Abstract
Traditionally, immunohistochemistry, immunoblotting, and histoblot have been used to detect protein in tissue samples. However, each of these techniques has a number of disadvantages. The sensitivity of protein detection in immunohistochemistry is lost due to fixation or paraffin embedding methods that modify antigenic sites. The anatomical resolution and specific cellular involvement are lost in immunoblotting. Histoblot, a hybrid of these two techniques, is able to resolve these issues, but it cannot be applied to formalin-fixed tissues. A recent technique, paraffin-embedded tissue (PET) blot, retains the superior protein detection and anatomical resolution of histoblot and is applicable to formalin-fixed tissues. Unfortunately, a major obstacle to the widespread application of PET is the lack of a detailed methodological description. In this paper, we describe a PET blotting method that was formulated from our own empirical and experimental research in Alzheimer disease and a systematic review of the current literature. From this, we conclude that PET can be applied to a variety of conditions with a wide spectrum of pathology.
Keywords: Alzheimer’s disease, amyloid, amyloid oligomer, paraffin-embedded tissue (PET) blot
INTRODUCTION
Many techniques are used to detect and measure proteins, such as immunohistochemistry, immunoblotting, and histoblot. However, each of these techniques comes with a set of unique disadvantages. Specifically, for some antigens, the sensitivity of protein detection with immunohistochemistry is lost due to fixation or paraffin embedding methods that modify antigenic sites. With immunoblotting, tissue samples are homogenized. Cellular distribution of the reaction, therefore, is lost. Histoblot, a hybrid of these two techniques that utilizes tissue sections applied directly to protein binding membranes, was able to resolve these issues. However, early methods required fresh tissue samples, precluding the possibility of using archived tissue samples for retrospective or prospective analysis (Dickson, 2002).
More recently, use of the paraffin-embedded tissue (PET) blot, has been shown to preserve the highly sensitive protein detection of histoblot while remaining applicable to formalin-fixed tissues (Neumann et al., 2002; Schulz-Schaeffer et al., 2000). While early studies of PET blot were successful in some disease-specific applications, the methodologies were not standardized for application to a range of diseases. In this study, we improve upon earlier methods and provide standardized and specific methodological details for fine tissue resolution using the PET blot staining method that is comparable to routine immunochemistry on glass microscope slides. Further, the tissue stabilization afforded by the protein binding membrane allows for a spectrum of harsh antigen retrieval techniques to unmask buried epitopes while retaining tissue integrity. As such, this method combines sensitive and specific protein detection, including the use of antigen retrieval methods, for detailed qualitative and quantitive analyses encompassing tissue distribution of proteins.
MATERIALS AND METHODS
Tissue collection
For this study, hippocampal brain tissue samples from Alzheimer disease and non-diseased aged-matched individuals were examined. Tissues were collected at autopsy using Case Western Reserve University IRB approved protocols, fixed in either routine formalin or methacarn (methanol:chloroform:acetic acid; 6:3:1) and embedded in paraffin.
Antibodies
Antibodies used included 4G8 (anti-amyloid-β; Covance) and PHF-1, which recognizes phosphorylated tau (gift of Sharon Greenberg).
Paraffin-embedded tissue blot
Immobilon (PVDF, Millipore) membranes are cut into 20 × 50 mm pieces (same size as a glass cover slip). The membranes are wetted with methanol and transferred to water in preparation for section collection. 5 μm sections of paraffin embedded tissue are cut using a microtome and placed into a water bath heated to 40°C. With broad-ended forceps, immobilon membranes are dipped into the water bath and tissue sections collected onto them in a similar fashion as with a glass slide. The PET blots are then placed onto a glass plate and labeled in a corner with a No. 2 pencil. Prior to use, samples were dehydrated by placing the glass plate and membranes into a 60°C oven for 30 min.
In glass staining dishes, the PET blots were immersed in 2 changes of xylene/100% ethanol/95% ethanol/70% ethanol/Tris buffered saline (TBS; 150 mM NaCl, 50 mM Tris, pH 7.6) for 5 min each. For the transition between xylene and 100% ethanol, care should be taken to air-dry the membranes on a glass plate before placing them in the 100% ethanol. In preliminary trials, often the xylene in the tissue was repulsed by the alcohol and sometimes caused the section to shear away from the membrane. Similar care should be taken to air dry the membranes during the transition between 70% ethanol and TBS as shearing often occurs between this step as well.
After the TBS bath, the PET blots are placed onto dental wax in a humidity chamber. To remove endogenous peroxidases, 125 μl of a 3% hydrogen peroxide solution was applied for 20 min at room temperature and then the PET blots rinsed in TBS. 10% normal goat serum (NGS) was applied as a blocking solution for 30 min to prepare the samples for antibody binding. The PET blots are cut individually with a razor blade or scissors and ~125 μl primary antibody solution applied and incubated for 1–12 h at room temperature.
After rinsing in a large volume of TBS for 1 min, 10% NGS was applied for 10 min, and the PET blots rinsed again with TBS. Secondary antibodies, either goat-anti-mouse or goat anti-rabbit, were then applied to the sample for 30 min, followed by another 10 min rinse with 10% NGS. The species-specific peroxidase antiperoxidase complex was then applied to the PET blots for 1 h. Finally, PET blots were rinsed in 2 changes of Tris buffer (50 mM Tris, pH 7.6) for 5 min and developed with a 3′-3′-diaminobenzidine (Dako) until the stains become apparent (~3 min).
After development, the PET blots were rinsed in Tris buffer and placed into a small petri dish containing a 3:2 mix of dimethyl sulfoxide (DMSO):ethanol. After a short incubation in this solution, the immobilon membranes became transparent, and the PET blots were placed onto a glass slide and directly cover slipped as the 3:2 DMSO:ethanol mix acts as a mounting solution. At this point, the sections are easily viewed with light microscopy (Zeiss Axiophot) and can be readily imaged (Zeiss Axiocam). After a time, the DMSO:ethanol dries, but PET blots can be remounted with the 3:2 DMSO:ethanol multiple times.
RESULTS
Comparison between PET blot and immunohistochemistry
For this study, at the time of tissue sectioning, adjacent sections to the PET blot samples were cut and placed onto glass microscope slides for routine immunohistochemistry, according to previously published methods (Sternberger, 1986; Zhu et al., 2003). Both the PET blots and glass slides were immunostained at the same time. A direct comparison between tissue morphology as well as antigenic expression was then made.
Pretreatments
For some experiments, various pretreatment parameters were examined. After PET blots were prepared and rehydrated, a post fixation in 10% buffered formalin for 30 min prior to immunostaining preserved both antigenic sites and tissue integrity for some antibody treatment paradigms. Treatments with 70% formic acid, 0.1% SDS, or 20 μg/mL proteinase K were analyzed.
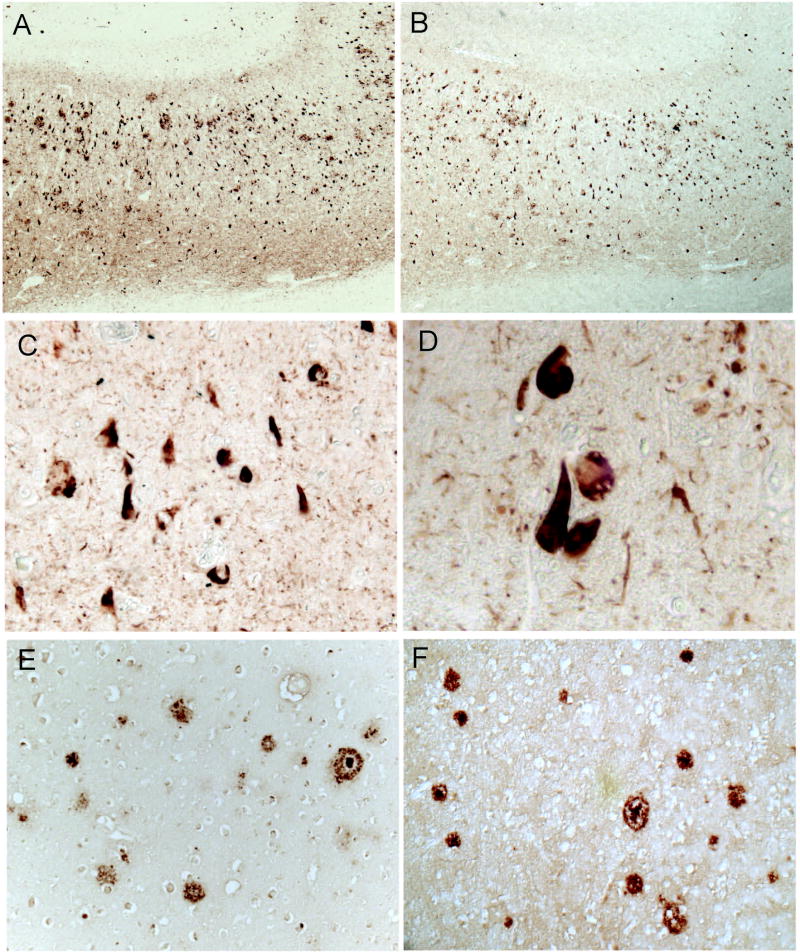
Preliminary experiments were performed that compared the use of nitrocellulose and immobilon for the PET blot technique. We found nitrocellulose to be inferior—the membrane was often too distorted to visualize and lacked a clear method for mounting and imaging. Thus, we developed a methodological technique using immobilon membranes instead. Use of the detailed PET blot technique resulted in clear immunostaining patterns virtually identical to that seen with traditional staining of tissue sections on glass microscope slides. Adjacent sections from same tissue samples prepared for either PET blot or placed onto glass slides were indistinguishable as to morphological structures stained or intensity of stain (Fig. 1). For instance, phosphorylated tau antibodies readily stained neurofibrillary pathology using both methodologies (Fig. 1A-D), and immunostaining with Aβ-specific antibodies demonstrated similar amyloid plaque localization using both techniques (Fig. 1E,F).
Figure 1.
Comparison of PET blot and routine glass slide immunohistochemistry. Sections that were sequentially cut from the same paraffin block and placed on either glass slide (A) or PET blot (B) reveal very similar pattern of immunoreactivity for PHF1 antibody. Both the number of pathological structures stained and intensity are similar using the two methods. Using PET blot, and the mounting method described in the methods, even high magnification images can be obtained. PHF1 immunostained NFTs can be easily photographed at 20X (C) and even 40X (D) magnification. Another antibody, 4G8, after treatment with formic acid readily stains amyloid plaques in both routine immunohistochemistry on glass slides (E) as well as on PET blot (F).
Pretreatments of the PET blots revealed both expected and novel findings when examining amyloid deposits in the Alzheimer disease brain. As expected, pretreatment with formic acid resulted in increased antigenicity for all amyloid antibodies analyzed. However, 0.5% SDS also increased amyloid plaque antigenicity. Of note, when the harsher treatments (i.e., SDS) were applied to the PET blot often the tissue became partially detached and resulted in folds. However, the 30 min post fixation in 10% buffered formalin corrected this and allowed for more stringent antigen retrieval methods (data not shown).
DISCUSSION
In this study, we describe a revised PET blot technique using Immobilin instead of nitrocellulose, and subjected the method to a variety of antigen retrieval techniques, including formic acid, which presumably degrades β-sheet formations and results in enhanced immunostaining of amyloid plaques, and SDS, a detergent that denatures proteins and may either increase or abolish antigenicity. As seen with traditional immunohistochemistry on glass slides, formic acid enhanced the visualization of amyloid plaques compared to the untreated sections. SDS treatment also enhanced plaque and diffuse amyloid detection, while the tissue section remained intact.
PET blot is overall a sensitive technique that increases the detection of antibodies. Although it was shown to be effective under very specific conditions, the published techniques lacked clear methodological details and were therefore difficult to replicate. Schulz-Schaeffer et al presented the technique in 2000 to describe early detection of prion proteins (Schulz-Schaeffer et al., 2000). However, the paper fails to clearly describe key points in the method, such as rehydration and its consequent curling of nitrocellulose membranes and descriptions of other possible pretreatments besides proteinase K (e.g., acid hydrolysis, autoclaving, etc.). In 2002, Neumann et al. successfully applied the PET blot technique to detect α-synuclein (Neumann et al., 2002). However, the technique’s description also failed to establish a proper solution to prevent curling of membranes or adequate descriptions of imaging techniques. Basing our experimental methods from the 2000 paper of Schulz-Schaeffer et al., our group was able to establish a clear method for nitrocellulose PET blots that could flatten the membranes in preparation for visualization.
We found that the visualization of results with nitrocellulose was often inadequate because of the tendency to retain some of its curling. Other authors were able to apply PET blot to their own published experiments, but we found the metholodogies unreliable in practice (Kramer and Schulz-Schaeffer, 2007; Lezmi et al., 2006; Ligios et al., 2007). Our improved procedure is both reliable and reproducible with the substitution of nitrocellulose with PVDF-immobilon membranes. These membranes do not curl, are resistant to a variety of conditions, and after mounting have a higher resolution of immunodetection.
In our systematic comparison of the published methods pertaining to PET blot we were able to establish the versatility of our method, including improved reliability with formic acid and SDS pretreatment using well-characterized antibodies in common use for immunohistochemical methods on glass slides. Our results demonstrated a marked improvement in the visualization of amyloid plaques and neurofibrillary tangles in Alzheimer disease brain through the retrieval of several epitopes by formic acid and, unexpectedly, SDS. Treatment of traditional tissue sections with solutions of SDS often results in the tissue falling off the slide or dissolving away. These problems are overcome by the use of PET blot.
In summary, we have elucidated a simple, reproducible, and sensitive method for protein detection that allows both protein quantitation and analysis of tissue distribution. Our method is superior to routine immunohistochemistry in that tissue integrity is better maintained after harsh pretreatments for antigen retrieval, and is superior to standard histoblot methods in that fixed tissues can be examined. Moreover, the simplicity and reproducibility is a marked improvement over published PET blot methodologies. Our PET blot technique can be completed in the same timeframe as immunohistochemical procedures, and yields immunodetection at a higher resolution than nitrocellulose membranes. Further, the results are virtually indistinguishable from techniques performed on tissue sections mounted on glass slides. We expect this technique will aid in the detection of a variety of antigens in situ using procedures that require harsh pretreatments that would otherwise hamper the experiments.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (AG028679, AG031364) and the Alzheimer’s Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dickson DW. Misfolded, protease-resistant proteins in animal models and human neurodegenerative disease. J Clin Invest. 2002;110:1403–5. doi: 10.1172/JCI17164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–10. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezmi S, Bencsik A, Baron T. PET-blot analysis contributes to BSE strain recognition in C57Bl/6 mice. J Histochem Cytochem. 2006;54:1087–94. doi: 10.1369/jhc.5A6892.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligios C, Cancedda GM, Margalith I, Santucciu C, Madau L, Maestrale C, Basagni M, Saba M, Heikenwalder M. Intraepithelial and interstitial deposition of pathological prion protein in kidneys of scrapie-affected sheep. PLoS ONE. 2007;2:e859. doi: 10.1371/journal.pone.0000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–39. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, Giese A, Groschup MH, Kretzschmar HA. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am J Pathol. 2000;156:51–6. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger LA. Immunocytochemistry. Wiley; New York: 1986. [Google Scholar]
- Zhu X, Sun Z, Lee HG, Siedlak SL, Perry G, Smith MA. Distribution, levels, and activation of MEK1 in Alzheimer’s disease. J Neurochem. 2003;86:136–42. doi: 10.1046/j.1471-4159.2003.01820.x. [DOI] [PubMed] [Google Scholar]