Abstract
Seizures are associated with adverse outcome in infants with hypoxic-ischemic encephalopathy. We hypothesized that early administration of the anticonvulsant phenobarbital after cerebral hypoxia-ischemia could enhance the neuroprotective efficacy of delayed-onset hypothermia. We tested this hypothesis in a neonatal rodent model. Seven-day-old rats (n=104) underwent right carotid ligation, followed by 90 min 8%O2 exposure; 15 min later, they received injections of phenobarbital (40 mg/kg) or saline. One or 3h later, all were treated with hypothermia (30°C, 3h). Function and neuropathology were evaluated after 7 days (“early outcomes”) or 1 month (“late outcomes”). Early outcome assessment demonstrated better sensorimotor performance and less cortical damage in phenobarbital-treated groups; there were no differences between groups in which the hypothermia delay was shortened from 3h to 1h. Late outcome assessment confirmed sustained benefits of phenobarbital+hypothermia treatment; sensorimotor performance was better (persistent attenuation of contralateral forepaw placing deficits and absence of contralateral forepaw neglect); neuropathology scores were lower (medians, phenobarbital 2, saline 8.5, p<0.05), and less ipsilateral cerebral hemisphere %Damage (mean±SD, 11±17 vs. 28±22, p<0.05). These results suggest that early post-hypoxia-ischemia administration of phenobarbital may augment the neuroprotective efficacy of therapeutic hypothermia.
Introduction
The results of four clinical trials support the safety and efficacy of hypothermia to decrease death and disability in infants with hypoxic-ischemic encephalopathy (HIE) (1–4). Yet, in these trials over 40% of hypothermia-treated infants died or had poor neurologic outcomes. Thus there is a need for strategies to improve the neuroprotective efficacy of hypothermia. One possible approach is to combine hypothermia with pharmacotherapy.
In experimental models of neonatal hypoxic-ischemic brain injury, several agents enhance the neuroprotective efficacy of hypothermia; these include topiramate, an anticonvulsant (5); xenon, an anesthetic (6); and N-acetylcysteine, an anti-oxidant (7). There is minimal neonatal clinical experience with these drugs (8). Yet, many neuroactive drugs are commonly administered to encephalopathic neonates, and an important question is whether any of these drugs could augment hypothermic neuroprotection.
Anticonvulsants are an attractive group of drugs to study in combination with hypothermia. Many have neuroprotective properties in cerebral ischemia models, although it is uncertain whether these effects are attributable to seizure cessation (9). Seizures are common in encephalopathic neonates, in whom they may exacerbate hypoxic-ischemic brain injury (10, 11). In one hypothermia trial, seizures were an independent predictor of adverse outcome (12). Phenobarbital is currently the anticonvulsant used most commonly to treat neonatal seizures (13). Moreover, in a small randomized trial, treatment of infants with HIE with phenobarbital within 6 hours of birth resulted in a decrease in death or disability at three years of age (14).
In this study, we used an experimental design that enabled us to model a clinically relevant scenario in a neonatal rodent model of acute hypoxic-ischemic brain injury (5) to study the interaction of phenobarbital with post-ischemia therapeutic hypothermia. Seven-day-old (P7) rats underwent right carotid ligation followed by exposure to 8% oxygen for 90 minutes; this procedure typically elicits quantifiable sensorimotor deficits and ipsilateral forebrain damage. Fifteen minutes after the end of hypoxia exposure, they received a single dose of phenobarbital, as could be administered after a clinical resuscitation; littermate controls received saline injections. Cooling was initiated either 1 or 3 hours later, replicating the range of likely lag periods that would typically occur before initiation of therapeutic hypothermia. Functional and pathological outcomes were compared in phenobarbital and saline-treated groups after 1-week and 5-week recovery periods.
Methods
Surgery
Seven-day-old (P7) Sprague-Dawley rats (9 experiments, 11–12 pups/experiment, n=104) (Charles River, Portage, MI), anesthetized with isoflurane, underwent double ligation and division of the right common carotid artery with 6-0 silk suture (15). After 90 min recovery in a 37°C incubator, rats were placed in 500 ml jars (2–3/jar), pre-warmed and partially immersed in a 36.5°C water bath. Eight % oxygen (balance nitrogen) was introduced at a constant flow rate for 90 min. Pups recovered for 1 h (three experiments) or 3h (six experiments), prior to initiation of hypothermia (see below). All procedures were approved by the University of Michigan Committee on Use and Care of Animals, and all efforts were made to minimize the numbers used.
Drug treatment
We selected the phenobarbital dose, 40 mg/kg, used in the clinical trial of Hall et al. (14). Preliminary experiments (not shown) demonstrated that post-hypoxia-ischemia administration of phenobarbital (40 mg/kg) did not confer neuroprotection. Fifteen minutes after the end of the hypoxia period, phenobarbital (sodium salt, Sigma, St. Louis, MO, USA) dissolved in phosphate-buffered saline (SAL) was injected intraperitoneally (i.p.) (n=52), and pups were returned to incubators. Controls (n=52) received injections of an equal volume of SAL at the same time point. Gender distribution was balanced between treatment groups in each litter.
Delayed Hypothermia
In the first six experiments, animals recovered in a 37°C circulating air incubator for either 1h (n=32, three experiments), or 3h (n=36), prior to initiation of hypothermia. In three subsequent experiments (n=36), after the injections, pups were returned to the dam for 2.75h prior to hypothermia. During hypothermia, rats were placed in a circulating air incubator set at 30°C. The incubator was partitioned into compartments (1 pup/compartment) to prevent pups from huddling. After 3h in the cooling incubator pups were returned to their dam. In prior experiments, we found that this duration of mild cooling, when initiated after a 1 or 3h delay, does not confer protection (5).
To evaluate whether phenobarbital administration altered body temperature, rectal temperatures were measured (YSI thermometer 43T with probe 554, Yellow Springs, OH) at 6 time points: immediately before surgery, at the end of hypoxia, 15 min after their injections (30 min after hypoxia), immediately before hypothermia (1 or 3h after hypoxia), 30 min after the initiation of hypothermia and at the conclusion of hypothermia.
Study design
The study included four experimental groups: saline and one-hour-delayed-onset hypothermia (SAL+1hdHT), phenobarbital and one-hour-delayed-onset hypothermia (PB+1hdHT), saline and three-hour-delayed-onset hypothermia (SAL+3hdHT), and phenobarbital and three-hour-delayed-onset hypothermia (PB+3hdHT). In the first group of experiments, functional and pathology outcomes were assessed after a one-week recovery (“Early outcome”); in the second group, functional and pathology outcomes were assessed after a longer recovery (32–40 days) (“Late outcome”).
Early outcome measures
On P14 animals underwent sensorimotor testing; animals were then euthanized, and brain damage was quantified.
Bilateral lateral vibrissae stimulated forepaw placement was tested, as previously described (5); the number of successful placements in 10 trials/side was recorded by an observer unaware of treatment. Contralateral deficits in forepaw placement can be detected consistently at P14 after P7 lesioning.
Animals were euthanized and brains were removed and frozen on P14. In this model, there is substantial variability in the severity of tissue injury within and between litters, even if lesioning conditions are rigorously controlled. Under the conditions used, the expected injury ranges from ipsilateral forebrain atrophy to tissue dissolution with cyst formation.
Coronal 20-micrometer sections were stained with cresyl violet. Bilateral cross-sectional areas of striatum, neocortex, hippocampus and cerebral hemisphere were measured on regularly spaced sections from the level of the anterior genu to the posterior genu of the corpus callosum, captured and analyzed in ImageJ (http://rsbweb.nih.gov/ij/) using the dot-grid method. The callosal landmarks were chosen to provide objective landmarks for starting and stopping measurements in serial coronal sections. Bilateral volumes were estimated by multiplying the sum of areas by the distance between regularly spaced sections.
Late outcome measures
These experiments included additional sensorimotor, and cognitive testing, as well as neuropathology scoring and volumetric measurements.
Vibrissae stimulated forepaw placement was evaluated on P14, P21, P28 and P35. In older animals, it was feasible to include two additional sensorimotor tests, the vertical cylinder forepaw preference test (16) and Rotarod gait testing.
In the vertical cylinder, each animal underwent a 2-minute trial that was videotaped for slow-motion analysis. Animals were placed in a clear plastic cylinder and the number of weight-bearing contacts with the right, left or both forepaws together, while exploring the cylinder wall, was counted. Normal animals use both forepaws equally; with right hemisphere lesions, reduced left forepaw usage is observed. Right forepaw preference scores were calculated with the formula 100*(Right-Left)/(Right+Left+Both).
On the Rotarod (Economex, Columbus Instruments, Columbus, OH), after acclimation to a static cylinder (7 cm diameter, 30 sec), and then to a non-accelerating cylinder (5 rpm, 30 sec), rats underwent 3 trials, beginning at 5 rpm and accelerating at 0.1 rpm/sec, until they fell off. Time until fall was recorded for 3 trials.
These animals also underwent Water Maze place navigation and retention testing (2 m diameter pool, 28–31°C water temperature), with a 10 cm diameter platform hidden 1 cm below water surface in a fixed location, as previously described (17). They were tested on five consecutive days from P32–36 or P40–44; differences in testing ages were determined by facility availability. Each day, animals underwent 8 one-minute trials, in which they were placed facing the pool wall at 7 of 8 starting positions, with the first and final trial of each day starting at the same spot. All rats swam in the same order of start positions on each day, but the order varied among days. Animals that did not find the platform were placed on it, with 10 sec on the platform between trials. Using a computerized video-tracking and analysis system (Water 2020, HVS Image, Buckingham, UK), escape latency was measured. Twenty-four hours after the final session, a 60 sec probe trial was conducted with no platform, and time in the target quadrant was recorded.
Animals were euthanized on P39–47. Brain sections were prepared as above. Severity of neuropathology was scored by an observer unaware of treatment group assignment, using a method modified from Thoresen et al. (18). At least 15 sections/brain were evaluated, with at least 5 at the level of the striatum rostral to the hippocampus, and 5 at the level of the dorsal hippocampus. Damage was scored in 7 regions: striatum, cortex at the level of the striatum rostral to the hippocampus, thalamus, cortex at the level of dorsal hippocampus, and CA1, CA3 and dentate gyrus sub-fields of dorsal hippocampus. Scores ranged from 0–4 in increments of 0.5, with 0 representing no detectable damage and 1–4 representing <10%, 20–30%, 40–60% and >75% tissue infarction (or neuronal loss in hippocampus) respectively.
Bilateral cerebral hemisphere and regional cross-sectional areas were measured, and volumes were estimated, as above. To provide comparable measures of tissue damage among regions, in each region, for each brain, bilateral volumes were compared, and %Damage was calculated using the formula [100*(left-right)/left].
Phenobarbital Pharmacokinetics
In two additional experiments rats received injections of phenobarbital 40 mg/kg immediately after lesioning. They recovered in a 37°C incubator for 3h, underwent hypothermia for 3h (at 30°C), and then blood samples were obtained immediately or 18h later (n=12/time point, equal gender distributions). Serum phenobarbital levels were measured by fluorescence polarization immunoassay (Abbott TDx/FLx, Abbott Labs., Abbott Park, IL, USA) at the Michigan State University Diagnostic Center for Population and Animal Health.
Data Analysis
For the early outcome experiments, differences in contralateral vibrissae-stimulated forepaw placing responses and in bilateral volumes were compared by two-way ANOVA, factoring drug treatment and delay to initiation of hypothermia, and, for morphometry, by 3-way ANOVA factoring treatment, delay to hypothermia, and region. We evaluated for a main effect of treatment, and for interactions with region, gender, and litter by two-way ANOVA, with Fisher protected least significant difference (PLSD) post-hoc tests. Differences in serial body temperatures were assessed by repeated measures ANOVA.
In the late outcome experiments between-group differences in serial vibrissae scores and in Watermaze escape latencies were evaluated by repeated measures ANOVA. Between-group differences in the probe test time in target quadrant, time to fall from the Rotarod, and paw preference score in the cylinder test were evaluated by parametric and non-parametric tests. To assess between-group differences in damage scores, the summed scores and regional scores were compared by Mann-Whitney U-tests. Damage was also evaluated by morphometry, as above. Statistical analyses were conducted using Statview 5.0 (SAS Institute, Cary, NC, USA) and Systat 5.2 (Systat Inc. Evanston, IL, USA).
Results
There was no difference in mortality between the drug and vehicle groups; there were 2 deaths in the phenobarbital-treated group (both before P14), and one death in the control group (before P14). There were no differences in mean temperatures between the two treatment groups at 6 time points (see Table 1). There were no differences in left (non-hypoxic-ischemic) hemisphere regional volumes between phenobarbital-treated and control groups in any experiments (see Tables 2 and 4).
Table 1.
Serial Temperature Measurements (°C, mean ±SD) Prior to and During Hypothermia (HT) *
| Experiments with pups in 37°C incubator between HI and HT | ||||||
|---|---|---|---|---|---|---|
| Pre-surgery | End of hypoxia |
30 min. after hypoxia |
Immediately pre-HT |
30 min after start of HT |
End of HT | |
| SAL+1h dHT | 32.3 ± 0.8 | 35.1 ± 0.6 | 36.1 ± 0.4 | 36.6 ± 0.3 | 31.0 ± 0.5 | 31.7 ± 0.5 |
| PB+1h dHT | 32.4 ± 0.8 | 35.4 ± 0.5 | 36.1 ± 0.3 | 36.6 ± 0.4 | 30.8 ± 0.4 | 31.4 ± 0.6 |
| SAL+3h dHT | 33.6 ± 1.3 | 34.5 ± 0.9 | 36.1 ± 0.8 | 36.4 ± 0.8 | 29.0 ± 0.7 | 27.3 ± 1.5 |
| PB+3h dHT | 33.7 ± 1.2 | 34.5 ± 1.1 | 36.0 ± 0.9 | 36.1 ± 0.5 | 28.2 ± 0.9 | 26.7 ± 1.0 |
| Experiments with pups returned to dam between HI and HT | ||||||
| Pre-surgery | End of hypoxia |
30 min. after hypoxia |
Immediately pre-HT |
30 min after start of HT |
End of HT | |
| SAL+3h dHT | 33.1 ± 0.7 | 34.0 ± 0.8 | 33.3 ± 0.8 | 34.4 ± 1.0 | 30.6 ± 0.5 | 30.9 ± 0.9 |
| PB+3h dHT | 33.4 ± 0.5 | 34.5 ± 0.5 | 32.5 ± 1.0 | 33.6 ± 1.0 | 30.3 ± 0.5 | 30.8 ± 0.7 |
by repeated measures ANOVA there were no between-group differences in serial temperature measurements.
Table 2.
Phenobarbital plus delayed hypothermia attenuates hypoxic-ischemic damage: Early outcome regional brain volumes
| Cortex | Striatum | Hippocampus | |||||
|---|---|---|---|---|---|---|---|
| n | Left | Right | Left | Right | Left | Right | |
| SAL+1h dHT | 16 | 62 ± 10 | 27 ± 25 | 23 ± 2 | 14 ± 7 | 9 ± 4 | 5 ± 5 |
| PB+1h dHT * | 16 | 65 ± 12 | 42 ± 26 † | 22 ± 4 | 17 ± 7 | 10 ± 4 | 6 ± 5 |
| SAL+3h dHT | 17 | 68 ± 12 | 37 ± 25 | 23 ± 2 | 14 ± 7 | 10 ± 4 | 6 ± 4 |
| PB+3h dHT * | 17 | 67 ± 12 | 50 ± 24 † | 22 ± 4 | 17 ± 8 | 10 ± 4 | 8 ± 6 |
| Total | |||||||
| SAL+dHT | 33 | 64 ± 11 | 32 ± 25 | 23 ± 3 | 14 ± 7 | 10 ± 4 | 5 ± 5 |
| PB+dHT * | 33 | 66 ± 12 | 46 ± 25 † | 23 ± 3 | 17 ± 7 | 10 ± 4 | 7 ± 5 |
Values represent left- and right-hemisphere volumes (mm3, mean±SD).
p<0.05 Main effect of phenobarbital treatment by 3-way ANOVA, factoring treatment, regional volume and delay. By post-hoc tests, delay to initiation of cooling (1 h vs. 3 h) did not affect outcome.
Fisher PLSD post-hoc tests (p<0.05).
Table 4.
Phenobarbital plus delayed hypothermia: Late outcome regional brain volumes
| Cortex | Striatum | Hippocampus | Hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Left | Right | Left | Right | Left | Right | Left | Right | |
| SAL+3h dHT | 18 | 86 ± 21 | 62 ± 29 | 36 ± 8 | 23 ± 11 | 8 ± 3 | 5 ± 2 | 215 ± 52 | 155 ± 62 |
| PB+3h dHT | 17 | 82 ± 17 | 72 ± 20 | 35 ± 8 | 30 ± 9 | 6 ± 2 | 5 ± 2 | 202 ± 42 | 178 ± 50 |
Values represent left- and right-sided volumes (mm3, mean±SD).
There was no between-group difference in right-sided volume by 2 way ANOVA factoring treatment and region (p=0.062), nor was there a difference in right hemisphere volumes.
Early outcomes
Sensorimotor testing. All animals demonstrated normal right forepaw responses. Combination treatment with phenobarbital+delayed hypothermia (1 or 3h delay) reduced the contralateral forepaw placing deficit. There were more successes in the phenobarbital+delayed hypothermia group (median 10/10, interquartile range (IQR) 1.25) compared to the saline+hypothermia group (median 7/10, IQR 4.5, p<0.001, Mann-Whitney). There were no effects or interactions of duration of delay to hypothermia (see Figure 1) or gender on scores.
Figure 1. Phenobarbital + delayed hypothermia attenuates early forepaw placing deficit.
Postnatal-day-7 (P7) rats (n=68) underwent unilateral cerebral hypoxia-ischemia and then received i.p. injections of phenobarbital 40 mg/kg or an equivalent volume of saline, recovered in a 37°C incubator for 1h (n=32) or 3h (n=36), then were cooled for 3h (30°C incubator). Lateral vibrissae-stimulated forepaw placing (10 trials/side) was evaluated on P14. Saline+delayed hypothermia (dHT)-treated animals had contralateral placing deficits; this deficit was attenuated in the group treated with phenobarbital prior to delayed HT (PB+dHT). Results were the same whether the delay to initiation of HT was 3h or 1h (*p<0.005 and †p<0.05, Mann-Whitney, vs. corresponding saline+dHT controls).
Phenobarbital+hypothermia treated rats had less right cerebral hemisphere damage than controls (Table 2). Overall, right hemisphere regional volumes were larger compared to the saline+hypothermia group (3-way ANOVA factoring treatment, region and hypothermia delay); on post-hoc tests to compare values in each region, only right cortex volumes differed (p<0.05, Fisher PLSD). The time delay to initiation of hypothermia and gender had no effect on regional volumes, and there were no significant interactions.
Late outcomes
Weekly vibrissae-stimulated forepaw placing testing from P14 to P35 revealed a persistent attenuation of the contralateral deficit in the phenobarbital+hypothermia group (see Figure 2, p<0.0001, repeated measures ANOVA). The contralateral placing deficit progressively resolved in the saline+hypothermia group (p<0.005 for interaction between group and testing age). This observation, suggestive of compensation for the deficit over time in the controls, led us to evaluate sensorimotor function with a complementary forepaw preference test, the cylinder test, in older animals.
Figure 2. Persistent attenuation of contralateral forepaw placing deficit.
P7 rats (n=36) underwent unilateral cerebral hypoxia-ischemia and then received injections of saline (SAL+3hdHT, solid bars) or phenobarbital 40 mg/kg (PB+3hdHT, hatched bars), with normothermia for 3h, then 3h hypothermia (30°C incubator). Rats underwent bilateral vibrissae-stimulated forepaw placing testing (10 trials/side) weekly from P14 to P35. The phenobarbital-treated group had a sustained advantage over the controls for several weeks (*p<0.0001, repeated-measures ANOVA). There was a significant interaction (p<0.005) between group and testing age; performance of the SAL+3hdHT controls converged towards the PB+3hdHT group. Values are means ±standard deviations.
In the cylinder test, the saline+hypothermia controls exhibited a right forepaw preference (i.e. relative left forepaw neglect) whereas in the phenobarbital+delayed hypothermia group, there was no asymmetry [mean±SD forepaw preference score (%): SAL+3hdHT 16.5±14.5 vs. PB+3hdHT −0.8±19, p=0.005, t-test].
On the Rotarod, there was no difference in performance between groups [mean±SD sum of latencies to fall (sec): SAL+3hdHT 199±108; PB+3hdHT 246±120].
Testing of spatial learning and memory in the Watermaze place navigation task demonstrated that performance in both groups improved over 5 days. There were no differences between groups in daily mean escape latency, or in the 24-hour probe test, with the platform removed, in time spent in the target quadrant (see Table 3).
Table 3.
Watermaze testing performance
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Probe (Day 6) |
||
|---|---|---|---|---|---|---|---|
| n | latency* | latency† | latency† | latency† | latency† | quad%‡ | |
| SAL+3h dHT | 18 | 51 ± 6 | 45 ± 9 | 42 ± 12 | 40 ± 13 | 35 ± 14 | 35 ± 16 |
| PB+3h dHT | 17 | 51 ± 6 | 45 ± 10 | 38 ± 11 | 35 ± 14 | 32 ± 13 | 38 ± 19 |
Mean escape latency (sec., ± SD) to reach the platform, of 8 trials/day/rat, maximum 60 sec.
By repeated measures ANOVA there was no between-group difference in escape latency, however rats in both groups exhibited a decrease in mean escape latency over time (p<0.0001 for testing day), i.e. both groups exhibited evidence of learning.
Mean (±SD) percent of total swim time spent in virtual quadrant that formerly contained platform. No between-group difference was detected.
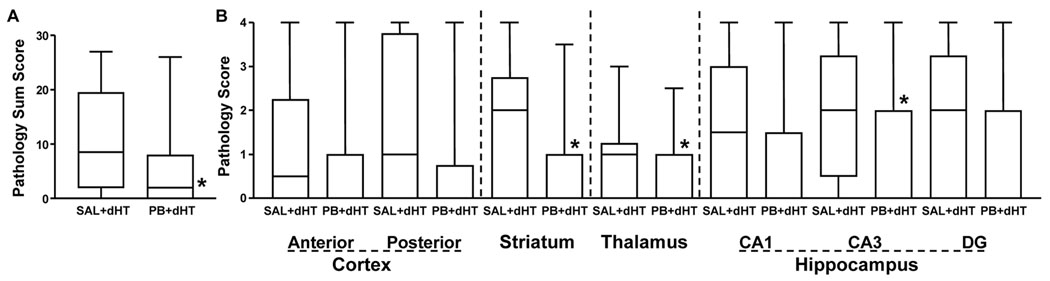
Neuropathology was scored in 7 regions. In both groups there was substantial variation in the total scores, yet overall the phenobarbital+hypothermia group total scores/brain were lower (p<0.05, Mann-Whitney; see Figure 3). Comparison of scores in each of the regions demonstrated similar trends with substantial variation in each group; there were significant reductions in the scores in the phenobarbital+hypothermia group in striatum, thalamus and hippocampal CA3 (p<0.05, Mann-Whitney).
Figure 3. Phenobarbital potentiates hypothermic neuroprotection: late outcome.
Injury severity was scored (0–4) in 7 brain regions, with substantial variation in scores in both groups. Total scores, providing an overall measure of damage severity, were lower in the phenobarbital-treated group (PB+dHT) (p<0.05, Mann-Whitney, panel A). Panel B includes scores in each region; anterior cortex at the level of striatum, posterior cortex at the level of hippocampus, striatum, thalamus, and hippocampal CA1, CA3 and dentate gyrus (DG). Data in box plots are: median (horizontal bar), IQR (25th–75th percentile, box) and range (whiskers). Regional scores were significantly lower in the PB+dHT group in striatum, thalamus and hippocampus CA3 (Mann-Whitney, p <0.05).
Analysis of right hemisphere regional volumes demonstrated similar but not statistically significant trends (p=0.062, 2-way ANOVA factoring treatment and regional volume, see Table 4). To take into account possible variation in brain size attributable to differences among litters and ages at euthanasia, we calculated hemisphere %Damage values. Overall, %Damage values were reduced in the phenobarbital+3h delayed hypothermia group, compared to the group treated with saline+hypothermia (mean±SD hemisphere %Damage: SAL+3hdHT 28±22, PB+3hdHT 11±17, p<0.05, t-test). Attenuation of %Damage in the phenobarbital+hypothermia group was confirmed by 2-way ANOVA factoring treatment and region (p<0.001).
The mean±SD phenobarbital concentrations at 6 and 24h after a 40 mg/kg injection, in post-hypoxic-ischemic rats treated with delayed-onset hypothermia, were 36±2 mcg/ml and 21±4 mcg/ml, respectively. The calculated half-life of phenobarbital was 23 hours (19).
Discussion
Phenobarbital remains the first line anticonvulsant in neonatology clinical practice (13). Nonetheless, concerns persist about potential adverse effects. The experimental data that demonstrated detrimental effects of phenobarbital in immature rodent brain have generally been obtained in normal animals (20). In contrast, we sought to determine whether beneficial or adverse effects of phenobarbital administration predominated in the most widely used animal model of asphyxial brain injury, in which all investigators report considerable, but poorly understood, variation in pathology (21, 22). This variability parallels clinical experience; neonates with similar insults have widely varying outcome. Since therapeutic hypothermia is now commonly implemented clinically, and many of these infants have seizures (23), we were particularly interested in assessing the combination intervention of phenobarbital and cooling.
In asphyxiated neonates, there is commonly a delay before initiation of therapeutic hypothermia. We developed a treatment protocol for the P7 rat hypoxia-ischemia model that incorporated this lag period in conjunction with preceding drug administration, as could be implemented post-resuscitation (5). With this protocol, we previously found that administration of topiramate, within 15 min after an acute hypoxic-ischemic insult, extended the therapeutic window for initiation of effective hypothermic neuroprotection up to 3 hours. In this study, we sought to determine whether phenobarbital could exert similar effects.
In a small randomized trial, treatment of term infants with hypoxic-ischemic encephalopathy with phenobarbital within 6 hours of birth resulted in a decrease in death or disability at three years of age (14), and this dose was selected. Serum phenobarbital levels at 6 hours after drug administration were consistent with previous reports in P7 rats (20), and were consistent with levels that would be attained in human neonates after a 40 mg/kg loading dose (24). This phenobarbital dose was not associated with any discernible adverse effects. There were no differences in mortality or in post-ischemic core temperatures.
Initial evaluation of sensorimotor function and neuropathology in animals treated with phenobarbital and delayed-onset cooling indicated that the efficacy of neuroprotection was less pronounced than attained with topiramate. For this reason, we also evaluated a second treatment protocol – with onset of cooling at one hour after hypoxia-ischemia (which could be feasible clinically e.g. by initiating cooling during transport), and trends were similar with both protocols. Therefore, we selected the three hour delay, which is more clinically relevant for most infants with HIE who undergo cooling, for the subsequent studies. Treatment with phenobarbital and delayed onset hypothermia conferred sustained improvements in sensorimotor function and attenuation of brain damage in animals assessed after a four-five week recovery period.
In asphyxiated neonates there is high risk for neuromotor deficit in survivors. In this neonatal model, several reproducible quantifiable measures of sensorimotor function have been utilized. Strengths of vibrissae-stimulated forepaw placing include that asymmetric function can be readily discerned, that the testing is feasible as early as P14, and that performance does not rely on motivation or exploratory behavior. All animals, at all ages tested, demonstrated normal responses in the right forepaw. In the phenobarbital+hypothermia group, better sensorimotor performance on the left side (contralateral to the lesion) was evident at P14, and the benefit was sustained. Of interest, performance in the control hypothermia-only group improved, and the gap in function between the two groups narrowed over time. Yet, at the final time-point evaluated, the cylinder test provided complementary evidence of persistent left forepaw sensorimotor deficits in controls, whereas there was no asymmetry in the phenobarbital+delayed hypothermia group.
However, no difference was discerned in performance on the Rotarod. We could not determine whether this reflected selection of too late a time-point for testing so that all animals substantially recovered function, low sensitivity of this test to a modest lateralized deficit, and/or modest effect size.
In the phenobarbital-treated group, performance in the Watermaze test was the same as in the control group. We did not compare cognitive performance with age-matched normal controls, but we were able to demonstrate that early single-dose phenobarbital administration did not have deleterious effects, in comparison with lesioned controls. When normal rats are treated in the first 2 weeks of life with repeated doses of phenobarbital, in adulthood (6 months age) these animals have spatial learning deficits (25). It is possible that moderate hypothermia might block drug-induced neurodegeneration in the immature brain; thus it is attractive to speculate that hypothermia might limit potential deleterious functional effects of phenobarbital. This complex question is beyond the scope of the current study.
In the early outcome experiments, there was a beneficial effect of phenobarbital plus hypothermia on preservation of right-hemisphere regional volumes, with the primary effect in the cortex. In the late outcome experiments, the beneficial effects of phenobarbital treatment combined with delayed hypothermia were discerned with application of a semi-quantitative injury scoring. Initial comparison of right hemisphere regional volume measurements did not confirm this treatment effect. We speculated that variation in brain sizes contributed to this finding, and also calculated %Damage values in each brain, to take into account differences in brain size. Using the %Damage formula, which compares volumes bilaterally in each brain, we confirmed that there was less damage in the phenobarbital plus delayed hypothermia group, although there was substantial variation in the range of neuroprotection.
In a previous study, in a similar experimental paradigm that incorporated sensorimotor and pathology outcomes, we found that treatment with a single early post-hypoxic-ischemic dose of topiramate in combination with brief delayed-onset hypothermia had robust, sustained neuroprotective effects (5); phenobarbital was less effective in the same study design. Since seizure burden cannot be reliably quantified in P7 rats with surface electroencephalogram, we could not assess whether differences in anticonvulsant efficacy contributed to the more potent neuroprotective effects of topiramate. However, in view of subsequent studies that demonstrated that topiramate treatment alone confers neuroprotection with more prolonged treatment (4 doses over 48h), it is more likely that greater neuroprotective efficacy is attributable to intrinsic AMPA-antagonist properties (26). Nonetheless, together these results provide support for the hypothesis that early seizures can contribute to the evolution of hypoxic-ischemic brain injury, and that anticonvulsant therapy could be an effective adjunct to therapeutic hypothermia.
It is also important to acknowledge that phenobarbital has multiple potential modes of action in addition to anticonvulsant effects that could contribute to neuroprotection in this setting, including reduced cerebral metabolic demand, antioxidant effects and decreased cerebral edema (27–30). It is also intriguing to consider that recent insights regarding the impact of maturational changes in neuronal chloride transporter expression on GABA receptor function may provide strategies that could improve the neuroprotective efficacy of phenobarbital in the neonate. Specifically, blocking the neonatal neuronal chloride transporter with bumetanide can augment the inhibitory activity of GABA agonists such as phenobarbital. Bumetanide enhances the anticonvulsant action of phenobarbital in neonatal rats (31). Whether this combination treatment could also result in improved neuroprotective efficacy in conjunction with cooling is an interesting question for future research.
Acknowledgments
Financial Support: NIH grants HD 060348 to [F.S.] and NS 045812 to [J.B.], by an American Academy of Pediatrics Neonatal Resuscitation Program Research Grant and by The GorgEffen Gift Fund.
Abbreviations
- HIE
Hypoxic-ischemic encephalopathy
- IQR
Interquartile range
- P7 etc.
Postnatal-day-7, postnatal day 14, etc.
- PB+1hdHT
Phenobarbital+one-hour-delayed-onset hypothermia
- PB+3hdHT
Phenobarbital+three-hour-delayed-onset hypothermia
- SAL
Saline
- SAL+1hdHT
Saline+one-hour-delayed-onset hypothermia
- SAL+3hdHT
Saline+three-hour-delayed-onset hypothermia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 3.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–1465. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 6.Ma D, Hossain M, Chow A, Arshad M, Battson RM, Sanders RD, Mehmet H, Edwards AD, Franks NP, Maze M. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol. 2005;58:182–193. doi: 10.1002/ana.20547. [DOI] [PubMed] [Google Scholar]
- 7.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2006;59:684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 8.Walls L, Baker CF, Sarkar S. Acetaminophen-induced hepatic failure with encephalopathy in a newborn. J Perinatol. 2007;27:133–135. doi: 10.1038/sj.jp.7211641. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Ann Neurol. 2003;53:693–702. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- 10.Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 11.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 13.Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero DM, Ment LR, Silverstein FS. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Hall RT, Hall FK, Daily DK. High-dose phenobarbital therapy in term newborn infants with severe perinatal asphyxia: a randomized, prospective study with three-year follow-up. J Pediatr. 1998;132:345–348. doi: 10.1016/s0022-3476(98)70458-5. [DOI] [PubMed] [Google Scholar]
- 15.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 16.Grow JL, Liu YQ, Barks JD. Can lateralizing sensorimotor deficits be identified after neonatal cerebral hypoxia-ischemia in rats? Dev Neurosci. 2003;25:394–402. doi: 10.1159/000075665. [DOI] [PubMed] [Google Scholar]
- 17.Chou I-C, Trakht T, Signori C, Smith J, Felt BT, Vazquez DM, Barks JD. Behavioral/environmental intervention improves learning after cerebral hypoxia-ischemia in rats. Stroke. 2001;32:2192–2197. doi: 10.1161/hs0901.095656. [DOI] [PubMed] [Google Scholar]
- 18.Thoresen M, Bågenholm R, Loberg EM, Apriccna F. The stress of being restrained reduces brain damage after a hypoxic-ischaemic insult in the 7-day-old rat. Neuroreport. 1996;7:481–484. doi: 10.1097/00001756-199601310-00025. [DOI] [PubMed] [Google Scholar]
- 19.Roberts RJ. Drug therapy in infants: Pharmacologic principles and clinical experience. Philadelphia: W. B. Saunders; 1984. Pharmacokinetics: Basic principles and clinical applications; pp. 13–24. [Google Scholar]
- 20.Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northington FJ. Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR J. 2006;47:32–38. doi: 10.1093/ilar.47.1.32. [DOI] [PubMed] [Google Scholar]
- 22.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Yap V, Engel M, Takenouchi T, Perlman JM. Seizures are common in term infants undergoing head cooling. Pediatr Neurol. 2009;41:327–331. doi: 10.1016/j.pediatrneurol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Donn SM, Grasela TH, Goldstein GW. Safety of a higher loading dose of phenobarbital in the term newborn. Pediatrics. 1985;75:1061–1064. [PubMed] [Google Scholar]
- 25.Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 26.Noh MR, Kim SK, Sun W, Park SK, Choi HC, Lim JH, Kim IH, Kim HJ, Kim H, Eun BL. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Exp Neurol. 2006;201:470–478. doi: 10.1016/j.expneurol.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson L. The influence of barbiturate anaesthesia upon the energy state and upon acid-base parameters of the brain in arterial hypotension and in asphyxia. Acta Neurol Scand. 1971;47:233–253. doi: 10.1111/j.1600-0404.1971.tb07479.x. [DOI] [PubMed] [Google Scholar]
- 28.Crane PD, Braun LD, Cornford EM, Cremer JE, Glass JM, Oldendorf WH. Dose dependent reduction of glucose utilization by pentobarbital in rat brain. Stroke. 1978;9:12–18. doi: 10.1161/01.str.9.1.12. [DOI] [PubMed] [Google Scholar]
- 29.Singh D, Kumar P, Majumdar S, Narang A. Effect of phenobarbital on free radicals in neonates with hypoxic ischemic encephalopathy--a randomized controlled trial. J Perinat Med. 2004;32:278–281. doi: 10.1515/JPM.2004.052. [DOI] [PubMed] [Google Scholar]
- 30.Smith AL, Marque JJ. Anesthetics and cerebral edema. Anesthesiology. 1976;45:64–72. doi: 10.1097/00000542-197607000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]