Abstract
The genetic contribution of antigen-presenting molecules and the environmental ignition of an antigen-specific immune attack to pancreatic β cells define autoimmune diabetes. We focused here on generating an antigen-specific model of autoimmune diabetes in humanized double-transgenic mice carrying antigen-presenting HLA-DQ8 diabetes-linked haplotype and expressing human autoantigen GAD65 in pancreatic β cells using a relatively diabetes-susceptible strain of mice. Double-transgenic (DQ8-GAD65) mice and controls were immunized with cDNA encoding human GAD65 in adenoviral vectors and monitored for glucose intolerance and diabetes. Human-GAD65 immunization induced insulitis, glucose intolerance and diabetes in double-transgenic mice, while controls were insulitis free and glucose tolerant. Glucose intolerance ten week post-immunization was followed by diabetes later on in most animals. Destructive insulitis characterized by inflammation and apoptosis correlated with the diabetes outcome. Humoral immune responses to hGAD65 were sustained in mice with diabetes while transient in non-responders. Insulitis was massive in mice with diabetes while mild in non-responders by the end of the study. Our results show for the first time the occurrence of antigen-specific induced insulitis, impaired glucose homeostasis and diabetes after immunization with a clinically relevant, human autoantigen in the context of HLA-DQ8 diabetes-susceptibility transgenes and human GAD65 expression in β cells. This animal model will facilitate studies of mechanisms of disease involved in development of autoimmunity to GAD65 in the context of HLA-DQ8. Furthermore, this model would be ideal for testing therapeutic strategies aimed at preventing human β cell loss and/or restoring function in the setting of autoimmune diabetes.
Keywords: Mouse model, autoimmune diabetes, type 1, MHC class II, GAD, GAD65
Introduction
Selective autoimmune destruction of pancreatic β cells, in a process that can span several years, results in glucose intolerance and type 1 diabetes (T1D) when the majority of β cells have been depleted. This process requires the genetic contribution of certain antigen-presenting molecules, and the environmental ignition of an antigen(s)-specific immune attack. Genetic contribution is provided by, among others, major histocompatibility complex (MHC) molecules of which HLA DQ8 is believed to be the dominant susceptibility tissue type in humans [1]. Environmental ignition is yet to be defined. However the earliest link to the initial event, the immune response against β cells, has been studied in detail. Two major autoantigens in human disease have been identified using sera immunoprecipitation experiments. One of them, the smaller isoform of the gamma amino butyric acid (GABA)-synthesizing enzyme, glutamic acid decarboxylase (GAD65) is recognized by 70–80% of diabetes patients’ sera [2]. The other one, identified as tyrosine phosphatase insulinoma-associated protein 2 (IA-2) [3], is recognized by 60–70% of patients’ sera [4]. More than 90% of patients have antibodies to one or both of these antigens in the pre-diabetes phase. A highly homologous isoform of GAD65, GAD67, is recognized by virtue of cross-reacting antibodies only in 11–18% of patients, but is not an independent autoantigen in human diabetes [5]. Whereas mouse β cells predominantly express GAD67, human β cells only express the GAD65 isoform [6].
Although MHC-class II genes are critical determinants of diabetes genetic susceptibility, it is presentation of primary target antigen(s) in the context of these genes that favors the development of disease [7]. An animal model of T1D, in which primary human beta cell autoantigen(s) are presented to effector cells in the context of human MHC class II diabetes-susceptibility genes, would be highly desirable for studies of molecular mechanisms of disease and for development of immune-modulation therapies applicable to man. Therefore, we generated transgenic mice that carry human diabetes-susceptibility by expressing human MHC-class II (DQ8) in antigen-presenting cells and human β-cell autoantigen GAD65 in β cells. The expression of hGAD65 in these mice is restricted to pancreatic β cells and the level of that expression is similar to endogenous expression of GAD65 in human β cells (as opposed to negligible expression in wild type mice; [6]). Immunization of these double transgenics (DQ8-GAD65) with cDNA encoding hGAD65 resulted in induction of autoimmunity and lymphocytic homing to islets of Langerhans [8]. However, diabetes did not develop in these C57BL/6 double transgenics. Aside from experiencing transient fluctuation of fasting blood glucose, mice were glucose tolerant. Signs of mitotic activity in some islets suggested that β-cell neogenesis/proliferation was present and could account for the glycemic control observed [8]. Hence, we searched for mouse-strain variations with compromised β cell neogenesis/proliferation expressed as relative insulin deficiency. BTBR mice have lower whole-pancreas insulin content and demonstrate a striking loss of islet tissue without developing diabetes [9]. Possible mechanisms that may account for this difference include deficient β cell neogenesis/proliferation, and/or increased apoptosis [9,10]. Therefore, we introduced the same transgenes we had incorporated in the C57BL/6 strain into the BTBR genetic background to convert a somewhat diabetes resistant animal model a more diabetes prone one. We report here the development of a new animal model of human diabetes where antigen-specific insulitis progresses to autoimmune diabetes.
Materials and Methods
Mice
HLA-DQA1*0301/DQB1*0302 (DQ8) transgenic, murine MHC-class II molecule–deficient (mII-) C57BL/6 mice (kindly provided by Dr. Wen, Yale, New Haven, CT; [11]) and RIP7-hGAD65 line 1 transgenic C57BL/6 mice (kindly provided by Dr. Baekkeskov, UCSF, San Francisco, CA; [12]) were crossed to BTBR (Jackson Laboratories, Bar Harbor, ME). DQ8 and RIP7-hGAD65 homozygosity was determined as previously described [8]. All mice used in this study were >N6 crosses. All animal protocols were approved by the University of Wisconsin and the Veterans Affair animal research committees.
Adenoviral constructs for immunization
Adenoviral constructs were made using the Gateway system (Invitrogen, Carlsbad, CA). hGAD65 was excised from pCI-hGAD65 with EcoRI and NotI (Invitrogen) and subcloned into the same sites in pENTR for pAD-CMV cloning and the sequence confirmed. pAD-CMVhGAD65 were propagated in AAV-293 cells (Stratagene, Garden Grove, CA), crude viral lysate purified by CsCl density gradient centrifugation and viral particle concentration determined by measuring the absorbance at 260 nm. All viruses used in this study were from the same preparation and were stored in aliquots at −80°C. hGAD65 expression from pAD-CMVhGAD65 was confirmed by Western blot. Immunizations were performed 2 times at 2 week intervals. Mice were intraperitonealy (ip) injected with 100 μl of PBS containing 1011 particles of pAD-CMVhGAD65 [13,14]. Immunization experiments were repeated twice.
Histology and Immunohistochemistry
Pancreata were snap-frozen in Tissue Tek (Miles Laboratories, Elkhart, IN). 5 μm-thick sections were stained with hematoxylin/eosin and biotinylated mAb directed against CD8 (Serotec, Raleigh, NC) followed by streptavidin-FITC conjugate and TO-PRO 3 (Molecular Probes, Eugene, OR) as counter-stain. Apoptotic cells were identified in pancreatic sections using transferase-mediated dUTP nick-end labeling (TUNEL) (Roche Applied Science, Indianapolis, IN). Then tissue slides were washed in PBS and double stained with anti-GAD65 antibody [8]. Nuclei were also counterstained with TO-PRO 3. All images were acquired by confocal microscopy.
Antibody measurement
Anti-GAD65 antibodies from mice serum samples were quantified with an ELISA kit (Kronus, Boise, ID) according with manufacturer instructions. Validation of the ELISA results were done with a parallel immunoprecipitation assay previously standardized in our lab [8]. Control-mouse serum samples show less than 5 U/ml of anti-GAD65 antibody concentration. At least two independent experiments in duplicated wells were performed, and average values for each sample was obtained.
Glucose tolerance test (GTT)
All experimental and control animals were subjected to GTT in at least 2 time points before and after immunization. Mice were fasted overnight for 14 hours followed by ip injection of glucose 2 g. Blood samples were obtained from tail vein at 0, 20, 40, 60, 80, 120 and 180 minutes after injection, and glucose level was assessed using a glucometer.
Serum insulin measurements
Blood was collected into microfuge tubes from tail vein using heparinized capillary tubes during the GTT. The blood samples were allowed to clot at room temperature (10 minutes) and then placed on ice. Samples were spun at 4°C and 3000 rpm for 20 minutes, and sera was transferred to new microfuge tubes. Sera was stored −20°C until analyzed. Serum insulin concentrations were assayed using a mouse insulin ELISA kit (Crystal Chem Inc, Downers Grove, IL) and mouse insulin used as standard.
Statistical analysis
Two tailed probability of the chi-square distribution was used to compare results.
Results
Glucose intolerance and diabetes correlates with insulitis
A colony of homozygous double-transgenic mice carrying DQ8 [11] and RIP7-hGAD65 [12] was established as previously done [8] on the BTBR background. Spontaneous diabetes was not observed in mice carrying one or both transgenes during a 6-month follow up period. We tested the ability of hGAD65-adenovirus immunization to generate insulitis and diabetes in groups of double-transgenic mice (DQ8-RIP7hGAD65+/+), single-transgenic mice (DQ8+/+ and RIP7hGAD65+/+) and non-transgenic BTBR/C57BL/6 controls. 6–8 week old mice in each group received 2 intraperitoneal injections (2 week apart) of 1011 hGAD65-adenovirus particles. Adenoviral vectors are known to induce innate immune responses similar to those observed post-viral infections and to enhance the development of Th1 CD4 and CD8 (+) cells [14].
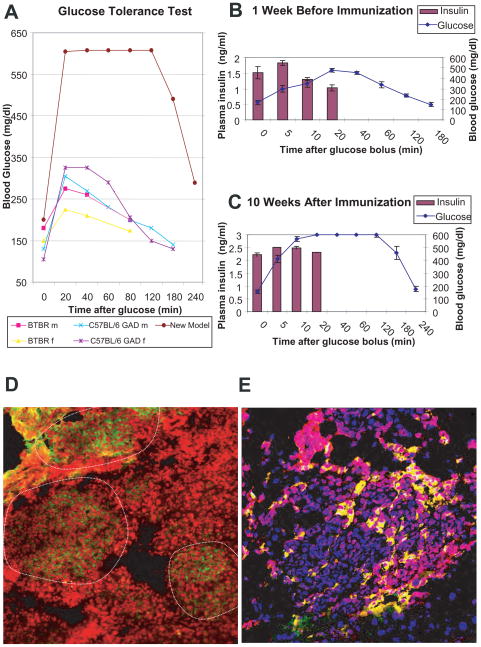
Animals were followed for 24 weeks post-immunization. 8 weeks after immunization, hGAD65-adenovirus immunized double-transgenic mice had statistically significantly higher blood glucoses (225 ± 52 mg/dl) than single transgenics (148 ± 13 mg/dl; p<0.001) and non-transgenic controls (137 ± 22 mg/dl; p<0.001). All animals were tested for glucose tolerance before and at 10 weeks post-immunization. After an overnight fasting, 2 g glucose bolus was given intraperitoneally to animals at time 0 and blood drawn immediately pre-bolus and at 5; 10; 20; 40; 60; 120; 180; minutes later for glucose measurement. Insulin was measured also at pre-bolus and 5; 10 and 20 minutes. Post-immunization glucose tolerance test of representative transgenics in different backgrounds and non-transgenic controls is shown (Fig. 1A). The results clearly show that our new model is glucose intolerant when compared with controls. Moreover, at 10-week post-immunization all immunized double transgenics had post-glucose bolus insulin-surge not capable of controlling glycemia (Fig. 1B & 1C). As in humans, our antigen-specific diabetes model shows evidence of impaired fasting glucose and glucose intolerance before the onset of diabetes.
Figure 1.
GTT, insulin response, insulitis and apoptosis. A. Panel shows glucose tolerance test at 10 week post-immunization of representative mice in different control background strains. B. Panel shows glucose and insulin values during a glucose tolerance test of 20, 6–8 week old double-transgenic (DQ8/RIP7hGAD65) BTBR mice. C. Panel shows glucose and insulin values from the same 19 of 20 mice (one animal died before 2nd immunization) during a similar glucose tolerance test performed 10 weeks post-immunization. Please note that while insulin was readily detectable in post-immunized animals, they were not capable of sustaining normal glycemia post-glucose challenge (glucose intolerant). D. Several markers were used to characterize the lymphocytic infiltration in islets of Langerhans in animals with diabetes, confocal resolution of anti-CD8 (green, nuclei red, 20X) is shown, islets perimeters delineated. E. Confocal images of islet co-stained with ant-GAD65 (red) and TUNEL (green), nuclei (blue, 40X) is shown. Note that green and red co-localize as yellow.
By week 16 post immunization animals manifested signs of diabetes. Animals were consequently euthanized upon development of diabetes as diagnosed by fasting glucose ≥250 mg/dl for two consecutive days. Staining of pancreatic sections of diabetic mice with different lymphocytic markers was consistent with a targeted immune response. Showed in Fig. 1D, the presence of CD8-positive T cells in the intra-islet infiltrates exemplifies the presence of an active inflammatory process. Co-staining for GAD65 and TUNEL confirmed the presence of an apoptotic process targeted to β cells (Fig. 1E). As previously showed [8], antigen-specific insulitis clinically manifested as glucose intolerance and diabetes in this new model.
Diabetes develops in some but not all double transgenics
Although most double-transgenic mice had glucose intolerance at 10 week post-immunization, not all developed overt diabetes during this 24 week study.
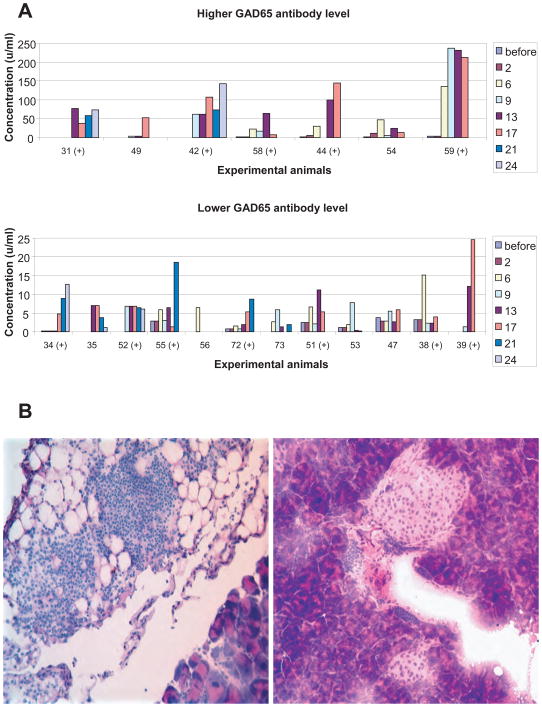
To monitor the immune response, blood samples were periodically obtained and analyzed for GAD65 autoantibodies (Fig. 2A). Antibodies to hGAD65 were detected post-immunization in most animals. This demonstrates lack of tolerance to the GAD65 antigen in non-transgenic controls (as expected) and also in GAD65 transgenics (despite the presence of the GAD65 transgene). Antibody levels fluctuated in frequency and amplitude but were sustained and correlated with destructive insulitis in mice with diabetes (Fig. 2A and B left). For mice that did not develop diabetes, antibody levels generally declined or became undetectable by the end of the study and the lymphocytic infiltration was localized mostly to the peri-islet areas (Fig. 2A and B right).
Figure 2.
Humoral immunity and insulitis. A. Panel shows antibody levels for individual double-transgenic mice overtime. While the humoral response to hGAD65 was sustained in mice that developed diabetes (+), other mice develop immune responses that either faded away or manifested later on. B. Frozen sections of pancreas of two different representative animals stained with hematoxylin and eosin is shown. Left, euthanized at week 17 after development of diabetes; right, euthanized at week 24 at the end of the study. Note the more aggressive characteristic of the lymphocytic infiltration in left (almost no islet tissue preserved), versus right (only peri-insular infiltration).
Discussion
We have previously generated mice that express high levels of human GAD65 in β cells and at the same time, have their endogenous mouse MHC-class II replaced by the human HLA-DQ8 diabetes-susceptibility locus [8]. After introducing genetic susceptibility for T1D, immunization with hGAD65 cDNA, produced a strong cellular immune response that homed to the islets of Langerhans [8]. Despite the presence of destructive insulitis, diabetes did not develop in these C57BL/6 transgenics [8]. We postulated that the observe insulitis was insufficient to affect glucose homeostasis may be due to β-cell proliferative/neogenic capacity [8]. Conversely, BTBR is a strain of mice with a relative deficiency in β cell functionality with lower whole-pancreas insulin content [9,10]. BTBR mice bear the black-and-tan at allele of agouti gene, associated with a tan coat color over the ventrum; this allele is dominant to the non-agouti a allele carried by C57BL/6 mice [15,16]. A mouse locus, which is strongly associated with high fasting plasma insulin neighbors the agouti gene and has important effects on diabetes syndromes in experimental systems [17]. BTBR mice however do not develop spontaneous diabetes. Hence, we developed our new model in this genetic background.
Our double-transgenic mice developed impaired fasting blood glucose, glucose intolerance and diabetes when immunized with adenoviral hGAD65. Quantification of antibody response showed that instead of retracting after the immunization stimulus finished, the response clearly progressed as animals developed diabetes. The characteristics of the infiltration with the simultaneous presence CD8 cells [8,18] and apoptosis of β cells indicate the presence of an active and specific immune attack [8,19].
We believe the availability of our animal model is of utmost importance for studying immune-modulation of disease especially in the setting of hGAD65 immunization. Recently, clinical trials have shown that immunization with hGAD65 protein in alum adjuvant may contribute to the preservation of residual insulin secretion in patients with recent-onset T1D [20]. As opposed to our DNA immunization, protein immunization seems to preserve β cell function. Considering that ultimately DNA is likely translated to protein in our model, it is intriguing that the two methods of immunization radically differ in their outcome (induction of diabetes versus preservation of β cell function).
Conclusion
All together, our results show for the first time, the occurrence of antigen-specific diabetes after immunization with a clinically relevant human autoantigen, in the context of human MHC-class II diabetes-susceptibility transgenes, while the autoantigen is being expressed in the target cell and in appropriate quantities. Since the nature of the immune attack is specific to a human autoantigen (GAD65), presented in the context of human diabetes-susceptibility genes (DQ8), we believe this new animal model provides an ideal tool in which to test therapeutic modalities appropriate for treatment of human autoimmune diabetes.
Acknowledgments
This work was funded by NIH and VA grants (JCJ). This work was presented in part at The Endocrine Society Annual Meeting, Washington, DC 2009. (oral communication, bench to bedside session).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1997;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 2.Baekkeskov S, Kanaani J, Jaume JC, Kash S. Does GAD have a unique role in triggering IDDM? J Autoimmun. 2000;15:279–286. doi: 10.1006/jaut.2000.0443. [DOI] [PubMed] [Google Scholar]
- 3.Christie MR, Vohra G, Champagne P, Daneman D, Delovitch TL. Distinct antibody specificities to a 64-kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med. 1990;172:789–794. doi: 10.1084/jem.172.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabin DU, Pleasic SM, Shapiro JA, Yoo-Warren H, Oles J, Hicks JM, Goldstein DE, Rae PM. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994;152:3183–3188. [PubMed] [Google Scholar]
- 5.Hagopian WA, Michelsen B, Karlsen AE, Larsen F, Moody A, Grubin CE, Rowe R, Petersen J, McEvoy R, Lernmark A. Autoantibodies in IDDM primarily recognize the 65,000-M(r) rather than the 67,000-M(r) isoform of glutamic acid decarboxylase. Diabetes. 1993;42:631–636. doi: 10.2337/diab.42.4.631. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Richter W, Aanstoot HJ, Shi Y, Fu Q, Rajotte R, Warnock G, Baekkeskov S. Differential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic islets. Diabetes. 1993;42:1799–1808. doi: 10.2337/diab.42.12.1799. [DOI] [PubMed] [Google Scholar]
- 7.Jaume JC. Endocrine autoimmunity. In: Gardner DG, Shoback DM, editors. Greenspan’s Basic & Clinical Endocrinology. 8. New York: McGraw-Hill Medical; 2007. pp. 59–79. [Google Scholar]
- 8.Elagin RB, Balijepalli S, Diacovo MJ, Baekkeskov S, Jaume JC. Homing of GAD65 specific autoimmunity and development of insulitis requires expression of both DQ8 and human GAD65 in transgenic mice. J Autoimmun. 2009;33:50–57. doi: 10.1016/j.jaut.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoehr JP, Nadler ST, Schueler KL, Rabaglia ME, Yandell BS, Metz SA, Attie AD. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes. 2000;49:1946–1954. doi: 10.2337/diabetes.49.11.1946. [DOI] [PubMed] [Google Scholar]
- 10.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008;18:706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen L, Wong FS, Burkly L, Altieri M, Mamalaki C, Kioussis D, Flavell RA, Sherwin RS. Induction of insulitis by glutamic acid decarboxylase peptide–specific and HLA-DQ8–restricted CD4+ T cells from human DQ transgenic mice. J Clin Invest. 1998;102:947–957. doi: 10.1172/JCI2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Kanaani J, Menard-Rose V, Ma YH, Chang PY, Hanahan D, Tobin A, Grodsky G, Baekkeskov S. Increased expression of GAD65 and GABA in pancreatic beta-cells impairs first-phase insulin secretion. Am J Physiol Endocrinol Metab. 2000;279:684–694. doi: 10.1152/ajpendo.2000.279.3.E684. [DOI] [PubMed] [Google Scholar]
- 13.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 14.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn LC. A fifth allelomorph in the agouti series of the house mouse. Proc Natl Acad Sci USA. 1928;14:816–819. doi: 10.1073/pnas.14.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 17.Mehrabian M, Wen P-Z, Fisher J, Davis RC, Lusis AJ. Genetic loci controlling body fat, lipoprotein metabolism, and insulin levels in a multifactorial mouse model. J Clin Invest. 1998;101:2485–2496. doi: 10.1172/JCI1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 19.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 20.Ludvigsson J, Faresj M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]