, Fraser H. Transport and targeting of scrapie infectivity and pathology in the optic nerve projections following intraocular infection. Progress in Clinical and Biological Research. 1989;317:645–652. [PubMed] [Google Scholar]
- 3.Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection. Journal of Virology. 2003;77(1):583–591. doi: 10.1128/JVI.77.1.583-591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kincaid AE, Bartz JC. The nasal cavity is a route for prion infection in hamsters. Journal of Virology. 2007;81(9):4482–4491. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sbriccoli M, Cardone F, Valanzano A, et al. Neuroinvasion of the 263 K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathologica. 2009;117(2):175–184. doi: 10.1007/s00401-008-0474-z. [DOI] [PubMed] [Google Scholar]
- 6.Bessen RA, Martinka S, Kelly J, Gonzalez D. Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. Journal of Virology. 2009;83(13):6435–6445. doi: 10.1128/JVI.00018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimberlin RH, Field HJ, Walker CA. Pathogenesis of mouse scrapie: evidence for spread of infection from central to peripheral nervous system. Journal of General Virology. 1983;64(3):713–716. doi: 10.1099/0022-1317-64-3-713. [DOI] [PubMed] [Google Scholar]
- 8.Bartz JC, Kincaid AE, Bessen RA. Retrograde transport of transmissible mink encephalopathy within descending motor tracts. Journal of Virology. 2002;76(11):5759–5768. doi: 10.1128/JVI.76.11.5759-5768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infection and Immunity. 1973;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kucera P, Dolivo M, Coulon P, Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. Journal of Virology. 1985;55(1):158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin S, Jeffrey M, González L, et al. Immunohistochemical and biochemical characteristics of BSE and CWD in experimentally infected European red deer (Cervus elaphus elaphus) BMC Veterinary Research. 2009;5, article 26 doi: 10.1186/1746-6148-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eklund CM, Kennedy RC, Hadlow WJ. Pathogenesis of scrapie virus infection in the mouse. Journal of Infectious Diseases. 1967;117(1):15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Kimberlin RH, Walker CA. Pathogenesis of scrapie in mice after intragastric infection. Virus Research. 1989;12(3):213–220. doi: 10.1016/0168-1702(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 14.Kimberlin RH, Walker CA. Novel Infectious Agents and the Central Nervous System. Vol. 135. Chichester, UK: Wiley; 1988. Pathogenesis of experimental scrapie; pp. 37–62. (Ciba Foundation Symposium). [DOI] [PubMed] [Google Scholar]
- 15.Kimberlin RH, Walker CA. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Research. 1989;12(3):201–212. doi: 10.1016/0168-1702(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 16.Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: effect of route of inoculation on infectivity titres and dose-response curves. Journal of Comparative Pathology. 1978;88(1):39–47. doi: 10.1016/0021-9975(78)90059-2. [DOI] [PubMed] [Google Scholar]
- 17.Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: patterns of agent replication in different parts of the CNS following intraperitoneal infection. Journal of the Royal Society of Medicine. 1982;75(8):618–624. doi: 10.1177/014107688207500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke MC, Kimberlin RH. Pathogenesis of mouse scrapie: distribution of agent in the pulp and stroma of infected spleens. Veterinary Microbiology. 1984;9(3):215–225. doi: 10.1016/0378-1135(84)90039-7. [DOI] [PubMed] [Google Scholar]
- 19.Buyukmihci N, Goehring-Harmon F, Marsh RF. Asymmetry of retinal lesions in experimental scrapie after intracerebral inoculation of hamsters. Experimental Neurology. 1985;87(1):172–176. doi: 10.1016/0014-4886(85)90143-8. [DOI] [PubMed] [Google Scholar]
- 20.Baldauf E, Beekes M, Diringer H. Evidence for an alternative direct route of access for the scrapie agent to the brain bypassing the spinal cord. Journal of General Virology. 1997;78(5):1187–1197. doi: 10.1099/0022-1317-78-5-1187. [DOI] [PubMed] [Google Scholar]
- 21.Beekes M, Baldauf E, Diringer H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. Journal of General Virology. 1996;77(8):1925–1934. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- 22.McBride PA, Schulz-Schaeffer WJ, Donaldson M, et al. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. Journal of Virology. 2001;75(19):9320–9327. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beekes M, McBride PA, Baldauf E. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. Journal of General Virology. 1998;79(3):601–607. doi: 10.1099/0022-1317-79-3-601. [DOI] [PubMed] [Google Scholar]
- 24.McBride PA, Beekes M. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neuroscience Letters. 1999;265(2):135–138. doi: 10.1016/s0304-3940(99)00223-2. [DOI] [PubMed] [Google Scholar]
- 25.Beekes M, McBride PA. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neuroscience Letters. 2000;278(3):181–184. doi: 10.1016/s0304-3940(99)00934-9. [DOI] [PubMed] [Google Scholar]
- 26.Mabbott NA, Bruce ME. The immunology of TSE diseases. Journal of General Virology. 2001;82:2307–2318. doi: 10.1099/0022-1317-82-10-2307. [DOI] [PubMed] [Google Scholar]
- 27.Diringer H. Sustained viremia in experimental hamster scrapie. Brief report. Archives of Virology. 1984;82(1-2):105–109. doi: 10.1007/BF01309373. [DOI] [PubMed] [Google Scholar]
- 28.Kimberlin RH, Walker CA. Pathogenesis of scrapie (strain 263k) in hamsters infected intracerebrally, intraperitoneally or intraocularly. Journal of General Virology. 1986;67(2):255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda Y, Gibbs CJ, Jr., Amyx HL, Gajdusek DC. Creutzfeldt-Jakob disease in mice: persistent viremia and preferential replication of virus in low-density lymphocytes. Infection and Immunity. 1983;41(1):154–161. doi: 10.1128/iai.41.1.154-161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein MA, Frigg R, Flechsig E, et al. A crucial role for B cells in neuroinvasive scraple. Nature. 1997;390(6661):687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 31.Klein MA, Frigg R, Raeber AJ, et al. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nature Medicine. 1998;4(12):1429–1433. doi: 10.1038/4022. [DOI] [PubMed] [Google Scholar]
- 32.Brown KL, Stewart K, Ritchie DL, et al. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nature Medicine. 1999;5(11):1308–1312. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 33.Mabbott NA, Williams A, Farquhar CF, Pasparakis M, Kollias G, Bruce ME. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. Journal of Virology. 2000;74(7):3338–3344. doi: 10.1128/jvi.74.7.3338-3344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beringue V, Demoy M, Lasmézas CI, et al. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. Journal of Pathology. 2000;190(4):495–502. doi: 10.1002/(SICI)1096-9896(200003)190:4<495::AID-PATH535>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 35.Davies GA, Bryant AR, Reynolds JD, Jirik FR, Sharkey KA. Prion diseases and gastrointestinal tract. Canadian Journal of Gastroenterology. 2006;20(1):18–24. doi: 10.1155/2006/184528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorban G, Defaweux V, Demonceau C, et al. Interaction between dendritic cells and nerve fibres in lymphoid organs after oral scrapie exposure. Virchows Archiv. 2007;451(6):1057–1065. doi: 10.1007/s00428-007-0476-6. [DOI] [PubMed] [Google Scholar]
- 37.Huang F-P, Farquhar CF, Mabbott NA, Bruce ME, MacPherson GG. Migrating intestinal dendritic cells transport PrPSc from the gut. Journal of General Virology. 2002;83(1):267–271. doi: 10.1099/0022-1317-83-1-267. [DOI] [PubMed] [Google Scholar]
- 38.Jeffrey M, González L, Chong A, et al. Ovine infection with the agents of scrapie (CH1641 isolate) and bovine spongiform encephalopathy: immunochemical similarities can be resolved by immunohistochemistry. Journal of Comparative Pathology. 2006;134(1):17–29. doi: 10.1016/j.jcpa.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Jeffrey M, González L, Espenes A, et al. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. Journal of Pathology. 2006;209(1):4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 40.Ford MJ, Burton LJ, Morris RJ, Hall SM. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience. 2002;113(1):177–192. doi: 10.1016/s0306-4522(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 41.Aguzzi A, Heppner FL, Heikenwalder M, et al. Immune system and peripheral nerves in propagation of prions to CNS. British Medical Bulletin. 2003;66:141–159. doi: 10.1093/bmb/66.1.141. [DOI] [PubMed] [Google Scholar]
- 42.Blattler T, Brandner S, Raeber AJ, et al. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature. 1997;389(6646):69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 43.Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron. 2001;31(1):25–34. doi: 10.1016/s0896-6273(01)00331-2. [DOI] [PubMed] [Google Scholar]
- 44.Carlson SL, Albers KM, Belting DJ, Parish M, Conner JM, Davis BM. NGF modulates sympathetic innervation of lymphoid tissues. Journal of Neuroscience. 1995;15(9):5892–5899. doi: 10.1523/JNEUROSCI.15-09-05892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadlow WJ, Kennedy RC, Race RE, Eklund CM. Virologic and neurohistologic findings in dairy goats affected with natural scrapie. Veterinary Pathology. 1980;17:187–199. doi: 10.1177/030098588001700207. [DOI] [PubMed] [Google Scholar]
- 46.Hadlow WJ, Kennedy RC, Race RE. Natural infection of Suffolk sheep with scrapie virus. Journal of Infectious Diseases. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 47.Groschup MH, Weiland F, Straub OC, Pfaff E. Detection of scrapie agent in the peripheral nervous system of a diseased sheep. Neurobiology of Disease. 1996;3(3):191–195. doi: 10.1006/nbdi.1996.0019. [DOI] [PubMed] [Google Scholar]
- 48.Groschup MH, Beekes M, McBride PA, Hardt M, Hainfellner JA, Budka H. Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathologica. 1999;98(5):453–457. doi: 10.1007/s004010051108. [DOI] [PubMed] [Google Scholar]
- 49.Andréoletti O, Berthon P, Marc D, et al. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. Journal of General Virology. 2000;81(12):3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 50.van Keulen LJM, Schreuder BEC, Vromans MEW, Langeveld JPM, Smits MA. Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. Journal of Comparative Pathology. 1999;121(1):55–63. doi: 10.1053/jcpa.1998.0300. [DOI] [PubMed] [Google Scholar]
- 51.van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Pathogenesis of natural scrapie in sheep. Archives of Virology Supplementum. 2000;(16):57–71. doi: 10.1007/978-3-7091-6308-5_5. [DOI] [PubMed] [Google Scholar]
- 52.Jeffrey M, Martin S, González L, et al. Cell-associated variants of disease-specific prion protein immunolabelling are found in different sources of sheep transmissible spongiform encephalopathy. Journal of General Virology. 2003;84:1033–1045. doi: 10.1099/vir.0.18825-0. [DOI] [PubMed] [Google Scholar]
- 53.van Keulen LJM, Vromans MEW, van Zijderveld FG. Early and late pathogenesis of natural scrapie infection in sheep. Acta Pathologica, Microbiologica et Immunologica Scandinavica. 2002;110(1):23–32. doi: 10.1034/j.1600-0463.2002.100104.x. [DOI] [PubMed] [Google Scholar]
- 54.Jeffrey M, Martin S, Thomson JR, Dingwall WS, Begara-McGorum I, González L. Onset and distribution of tissue PrP accumulation in scrapie-affected Suffolk sheep as demonstrated by sequential necropsies and tonsillar biopsies. Journal of Comparative Pathology. 2001;125(1):48–57. doi: 10.1053/jcpa.2001.0476. [DOI] [PubMed] [Google Scholar]
- 55.Jeffrey M, Ryder S, Martin S, et al. Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. Journal of Comparative Pathology. 2001;124(4):280–289. doi: 10.1053/jcpa.2001.0465. [DOI] [PubMed] [Google Scholar]
- 56.McGovern G, Jeffrey M. Scrapie-specific pathology of sheep lymphoid tissues. PLoS One. 2007;2(12, article e1304) doi: 10.1371/journal.pone.0001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heggebø R, Press CMcL, Gunnes G, et al. Distribution of prion protein in the ileal Peyer’s patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. Journal of General Virology. 2000;81(9):2327–2337. doi: 10.1099/0022-1317-81-9-2327. [DOI] [PubMed] [Google Scholar]
- 58.Ersdal C, Ulvund MJ, Benestad SL, Tranulis MA. Accumulation of pathogenic prion protein (PrPSc) in nervous and lymphoid tissues of sheep with subclinical scrapie. Vet Pathol. 2003;40(2):164–174. doi: 10.1354/vp.40-2-164. [DOI] [PubMed] [Google Scholar]
- 59.Heggebø R, González L, Press CMcL, Gunnes G, Espenes A, Jeffrey M. Disease-associated PrP in the enteric nervous system of scrapie-affected Suffolk sheep. Journal of General Virology. 2003;84(5):1327–1338. doi: 10.1099/vir.0.18874-0. [DOI] [PubMed] [Google Scholar]
- 60.van Keulen LJM, Vromans MEW, Dolstra CH, Bossers A, van Zijderveld FG. Pathogenesis of bovine spongiform encephalopathy in sheep. Archives of Virology. 2008;153(3):445–453. doi: 10.1007/s00705-007-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heggebø R, Press CMcL, Gunnes G, González L, Jeffrey M. Distribution and accumulation of PrP in gut-associated and peripheral lymphoid tissue of scrapie-affected Suffolk sheep. Journal of General Virology. 2002;83(2):479–489. doi: 10.1099/0022-1317-83-2-479. [DOI] [PubMed] [Google Scholar]
- 62.Bellworthy SJ, Hawkins SAC, Green RB, et al. Tissue distribution of bovine spongiform encephalopathy infectivity in Romney sheep up to the onset of clinical disease after oral challenge. Veterinary Record. 2005;156(7):197–202. doi: 10.1136/vr.156.7.197. [DOI] [PubMed] [Google Scholar]
- 63.González L, Martin S, Houston FE, et al. Phenotype of disease-associated PrP accumulation in the brain of bovine spongiform encephalopathy experimentally infected sheep. Journal of General Virology. 2005;86(3):827–838. doi: 10.1099/vir.0.80299-0. [DOI] [PubMed] [Google Scholar]
- 64.Houston F, Goldmann W, Chong A, et al. Prion diseases: BSE in sheep bred for resistance to infection. Nature. 2003;423(6939):p. 498. doi: 10.1038/423498a. [DOI] [PubMed] [Google Scholar]
- 65.Martin S, González L, Chong A, Houston FE, Hunter N, Jeffrey M. Immunohistochemical characteristics of disease-associated PrP are not altered by host genotype or route of inoculation following infection of sheep with bovine spongiform encephalopathy. Journal of General Virology. 2005;86(3):839–848. doi: 10.1099/vir.0.80364-0. [DOI] [PubMed] [Google Scholar]
- 66.Terry LA, Marsh S, Ryder SJ, Hawkins SAC, Wells GAH, Spencer YI. Detection of disease-specific Prp in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Veterinary Record. 2003;152(13):387–392. doi: 10.1136/vr.152.13.387. [DOI] [PubMed] [Google Scholar]
- 67.Buschmann A, Groschup MH. Highly bovine spongiform encephalopathy-sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. Journal of Infectious Diseases. 2005;192(5):934–942. doi: 10.1086/431602. [DOI] [PubMed] [Google Scholar]
- 68.Wells GA, Dawson M, Hawkins SA, et al. Infectivity in the ileum of cattle challenged orally with bovine spongiform encephalopathy. Veterinary Record. 1994;135(2):40–41. doi: 10.1136/vr.135.2.40. [DOI] [PubMed] [Google Scholar]
- 69.Wells GAH, Hawkins SAC, Green RB, et al. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Veterinary Record. 1998;142(5):103–106. doi: 10.1136/vr.142.5.103. [DOI] [PubMed] [Google Scholar]
- 70.Wells GAH, Spiropoulos J, Hawkins SAC, Ryder SJ. Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Veterinary Record. 2005;156(13):401–407. doi: 10.1136/vr.156.13.401. [DOI] [PubMed] [Google Scholar]
- 71.Hoffmann C, Ziegler U, Buschmann A, et al. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. Journal of General Virology. 2007;88(3):1048–1055. doi: 10.1099/vir.0.82186-0. [DOI] [PubMed] [Google Scholar]
- 72.Williams ES. Chronic wasting disease. Veterinary Pathology. 2005;42(5):530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- 73.Sigurdson CJ. A prion disease of cervids: chronic wasting disease. Veterinary Research. 2008;39(4, article 41) doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- 74.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. Journal of General Virology. 2001;82(10):2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 75.Bessen RA, Marsh RF. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. Journal of General Virology. 1992;73(2):329–334. doi: 10.1099/0022-1317-73-2-329. [DOI] [PubMed] [Google Scholar]
- 76.Bartz JC, DeJoia C, Tucker T, Kincaid AE, Bessen RA. Extraneural prion neuroinvasion without lymphoreticular system infection. Journal of Virology. 2005;79(18):11858–11863. doi: 10.1128/JVI.79.18.11858-11863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill AF, Butterworth RJ, Joiner S, et al. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. The Lancet. 1999;353(9148):183–189. doi: 10.1016/s0140-6736(98)12075-5. [DOI] [PubMed] [Google Scholar]
- 78.Hainfellner JA, Budka H. Disease associated prion protein may deposit in the peripheral nervous system in human transmissible spongiform encephalopathies. Acta Neuropathologica. 1999;98(5):458–460. doi: 10.1007/s004010051109. [DOI] [PubMed] [Google Scholar]
- 79.Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. New England Journal of Medicine. 2003;349(19):1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- 80.McGovern G, Martin S, González L, Witz J, Jeffrey M. Frequency and distribution of nerves in scrapie-affected and unaffected peyer’s patches and lymph nodes. Veterinary Pathology. 2009;46(2):233–240. doi: 10.1354/vp.46-2-233. [DOI] [PubMed] [Google Scholar]
- 81.González L, Dagleish MP, Martin S, et al. Ante-mortem diagnosis of preclinical sheep scrapie by immunohistochemical examination of rectal biopsy samples. Veterinary Record. 2008;162(13):397–403. doi: 10.1136/vr.162.13.397. [DOI] [PubMed] [Google Scholar]
- 82.van Keulen LJM, Schreuder BEC, Meloen RH, Mooij-Harkes G, Vromans MEW, Langeveld JPM. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. Journal of Clinical Microbiology. 1996;34(5):1228–1231. doi: 10.1128/jcm.34.5.1228-1231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeffrey M, Begara-McGorum I, Clark S, et al. Occurrence and distribution of infection-specific PrP in tissues of clinical scrapie cases and cull sheep from scrapie-affected farms in Shetland. Journal of Comparative Pathology. 2002;127(4):264–273. doi: 10.1053/jcpa.2002.0592. [DOI] [PubMed] [Google Scholar]
- 84.González L, Martin S, Sisó S, et al. High prevalence of scrapie in a dairy goat herd: tissue distribution of disease-associated PrP and effect of PRNP genotype and age. Veterinary Research. 2009;40(6, article 65) doi: 10.1051/vetres/2009048. [DOI] [PubMed] [Google Scholar]
- 85.Head MW, Ritchie D, Smith N, et al. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. American Journal of Pathology. 2004;164(1):143–153. doi: 10.1016/S0002-9440(10)63105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benestad SL, Arsac J-N, Goldmann W, Nöremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Veterinary Research. 2008;39(4, article 19) doi: 10.1051/vetres:2007056. [DOI] [PubMed] [Google Scholar]
- 87.Felten DL, Felten SY, Carlson SL. Noradrenergic and peptidergic innervation of lymphoid tissue. Journal of Immunology. 1985;135(supplement 2):755s–765s. [PubMed] [Google Scholar]
- 88.Felten DL, Felten SY. Sympathetic noradrenergic innervation of immune organs. Brain Behavior and Immunity. 1988;2(4):293–300. doi: 10.1016/0889-1591(88)90031-1. [DOI] [PubMed] [Google Scholar]
- 89.Bencsik A, Lezmi S, Baron T. Autonomous nervous system innervation of lymphoid territories in spleen: a possible involvement of nonadrenergic neurons for prion neuroinvasion in natural scrapie. Journal of Neurovirology. 2001;7(5):447–453. doi: 10.1080/135502801753170309. [DOI] [PubMed] [Google Scholar]
- 90.Bencsik A, Lezmi S, Hunsmann G, Baron T. Close vicinity of PrP expressing cells (FDC) with noradrenergic fibers in healthy sheep spleen. Developmental Immunology. 2001;8(3-4):235–241. doi: 10.1155/2001/40871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Felten DL, Felten SY, Bellinger DL, et al. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunological Reviews. 1987;(100):225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 92.Sisó S, Jeffrey M, González L. Neuroinvasion in sheep transmissible spongiform encephalopathies: the role of the haematogenous route. Neuropathology and Applied Neurobiology. 2009;35(3):232–246. doi: 10.1111/j.1365-2990.2008.00978.x. [DOI] [PubMed] [Google Scholar]
- 93.Head MW, Yull HM, Ritchie DL, Bishop MT, Ironside JW. Pathological investigation of the first blood donor and recipient pair linked by transfusion-associated variant Creutzfeldt-Jakob disease transmission. Neuropathology and Applied Neurobiology. 2009;35(4):433–436. doi: 10.1111/j.1365-2990.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 94.Millson GC, Kimberlin RH, Manning EJ, Collis SC. Early distribution of radioactive liposomes and scrapie infectivity in mouse tissues following administration by different routes. Veterinary Microbiology. 1979;4(2):89–99. [Google Scholar]
- 95.Houston F, McCutcheon S, Goldmann W, et al. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood. 2008;112(12):4739–4745. doi: 10.1182/blood-2008-04-152520. [DOI] [PubMed] [Google Scholar]
- 96.Sisó S, González L, Houston F, Hunter N, Martin S, Jeffrey M. The neuropathologic phenotype of experimental ovine BSE is maintained after blood transfusion. Blood. 2006;108(2):745–748. doi: 10.1182/blood-2005-12-5156. [DOI] [PubMed] [Google Scholar]
- 97.Sisó S, González L, Jeffrey M, Martin S, Chianini F, Steele P. Prion protein in kidneys of scrapie-infected sheep. Veterinary Record. 2006;159(10):327–328. doi: 10.1136/vr.159.10.327-b. [DOI] [PubMed] [Google Scholar]
- 98.Sisó S, Jeffrey M, Martin S, Houston F, Hunter N, González L. Pathogenetical significance of porencephalic lesions associated with intracerebral inoculation of sheep with the bovine spongiform encephalopathy (BSE) agent. Neuropathology and Applied Neurobiology. 2009;35(3):247–258. doi: 10.1111/j.1365-2990.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 99.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in sheep. The Lancet. 2000;356(9234):999–1000. doi: 10.1016/s0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 100.Hunter N, Foster J, Chong A, et al. Transmission of prion diseases by blood transfusion. Journal of General Virology. 2002;83(11):2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 101.Sisó S, Jeffrey M, Houston F, Hunter N, Martin S, González L. Pathological phenotype of sheep scrapie after blood transfusion. Journal of Comparative Pathology. 2010;142(1):27–35. doi: 10.1016/j.jcpa.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Mathiason CK, Powers JG, Dahmes SJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314(5796):133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 103.Llewelyn CA, Hewitt PE, Knight RSG, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. The Lancet. 2004;363(9407):417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 104.Peden AH, Head MW, Ritchie DL, Bell PJE, Ironside PJW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. The Lancet. 2004;364(9433):527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 105.Wroe SJ, Pal S, Siddique D, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. The Lancet. 2006;368(9552):2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 106.HPA Press Statement. 4th case of variant CJD infection associated with blood transfusion. Health Protection Agency, 2007, http://www.hpa.org.uk/hpa/news/articles/press_releases/2007/070118_vCJD.htm.
- 107.HPA Press Statement. vCJD abnormal prion protein found in a patient with haemophilia at post mortem. Health Protection Agency, 2009, http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1234859690542?p=1231252394302.
- 108.Saá P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313(5783):92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 109.Andréoletti O, Lacroux C, Chabert A, et al. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. Journal of General Virology. 2002;83(10):2607–2616. doi: 10.1099/0022-1317-83-10-2607. [DOI] [PubMed] [Google Scholar]
- 110.Ligios C, Sigurdson CJ, Santucciu C, et al. PrPSc in mammary glands of sheep affected by scrapie and mastitis. Nature Medicine. 2005;11(11):1137–1138. doi: 10.1038/nm1105-1137. [DOI] [PubMed] [Google Scholar]
- 111.Vascellari M, Nonno R, Mutinelli F, et al. PrPSc in salivary glands of scrapie-affected sheep. Journal of Virology. 2007;81(9):4872–4876. doi: 10.1128/JVI.02148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sisó S, Jeffrey M, Steele P, et al. Occurrence and cellular localization of PrPd in kidneys of scrapie-affected sheep in the absence of inflammation. Journal of Pathology. 2008;215(2):126–134. doi: 10.1002/path.2336. [DOI] [PubMed] [Google Scholar]
- 113.Lacroux C, Simon S, Benestad SL, et al. Prions in milk from ewes incubating natural scrapie. PLoS Pathogens. 2008;4(12, article e1000238) doi: 10.1371/journal.ppat.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Konold T, Moore SJO, Bellworthy SJ, Simmons HA. Evidence of scrapie transmission via milk. BMC Veterinary Research. 2008;4, article 14 doi: 10.1186/1746-6148-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.González L, Martin S, Hawkins SA, et al. Pathogenesis of natural goat scrapie: modulation by host PRNP genotype and effect of co-existent conditions. Veterinary Research. 2010;41(4):p. 48. doi: 10.1051/vetres/2010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.González L, Martin S, Begara-McGorum I, et al. Effects of agent strain and host genotype on PrP accumulation in the brain of sheep naturally and experimentally affected with scrapie. Journal of Comparative Pathology. 2002;126(1):17–29. doi: 10.1053/jcpa.2001.0516. [DOI] [PubMed] [Google Scholar]
- 117.Spraker TR, Zink RR, Cummings BA, Sigurdson CJ, Miller MW, O’Rourke KI. Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Veterinary Pathology. 2002;39(5):546–556. doi: 10.1354/vp.39-5-546. [DOI] [PubMed] [Google Scholar]