Abstract
The biological basis of variability in histological progression of nonalcoholic fatty liver disease (NAFLD) is unknown. Dehydroepiandrosterone(DHEA) is the most abundant steroid hormone and has been shown to influence sensitivity to oxidative stress, insulin sensitivity, and expression of peroxisome proliferator-activated receptor alpha and procollagen messenger RNA. Our aim was to determine whether more histologically advanced NAFLD is associated with low circulating levels of DHEA. Serum samples were obtained prospectively at the time of liver biopsy in 439 patients with NAFLD (78 in an initial and 361 in validation cohorts) and in controls with cholestatic liver disease (n = 44). NAFLD was characterized as mild [simple steatosis or nonalcoholic steatohepatitis (NASH) with fibrosis stage 0–2] or advanced (NASH with fibrosis stage 3–4). Serum levels of sulfated DHEA (DHEA-S) were measured by enzyme-linked immunosorbent assay. Patients with advanced NAFLD had lower plasma levels of DHEA-S than patients with mild NAFLD in both the initial (0.25 ± 0.07 versus 1.1 ± 0.09 µg/mL, P < 0.001) and validation cohorts (0.47 ± 0.06 versus 0.99 ± 0.04 µg/mL, P < 0.001). A “dose effect” of decreasing DHEA-S and incremental fibrosis stage was observed with a mean DHEA-S of 1.03 ± 0.05, 0.96 ± 0.07, 0.83 ± 0.11, 0.66 ± 0.11, and 0.35 ± 0.06 µg/mL for fibrosis stages 0, 1, 2, 3, and 4, respectively. All patients in both cohorts in the advanced NAFLD group had low DHEA-S levels, with the majority in the hypoadrenal range. The association between DHEA-S and severity of NAFLD persisted after adjusting for age. A relationship between disease/fibrosis severity and DHEA-S levels was not seen in patients with cholestatic liver diseases.
Conclusion
More advanced NAFLD, as indicated by the presence of NASH with advanced fibrosis stage, is strongly associated with low circulating DHEA-S. These data provide novel evidence for relative DHEA-S deficiency in patients with histologically advanced NASH.
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent form of liver disease in North America and is an increasingly frequent cause of cirrhosis and liver failure.1–4 Although several factors have been associated with more advanced NAFLD, the biological basis of the histological diversity of severity of NAFLD [i.e., why some patients develop simple steatosis and others develop nonalcoholic steatohepatitis (NASH) with advanced fibrosis] remains unknown. More advanced NAFLD is characterized by insulin resistance,5,6 oxidative stress,7 and advanced fibrosis. Although several relatively noninvasive parameters have been identified as predictive of more advanced fibrosis stage in patients with NAFLD,8 none has sufficient sensitivity or specificity to be of clinical utility to negate the need for liver biopsy.9
We have previously reported the specific relative underexpression of scavengers of reactive oxygen species, including superoxide dismutase, in patients with histologically advanced NASH,10 suggesting a pretranscriptional basis of susceptibility to oxidative stress in histologically advanced NAFLD. Dehydroepiandrosterone (DHEA) is a potential mediator of reactive oxygen species scavenger synthesis11 and has also been reported to augment insulin sensitivity12–15 and peroxisome proliferator activation.16,17 These observations, together with a recent report of a high frequency of NASH with rapid progression to cirrhosis in patients with panhypopituitarism,18 who are deficient in DHEA, led us to hypothesize that DHEA levels may be low in patients with more advanced NAFLD. If found to be differentially abundant across the histological spectrum of NAFLD, circulating DHEA levels might be used for the identification of patients at risk for the development of NASH with advanced fibrosis and provide a novel therapeutic target for NASH.
Patients and Methods
We studied the association of a broad range of parameters, including sulfated DHEA (DHEA-S), with histological severity of NAFLD. The study was conducted in 2 phases. The first phase involved a detailed analysis of a cohort of patients from a single center (Mayo Clinic, Rochester, MN)in which DHEA-S, adipokines and other parameters were measured (initial cohort). Once the initial cohort had been analyzed, confirmation of findings was sought through a focused analysis of DHEA-S levels and liver histology in a separate group of participants from 2 other academic medical centers in a blinded fashion for validation of the observations made in the initial cohort.
Initial Cohort
A total of 122 participants at a single center were studied in the initial cohort, including:
Patients with liver biopsy demonstrating simple steatosis or NASH with fibrosis stage 0–2 (mild group, n = 53).
Patients with liver biopsy demonstrating NASH with more advanced fibrosis (advanced group, n = 25).
Subjects with other liver diseases (primary biliary cirrhosis and primary sclerosing cholangitis, n = 44).
Validation Cohort
Following the initial analysis, a validation cohort was also studied from 2 separate academic medical centers (Indiana University, n = 96; University of California, San Francisco, n = 265). The validation cohort comprised a total of 361 patients with NAFLD, grouped using the same criteria as having either mild or advanced NAFLD.
The study was approved by the Mayo Institutional Review Board and all participants gave written informed consent for participation in medical research. Participants with NAFLD (mild and advanced NAFLD groups) in the initial cohort were recruited from patients seen at the Hepatobiliary Clinic at Mayo Clinic, Rochester, MN, between January 2002 and December 2004. Serum samples were obtained prospectively at the same time as liver biopsies from participating patients who were scheduled to undergo liver biopsy for investigation of suspected liver disease (NAFLD or cholestatic liver disease). Patients with NAFLD who had secondary causes of steatohepatitis (drugs), and patients with other etiologies of chronic liver disease (excessive alcohol consumption, viral hepatitis (B, C), hemochromatosis, Wilson’s disease, drug-induced liver disease, and alpha 1-antitrypsin deficiency) were excluded from the study. Patients with cholestatic liver disease were recruited as disease controls.
Participants with NAFLD (mild and advanced NAFLD groups) in the validation cohort were recruited from patients seen at the Hepatobiliary Clinics at Indiana University and from the Hepatobiliary Clinic and patients undergoing bariatric surgery at the University of California, San Francisco.
Primary biliary cirrhosis was diagnosed based upon an antimitochondrial antibody of ≤1:40, biochemical abnormalities, and a liver biopsy demonstrating histological features of primary biliary cirrhosis (portal hepatitis with granulomatous destruction of the bile ducts). Stage 2 is characterized by periportal hepatitis and bile duct proliferation. The presence of fibrous septa or bridging necrosis is classified as stage 3 and cirrhosis as stage 4. The presence of fibrosis or cirrhosis does indicate a worse prognosis than if no fibrosis is seen on biopsy. Primary sclerosing cholangitis was diagnosed by the findings of multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography, biochemical abnormalities, and the presence of histological finding of fibrous obliteration of small bile ducts.
A detailed alcohol consumption history was obtained from all participants. All participants drank less than 20 g/day of ethanol. Diagnosis of diabetes mellitus was based on the American Diabetes Association or World Health Organization criteria.
Liver Histology in NAFLD
Experienced liver pathologists who were blinded to the clinical data reviewed the liver biopsy specimens. Patients with NAFLD were divided into mild [simple steatosis and NASH (defined using the Brunt criteria19) with fibrosis stage 0–2] and advanced (NASH with fibrosis stage 3–4). The fibrosis staging system was classified as follows: stage 0 = no fibrosis, stage 1 = zone 3 predominant pericellular fibrosis, stage 2 = zone 3 fibrosis plus periportal fibrosis, stage 3 = bridging fibrosis, stage 4 = cirrhosis. Scoring of steatosis included both microvesicular and macrovesicular steatosis and was based on the percentage area of the parenchyma that was fatty. Mild was considered less than 33%, moderate 33%–65%, and advanced if greater than 66% was observed.
Adipokines and DHEA and Glucose
Leptin, resistin, adiponectin, C-reactive protein, and DHEA-S concentrations were measured by radioimmunoassays using a commercial kit (Diagnostic Systems Laboratories, Webster, TX) in blood drawn at the time of the liver biopsies. All analyses were carried out in blinded fashion.
Plasma glucose concentrations were measured enzymatically with an auto-analyzer (Beckman Instruments, Fullerton, CA).
Patient Characteristics
Patient age, sex, body mass index (BMI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST/ALT ratio, total bilirubin, fasting glucose, triglycerides, cholesterol, creatinine, hematological profile, detailed alcohol consumption history, smoking history, and diagnosis of diabetes were all recorded.
Statistical Methods
Continuous variables were summarized with means and standard deviations, while categorical variables were summarized with frequencies and percentages. Continuous data were analyzed using analysis of variance if normally distributed, or the nonparametric Wilcoxon rank sums test if nonnormally distributed. The chi-squared test or Fisher’s exact test was used for comparison of frequency data where appropriate. Linear and logistic regression models were fit to correlate variables, including DHEA-S and adipokine levels with clinical variables, and between patients with mild versus advanced NAFLD. The area under the receiver operating characteristics (AUROC) curve was calculated for every model. Subsequently, the accuracy of DHEA-S levels in separating patients with mild and advanced NAFLD was determined by calculating sensitivity, specificity, and positive and negative predictive values.
Results
Initial Cohort
Clinical, biochemical, and histological stage of disease in participants with NAFLD and controls with chronic cholestatic liver disease are summarized in Table 1. Components of the metabolic syndrome including increased BMI (and thus overweight and obesity), type 2 diabetes, hypertension, and low high-density lipoprotein cholesterol were significantly more common in patients with NAFLD than in participants with cholestatic liver disease. Participants with cholestatic liver disease had significantly higher levels of aminotransferases, bilirubin, alkaline phosphatase, and total cholesterol, and lower albumin levels when compared with NAFLD patients.
Table 1.
Clinical and Laboratory Data of Patients with NAFLD and Controls with Chronic Cholestatic Liver Disease
| Characteristic | NAFLD (n = 78) | Controls (n = 44) | P Value |
|---|---|---|---|
| Age (years) | 50.0 ± 11.1 | 47.1 ± 11.7 | 0.2 |
| Sex (female/male) | 50/28 | 31/13 | 0.6 |
| Race (white) | 76 (97%) | 44 (100%) | 0.6 |
| BMI (kg/m2) | 33.0 ± 7.4 | 26.0 ± 6.2 | <0.0001 |
| Overweight (BMI 25–30 kg/m2) | 22 (28%) | 10 (23%) | <0.0001 |
| Obesity (BMI >30 kg/m2) | 51 (65%) | 10 (23%) | <0.0001 |
| Type 2 diabetes mellitus | 32 (41%) | 5 (14%) | 0.003 |
| Hypertension | 39 (50%) | 10 (27%) | 0.03 |
| Hyperlipidemia | 57 (73%) | 27 (73%) | 1.0 |
| History of smoking | |||
| None | 52 (67%) | 31 (84%) | 0.001 |
| Ex-smoking | 26 (33%) | 3 (8%) | |
| Current smoking | - | 3 (8%) | |
| Fasting glucose (mg/dL) | 116.4 ± 41.8 | 106.5 ± 46.2 | 0.3 |
| Cholesterol (mg/dL) | 194.6 ± 43.0 | 235.1 ± 58.7 | <0.0001 |
| Triglyceride (mg/dL) | 177.7 ± 86.0 | 152.1 ± 86.5 | 0.1 |
| HDL-C (mg/dL) | 42.8 ± 10.2 | 61.8 ± 22.0 | <0.0001 |
| AST (U/L) | 60.0 ± 44.2 | 88.9 ± 62.0 | 0.008 |
| ALT (U/L) | 85.1 ± 88.0 | 181.1 ± 114.4 | 0.04 |
| AST/ALT ratio | 0.98 ± 0.97 | 0.71 ± 0.22 | 0.05 |
| Alkaline phosphatase (U/L) | 210.5 ± 103.4 | 914.3 ± 737.9 | <0.0001 |
| Total bilirubin (mg/dL) | 0.9 ± 0.5 | 2.5 ± 3.4 | 0.004 |
| Albumin (g/dL) | 4.2 ± 0.4 | 3.9 ± 0.5 | 0.001 |
| Prothrombin time (seconds) | 9.8 ± 0.7 | 10.5 ± 2.9 | 0.1 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.6 |
| Hemoglobin (g/dL) | 13.7 ± 1.3 | 13.1 ± 1.3 | 0.03 |
| Leukocyte count (cells/mL) | 5804.7 ± 1701.2 | 5908.1 ± 2451.3 | 0.8 |
| Platelet (×103/mL) | 202.2 ± 68.7 | 190.6 ± 96.6 | 0.5 |
| DHEA-S (µg/mL) | 0.8 ± 1.0 | 0.8 ± 0.7 | 0.9 |
| Fibrosis stage | |||
| Stage 0 | 21 (27%) | 0 (0%) | 0.002 |
| Stage 1 | 13 (17%) | 9 (20%) | |
| Stage 2 | 19 (24%) | 10 (23%) | |
| Stage 3 | 13 (17%) | 11 (25%) | |
| Stage 4 | 12 (15%) | 14 (32%) |
Data are expressed as the mean ± standard deviation.
Abbreviation: HDL-C, high-density lipoprotein cholesterol.
The histological characteristics of participants with NAFLD are shown in Table 2. Table 2 also summarizes the comparison between participants with mild and advanced NAFLD in the training cohort. Mild and advanced NAFLD groups had similar proportions of men and women. Participants in the advanced NAFLD group were significantly older, and were more commonly obese and diabetic compared with participants with mild NAFLD. Participants with advanced NAFLD had significantly lower levels of ALT, higher AST/ALT ratio, lower albumin and hemoglobin levels, and lower platelet counts.
Table 2.
Clinical and Laboratory Data for Initial Cohort Patients with Mild and Advanced NAFLD
| Characteristic | Mild NAFLD (n = 53) | Advanced NAFLD (n = 25) | P Value |
|---|---|---|---|
| Age (years) | 47.3 ± 11.8 | 55.9 ± 6.4 | 0.0007 |
| Sex (female/male) | 32/21 | 18/7 | 0.3 |
| Race (white) | 51 (96%) | 25 (100%) | 0.6 |
| BMI (kg/m2) | 31.8 ± 6.8 | 35.4 ± 8.2 | 0.05 |
| Obesity (BMI > 30 kg/m2) | 31 (58%) | 20 (80%) | 0.06 |
| Type 2 diabetes mellitus | 17 (32%) | 15 (60%) | 0.02 |
| Hypertension | 24 (45%) | 15 (60%) | 0.2 |
| Hyperlipidemia | 41 (77%) | 16 (64%) | 0.2 |
| History of smoking | 19 (36%) | 7 (28%) | 0.5 |
| Fasting glucose (mg/dL) | 117.1 ± 48.2 | 114.7 ± 23.9 | 0.3 |
| Cholesterol (mg/dL) | 199.8 ± 46.2 | 183.4 ± 33.1 | 0.2 |
| Triglyceride (mg/dL) | 186.2 ± 92.0 | 159.6 ± 70.1 | 0.2 |
| HDL-C (mg/dL) | 43.0 ± 10.4 | 42.2 ± 10.2 | 0.7 |
| AST (U/L) | 56.8 ± 38.3 | 67.0 ± 54.7 | 0.9 |
| ALT (U/L) | 90.9 ± 92.4 | 73.4 ± 78.6 | 0.04 |
| AST/ALT ratio | 0.8 ± 0.4 | 1.4 ± 1.5 | <0.0001 |
| Alkaline phosphatase (U/L) | 207.1 ± 112.7 | 217.6 ± 81.9 | 0.5 |
| Total bilirubin (mg/dL) | 0.8 ± 0.4 | 1.0 ± 0.7 | 0.07 |
| Albumin (g/dL) | 4.3 ± 0.4 | 4.0 ± 0.3 | 0.004 |
| Prothrombin time (seconds) | 9.7 ± 0.7 | 9.9 ± 0.7 | 0.08 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.9 |
| Hemoglobin (g/dL) | 14.0 ± 1.1 | 13.2 ± 1.5 | 0.01 |
| Leukocyte count (cells/mL) | 5942.8 ± 1760.7 | 5512.0 ± 1561.1 | 0.5 |
| Platelet (×103/ mL) | 222.6 ± 66.8 | 158.9 ± 51.2 | <0.0001 |
| DHEA-S (µg/mL) | 1.1 ± 0.09 | 0.25 ± 0.07 | <0.0001 |
| Leptin (ng/mL) | 24.5 ± 15.2 | 33.9 ± 20.6 | 0.06 |
| Adiponectin (µg/mL) | 20.3 ± 10.6 | 21.6 ± 10.5 | 0.4 |
| Resistin (ng/mL) | 1.7 ± 0.6 | 2.4 ± 0.8 | 0.0008 |
| C-reactive protein (mg/L) | 0.4 ± 0.4 | 0.7 ± 0.7 | 0.05 |
Data are expressed as the mean ± standard deviation.
Abbreviation: HDL-C, high-density lipoprotein cholesterol.
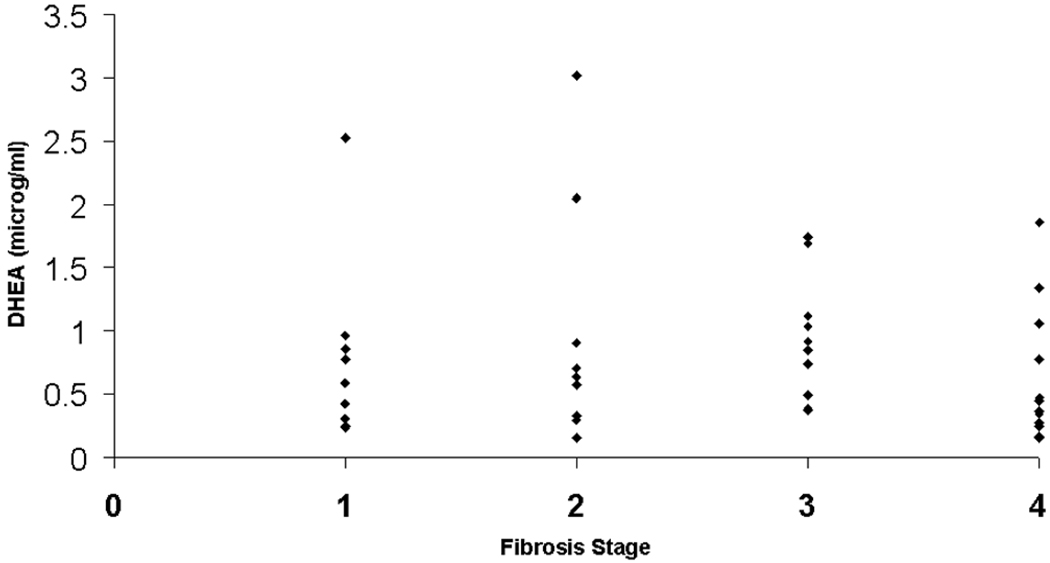
Participants in the advanced NAFLD group had lower levels of DHEA-S (1.11 ± 0.09 versus 0.25 ± 0.07 µg/mL, P<0.001), and higher levels of resistin (P=0.0008) and C-reactive protein (P = 0.05). A “dose effect” of lower DHEA-S levels and fibrosis stage was observed in patients with NAFLD, with mean DHEA-S levels decreasing with stepwise increases in fibrosis stage. Conversely, levels of DHEA-S did not correlate significantly with severity of the liver disease in participants with cholestatic liver disease (Fig. 1). Levels of DHEA-S were not significantly different between participants with NAFLD and participants with cholestatic liver disease (P = 0.9).
Fig. 1.
Variations in DHEA-S levels with fibrosis stage for participants in the control group with cholestatic liver diseases.
Mean DHEA levels were significantly lower in patients with steatohepatitis grades 2–4 than 0–1 (0.71 ± 0.19 versus 1.17 ± 0.24 µg/mL, P = 0.05).
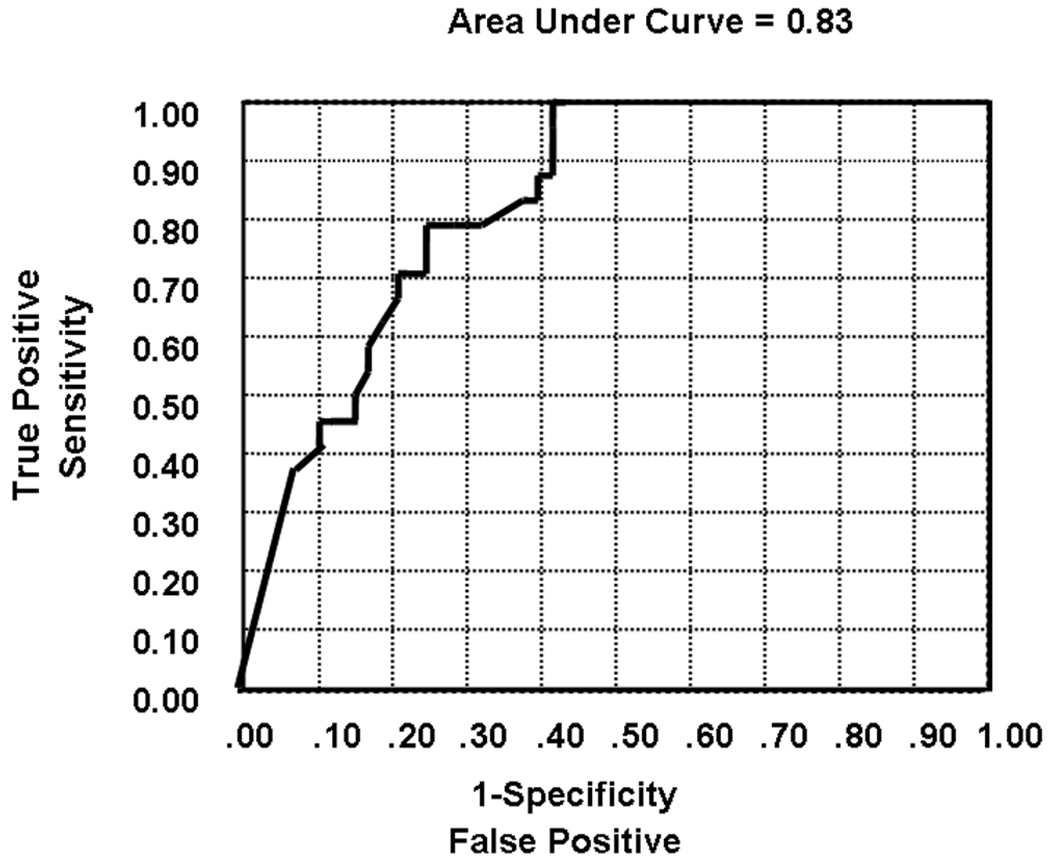
As levels of DHEA-S are different between men and women and lower in older individuals, DHEA-S levels were adjusted for age and sex. As expected, using simple linear regression, DHEA-S levels were seen to significantly correlate with age (P < 0.0001) and sex (male > female, P < 0.0001). Several multivariate logistic regression models were run in order to determine the association of DHEA levels with the presence or absence of advanced NAFLD while adjusting for the effect of age and sex. As illustrated in Table 3, the unadjusted (model 1, Fig. 2) association of DHEA levels with severity of NAFLD remained highly significant when adjusted by age (model 2) and age plus sex (model 3). The AUROC curve values of the models are shown in the last column of Table 3. The AUROC for DHEA in separating patients with and without significant fibrosis was 0.83. This clearly indicates that the biological association of DHEA levels with severity of NAFLD is independent of age and sex. Almost all of the predictivity for histological severity of NAFLD could be attributed to DHEA-S levels independent of age and sex.
Table 3.
Logistic Regression Models of the Association of NAFLD (Advanced Versus Mild) with DHEA Levels and Other Clinical Variables
| Variables | P Value | AUROC Model |
|---|---|---|
| Model 1 | ||
| DHEA | <0.0001 | 0.83 |
| Model 2 | ||
| DHEA | <0.0001 | 0.83 |
| Age | 0.7 | |
| Model 3 | ||
| DHEA | <0.0001 | 0.84 |
| Age | 0.8 | |
| Sex | 0.4 | |
| Model 4 | ||
| DHEA | <0.0001 | 0.87 |
| BMI | 0.02 | |
| Diabetes mellitus | 0.8 | |
| Hypertension | 0.5 | |
| Hypertriglyceridemia | 0.1 | |
| Low HDL-C cholesterol | 0.2 | |
| Model 5 | ||
| DHEA | <0.0001 | 0.88 |
| Age | 0.4 | |
| Sex | 0.9 | |
| BMI | 0.02 | |
| Diabetes mellitus | 0.9 | |
| Hypertension | 0.4 | |
| Hypertriglyceridemia | 0.1 | |
| Low HDL-C | 0.2 |
Abbreviation: HDL-C, high-density lipoprotein cholesterol.
Fig. 2.
The AUROC curve for DHEA in separating patients with and without significant fibrosis was 0.83.
In order to determine if the significant association of DHEA levels and more advanced NAFLD was simply because patients with more advanced NAFLD had more metabolic abnormalities, 2 additional multivariate logistic regression models were run (models 4 and 5 in Table 3). As illustrated in Table 3, DHEA levels remained highly significantly associated with advanced NAFLD after adjusted by all components of the insulin resistance syndrome (model 4) and when age and sex were added to those components as well (model 5). BMI was the only variable independently associated with severity of NAFLD in models 4 and 5 but to a much lower degree than DHEA levels. Waist-to-hip ratio, with which DHEA levels can vary, was not recorded in this study.
Validation Cohort
The validation cohort comprised a total of 361 patients (230 female) with NAFLD. A total of 327 patients met criteria for mild NAFLD and 36 met criteria for advanced NAFLD.
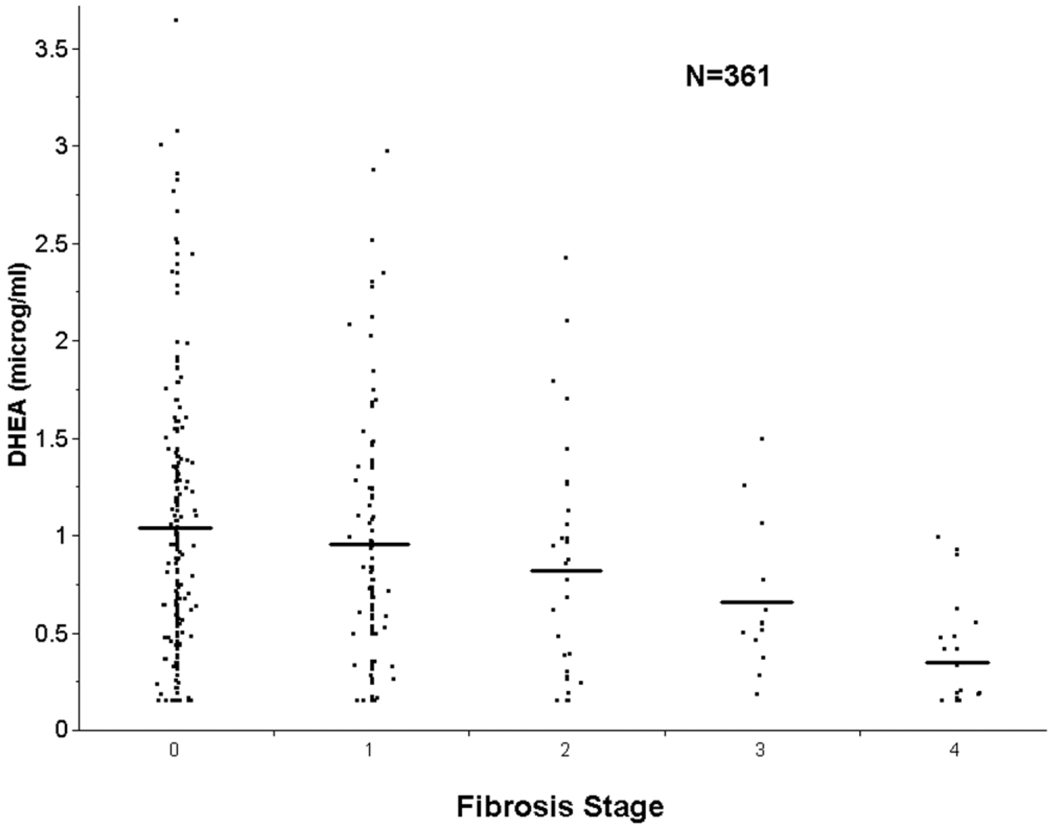
As in the training cohort, mild and advanced NAFLD groups had similar proportions of men and women. Participants in the advanced NAFLD group were older than participants with mild NAFLD (51.3 ± 1.8 versus 44.5 ± 0.6 years, P < 0.0001). As in the training cohort, levels of DHEA-S strongly correlated with histological severity of disease. Participants with advanced NAFLD had significantly lower levels of DHEA-S compared with participants with mild NAFLD (0.47 ± 0.06 versus 0.99 ± 0.07 µg/mL, P < 0.0001). To examine more carefully for a simple age effect, we compared DHEA-S levels in patients with more advanced NAFLD, age 40–65 years (mean age 53.1 ± 0.9 years), with patients with mild NAFLD, age 40–65 years (mean age 49.8 ± 0.5 years, P value not significant). DHEA-S levels were still significantly lower in patients with advanced NAFLD than in patients with mild NAFLD (0.43 ± 0.05 versus 0.85 ± 0.04 µg/mL, P < 0.0005). As in the initial cohort, a “dose effect” of lower DHEA-S and advanced fibrosis was observed with a mean DHEA-S of 1.03 ± 0.05, 0.96 ± 0.07, 0.83 ± 0.11, 0.66 ± 0.11, and 0.35 ± 0.06 µg/mL for fibrosis stages 0, 1, 2, 3, and 4, respectively. All patients with advanced NAFLD had physiologically low levels of DHEA-S, with the majority in the hypoadrenal range.
Variation of fibrosis stage with age for NAFLD in the validation cohort is shown in Fig. 3. The sensitivity of a DHEA-S value of >1.0 µg/mL for the presence of more advanced NAFLD was 95% (57/60) and specificity was 58%.
Fig. 3.
Variation in DHEA-S levels with fibrosis stage for participants with NAFLD in the validation cohort. Mean DHEA-S levels are indicated by horizontal lines. A “dose effect” of lower DHEA-S and advanced fibrosis was observed, with a mean DHEA-S of 1.03 ± 0.05, 0.96 ± 0.07, 0.83 ± 0.11, 0.66 ± 0.11, and 0.35 ± 0.06 µg/mL for fibrosis stages 0, 1, 2, 3, and 4, respectively.
Discussion
Although NAFLD is associated with many abnormalities, especially the metabolic syndrome and insulin resistance/hyperinsulinemia occurring in the setting of obesity, little is known about the physiological basis of the histological diversity of NAFLD. Specifically, why patients with similar metabolic profiles, BMI, age, and sex can develop disparate histological findings is not known. Any explanation of the variable histological progression of NAFLD is likely to involve the differential abundance of an effector of susceptibility to insulin resistance, oxidative stress, and/or mediators of hepatic fibrosis. The reported metabolic and intracellular effects of DHEA-S led to the hypothesis that relative deficiency of DHEA-S may play a role in the histological progression of NAFLD. The principal finding of this study is that circulating DHEA-S levels are strongly associated with the most important feature of histologically advanced NAFLD—steatohepatitis in association with advanced fibrosis stage. Indeed, all patients with advanced NAFLD in this study were seen to have low circulating levels of DHEA-S.
DHEA, and its interchangeable sulfated form, DHEA-S, is the most abundant circulating steroid hormone and is produced primarily by the zona reticularis of the adrenal cortex in response to adrenocorticotropic hormone. DHEA and DHEA-S levels peak at approximately age 25 years and decrease progressively thereafter, falling to 5% of peak levels by the ninth decade.20 The levels of DHEA seen in patients with advanced NAFLD in our current study were similar to those reported in patients with hypoadrenalism and old age.15 Because DHEA levels decline with age, it is important to consider whether the lower DHEA levels observed in patients with advanced NAFLD in our study were simply a surrogate of older age. Although, as expected, the mean age was greater in patients with advanced NAFLD when compared with those with mild NAFLD, probably reflecting greater duration of disease, age was less predictive of severity of NAFLD than DHEA-S levels and did not exhibit the “dose effect” seen with DHEA-S levels. Similarly, when patients with more advanced NAFLD were compared with age-matched patients with mild NAFLD, the differences in circulating DHEA levels persisted. We did not measure the health of the adrenocorticol axis in this study. Circulating DHEA-S levels are, however, thought to be chiefly regulated by 17,20-lyase activity,21 and changes in circulating DHEA-S occur independently of changes in levels of other adrenal hormones, such as cortisol.22 It was also possible that the variation in DHEA levels was confounded by one or more of the parameters of insulin resistance present in patients with more advanced NAFLD. Based on models 4 and 5 (Table 3), it is highly likely that the association of DHEA levels and severity of NAFLD found in our patients was not confounded by any of the metabolic abnormalities that compose the insulin resistance syndrome, nor by the degree of insulin resistance. However, we recognize that further studies are necessary to determine the relationship of DHEA, NAFLD severity, and degree of insulin resistance using specific measurement of insulin sensitivity such as HOMA, the glucose clamp, and so forth. Because DHEA-S levels were strongly associated with more histologically advanced NAFLD, other variables that were associated with more histologically advanced NAFLD were also associated with low circulating DHEA-S levels.
It was also important to consider whether low levels of DHEA-S might occur as a result of chronic liver disease in general versus a specific phenomenon of histologically more advanced NAFLD. In order to determine the specificity of low DHEA-S levels as an association with more histologically advanced NAFLD, we measured DHEA-S levels in a contemporary cohort of patients with cholestatic liver disease—primary biliary cirrhosis and primary sclerosing cholangitis. DHEA-S levels were not significantly predictive of severity of disease in patients with cholestatic liver disease (Fig. 3). Low levels of DHEA-S in our study participants with more advanced NAFLD thus could not be attributed to a nonspecific effect of liver disease in general. It is possible that impaired enterohepatic circulation, as occurs in patients with stage 3/4 cholestatic liver disease, alters DHEA-S metabolism. Circulating DHEA-S levels are not known to be affected by alterations in enterohepatic circulation. Adrenal hormones, such as estriol, are lowered secondary to increased fecal excretion in the context of impaired enterohepatic circulation, while DHEA-S metabolism is preserved.23 If impaired enterohepatic circulation, as occurs in more advanced stages of cholestatic liver disease, were to have an effect on DHEA-S levels, it is likely that the effect would be, if anything, to decrease circulating DHEA-S levels. Such an effect was not seen in our control subjects.
The striking difference in DHEA-S levels seen in participants with advanced NAFLD when compared with participants with mild NAFLD in our study raises the obvious mechanistic question of how DHEA-S deficiency might mediate histological severity of NAFLD. A role of DHEA-S deficiency in histological progression of NAFLD is likely to involve effects on insulin sensitivity, hepatic susceptibility to oxidative stress injury, and/or stimulation of fibrosis. Although the literature concerning the role of DHEA in mediating insulin sensitivity in humans is conflicting,12–15,24–27 evidence generated from randomized controlled trials suggests that DHEA enhances insulin sensitivity.13,14 In a recent randomized, placebo-controlled crossover study in hypoadrenal subjects, who had circulating DHEA-S levels at baseline that were similar to those seen in our study subjects with advanced NAFLD, DHEA supplementation significantly lowered fasting insulin and glucose levels and increased insulin sensitivity, as measured by hyperinsulinemic glucose clamp.15 In a larger randomized, placebo-controlled study, DHEA therapy induced significant decreases in visceral and subcutaneous fat and improved indices of insulin sensitivity.28 DHEA stimulation of insulin sensitivity is thought to involve the reported activation of peroxisome proliferator-activated receptor alpha.16,17 DHEA is also known to inhibit the local amplification of glucocorticoids by 11-beta-hydroxysteroid dehydrogenase in adipose tissue, thereby decreasing cortisol activity,29 and to increase serum levels of IGF-1.24,26,30,31 Intrahepatic expression of IGF-1 has been shown to be decreased in histologically advanced NAFLD.10 Conversely, low DHEA levels, as seen our study subjects with advanced NAFLD, are associated with hyperglycemia and insulin resistance.32 Although mediation of insulin action might explain a potential role of circulating DHEA-S levels in effecting histological severity of NAFLD, indices of insulin sensitivity are at best only partly predictive of histology in NAFLD and are weakly predictive of fibrosis stage, suggesting an alternative or additional mechanism.
A mechanistic role of DHEA-S in histological progression of NAFLD to NASH with advanced fibrosis, irrespective of any effect on insulin sensitivity, would be likely to involve increased susceptibility to hepatocellular oxidative injury and/or a profibrotic effect. Increased oxidative stress with subsequent mitochondrial dysfunction are features of both animal models of steatohepatitis7 and humans with NAFLD.6,33–35 DHEA has been shown to exert a protective effect in hepatocytes against oxidative injury by decreasing malondialdehyde concentration and increasing superoxide dismutase activity11 and total glutathione concentrations in animal models of oxidative stress.36,37 Lowered hepatic messenger RNA for superoxide dismutase has been demonstrated in patients with histologically advanced NAFLD.10 In healthy humans, DHEA and its metabolite, 7-alpha-hydroxy-DHEA, reduce tissue susceptibility to oxidation of both lipids and proteins.38 DHEA has also been shown to directly inhibit procollagen type I synthesis at the transcriptional level in vivo and in vitro in animals,39 and in vitro in human fibroblasts.39 Finally, DHEA might exert a hepatoprotective effect in NAFLD through attenuation of reactive oxygen species–mediated release of cytokines by Kupffer cells, adipose tissue, and hepatocytes.40–42 DHEA inhibits tumor necrosis factor alpha–induced nuclear factor kappa B–dependent transcription in human hepatocytes in a time-dependent and dose-dependent manner.39 Similarly, DHEA-treated animals demonstrate reduced levels of the proinflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, and interleukin-6 and the anti-inflammatory cytokine interleukin-10.43,44 There are thus several potential mechanisms for DHEA deficiency to promote histological progression in NAFLD. Of particular interest is that panhypopituitarism is associated with a high frequency of NASH with rapid progression to cirrhosis. 18 Although many hormones are replaced in patients with panhypopituitarism, DHEA—the levels of which are typically undetectable—is not.
We are aware of no other reports of an association of DHEA (or DHEA-S) abundance and histological severity of NAFLD and only 1 published report of DHEA metabolism in NAFLD, although a protective effect of DHEA was reported in an orotic acid–induced animal model of fatty liver disease.37
The association of low circulating levels of DHEA-S with more histologically advanced NAFLD has several important implications. Firstly, despite an association of metabolic abnormalities, including indices of insulin resistance, with NAFLD, grading and staging of NAFLD requires a liver biopsy. Our data suggest that patients with DHEA-S levels greater than 1.0 µg/mL are highly unlikely to have advanced NAFLD (3/439 patients, sensitivity 95% and specificity 58%). Perhaps most significantly, DHEA deficiency (patients with advanced NAFLD had levels of DHEA-S associated with hypoadrenalism) presents an appealing new therapeutic target for the treatment and prevention of NAFLD. DHEA has substantial use and safety data in humans and, in contrast to peroxisome proliferator-activated receptor alpha agonists,45–47 is not adipogenic. The basis of differential circulating DHEA-S levels in patients with histologically advanced NAFLD should be the subject of further investigation.
Our study has several important limitations. First, because we did not capture a specific index of insulin resistance, such as HOMA (although we did capture data on all clinical components of the insulin resistance /metabolic syndrome), further studies are necessary to determine the relationship of DHEA, NAFLD severity, and the degree of insulin resistance. Secondly, the validation cohort consisted of 2 groups of patients that were analyzed together but comprised very different populations: morbid obese patients undergoing bariatric surgery (University of California, San Francisco) and suspected NAFLD patients seen at the hepatobiliary clinic (Indiana University). It is possible that the role of obesity and other metabolic comorbidities was different between these groups.
In conclusion, we have found that patients with more advanced NAFLD have low circulating levels of DHEA-S. These data provide novel evidence for relative DHEA deficiency in patients with histologically advanced NASH.
Acknowledgments
Supported by Public Health Service Grants NIDDK RO1 DK069757-01 and GCRC RR00585.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristics
- BMI
body mass index
- DHEA
dehydroepiandrosterone
- DHEA-S
sulfated dehydroepiandrosterone
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. HEPATOLOGY. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 2.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Poterucha JJ, Porayko MK, Dickson ER, Steers JL, Wiesner RH. Recurrence of nonalcoholic steatohepatitis following liver transplantation. Transplantation. 1996;62:1802–1805. doi: 10.1097/00007890-199612270-00021. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, et al. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. HEPATOLOGY. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. Erratum in: HEPATOLOGY 2003;38:536. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira CP, Costa Gayotto LC, Tatai C, Della Bina BI, Janiszewski M, Lima ES, et al. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mol Med. 2002;6:399–406. doi: 10.1111/j.1582-4934.2002.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. HEPATOLOGY. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 10.Sreekumar R, Rosado B, Rasmussen D, Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. HEPATOLOGY. 2003;38:244–251. doi: 10.1053/jhep.2003.50290. [DOI] [PubMed] [Google Scholar]
- 11.Bednarek-Tupikowska G, Gosk I, Szuba A, Bohdanowicz-Pawlak A, Kosowska B, Bidzinska B, et al. Influence of dehydroepiandrosterone on platelet aggregation, superoxide dismutase activity and serum lipid peroxide concentrations in rabbits with induced hypercholesterolemia. Med Sci Monit. 2000;6:40–45. [PubMed] [Google Scholar]
- 12.Lasco A, Frisina N, Morabito N, Gaudio A, Morini E, Trifiletti A, et al. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol. 2001;145:457–461. doi: 10.1530/eje.0.1450457. [DOI] [PubMed] [Google Scholar]
- 13.Jakubowicz D, Beer N, Rengifo R. Effect of dehydroepiandrosterone on cyclic-guanosine monophosphate in men of advancing age. Ann N Y Acad Sci. 1995;774:312–315. doi: 10.1111/j.1749-6632.1995.tb17395.x-i1. [DOI] [PubMed] [Google Scholar]
- 14.Kawano M. Complement regulatory proteins and autoimmunity. Arch Immunol Ther Exp (Warsz) 2000;48:367–372. [PubMed] [Google Scholar]
- 15.Dhatariya K, Bigelow ML, Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes. 2005;54:765–769. doi: 10.2337/diabetes.54.3.765. [DOI] [PubMed] [Google Scholar]
- 16.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 17.Peters JM, Zhou YC, Ram PA, Lee SS, Gonzalez FJ, Waxman DJ. Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol Pharmacol. 1996;50:67–74. [PubMed] [Google Scholar]
- 18.Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. HEPATOLOGY. 2004;39:909–914. doi: 10.1002/hep.20140. [DOI] [PubMed] [Google Scholar]
- 19.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 21.Labrie F, Belanger A, Simard J, Van LT, Labrie C. DHEA and peripheral androgen and estrogen formation: intracinology. Ann N Y Acad Sci. 1995;774:16–28. doi: 10.1111/j.1749-6632.1995.tb17369.x. [DOI] [PubMed] [Google Scholar]
- 22.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. TrendsEndocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 23.Osathanondh R, Fencl MD, Schiff I, Himmel M, Tulchinsky D. Reduced urinary and serum total estriol levels in pregnancies after colectomy. Obstet Gynecol. 1979;53:664–667. [PubMed] [Google Scholar]
- 24.Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol. 1998;49:421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- 25.Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. Erratum in: J Clin Endocrinol Metab 1995;80:2799. [DOI] [PubMed] [Google Scholar]
- 26.Vogiatzi MG, Boeck MA, Vlachopapadopoulou E, el Rashid R, New MI. Dehydroepiandrosterone in morbidly obese adolescents: effects on weight, body composition, lipids, and insulin resistance. Metabolism. 1996;45:1011–1015. doi: 10.1016/s0026-0495(96)90272-3. [DOI] [PubMed] [Google Scholar]
- 27.Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab. 1990;71:696–704. doi: 10.1210/jcem-71-3-696. [DOI] [PubMed] [Google Scholar]
- 28.Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292:2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- 29.Gu S, Ripp SL, Prough RA, Geoghegan TE. Dehydroepiandrosterone affects the expression of multiple genes in rat liver including 11 beta-hydroxysteroid dehydrogenase type 1: a cDNA array analysis. Mol Pharmacol. 2003;63:722–731. doi: 10.1124/mol.63.3.722. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42:247–253. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- 31.Nestler JE, Barlascini CO, Clore JN, Blackard WG. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988;66:57–61. doi: 10.1210/jcem-66-1-57. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–811. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 33.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 34.Rashid A, Wu TC, Huang CC, Chen CH, Lin HZ, Yang SQ, et al. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. HEPATOLOGY. 1999;29:1131–1138. doi: 10.1002/hep.510290428. [DOI] [PubMed] [Google Scholar]
- 35.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 36.Celebi F, Yilmaz I, Aksoy H, Gumus M, Taysi S, Oren D. Dehydroepiandrosterone prevents oxidative injury in obstructive jaundice in rats. J Int Med Res. 2004;32:400–405. doi: 10.1177/147323000403200408. [DOI] [PubMed] [Google Scholar]
- 37.Goto H, Yamashita S, Makita T. Preventive effects of dehydroepiandrosterone acetate on the fatty liver induced by orotic acid in male rats. Exp Anim. 1998;47:257–260. doi: 10.1538/expanim.47.257. [DOI] [PubMed] [Google Scholar]
- 38.Mastrocola R, Aragno M, Betteto S, Brignardello E, Catalano MG, Danni O, et al. Pro-oxidant effect of dehydroepiandrosterone in rats is mediated by PPAR activation. Life Sci. 2003;73:289–299. doi: 10.1016/s0024-3205(03)00287-x. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaki T, Mukasa K, Yoneda M, Ito S, Yamada Y, Mori Y, et al. Marked attenuation of production of collagen type I from cardiac fibroblasts by dehydroepiandrosterone. Am J Physiol Endocrinol Metab. 2005;288:E1222–E1228. doi: 10.1152/ajpendo.00370.2004. [DOI] [PubMed] [Google Scholar]
- 40.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. HEPATOLOGY. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 41.Tafani M, Schneider TG, Pastorino JG, Farber JL. Cytochrome c-dependent activation of caspase-3 by tumor necrosis factor requires induction of the mitochondrial permeability transition. Am J Pathol. 2000;156:2111–2121. doi: 10.1016/S0002-9440(10)65082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–29798. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- 43.Hildebrand F, Pape HC, Hoevel P, Krettek C, van Griensven M. The importance of systemic cytokines in the pathogenesis of polymicrobial sepsis and dehydroepiandrosterone treatment in a rodent model. Shock. 2003;20:338–346. doi: 10.1097/01.shk.0000081408.57952.22. [DOI] [PubMed] [Google Scholar]
- 44.Chang DM, Chu SJ, Chen HC, Kuo SY, Lai JH. Dehydroepiandrosterone suppresses interleukin 10 synthesis in women with systemic lupus erythematosus. Ann Rheum Dis. 2004;63:1623–1626. doi: 10.1136/ard.2003.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. HEPATOLOGY. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 46.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Sponseller CA, Hampton K, Bacon BR. Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with nonalcoholic steatohepatitis. J Hepatol. 2003;38:434–440. doi: 10.1016/s0168-8278(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 47.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]