Abstract
Objective:
A variety of measurements have been individually linked to decline in mild cognitive impairment (MCI), but the identification of optimal markers for predicting disease progression remains unresolved. The goal of this study was to evaluate the prognostic ability of genetic, CSF, neuroimaging, and cognitive measurements obtained in the same participants.
Methods:
APOE ε4 allele frequency, CSF proteins (Aβ1-42, total tau, hyperphosphorylated tau [p-tau181p]), glucose metabolism (FDG-PET), hippocampal volume, and episodic memory performance were evaluated at baseline in patients with amnestic MCI (n = 85), using data from a large multisite study (Alzheimer's Disease Neuroimaging Initiative). Patients were classified as normal or abnormal on each predictor variable based on externally derived cutoffs, and then variables were evaluated as predictors of subsequent conversion to Alzheimer disease (AD) and cognitive decline (Alzheimer's Disease Assessment Scale–Cognitive Subscale) during a variable follow-up period (1.9 ± 0.4 years).
Results:
Patients with MCI converted to AD at an annual rate of 17.2%. Subjects with MCI who had abnormal results on both FDG-PET and episodic memory were 11.7 times more likely to convert to AD than subjects who had normal results on both measures (p ≤ 0.02). In addition, the CSF ratio p-tau181p/Aβ1-42 (β = 1.10 ± 0.53; p = 0.04) and, marginally, FDG-PET predicted cognitive decline.
Conclusions:
Baseline FDG-PET and episodic memory predict conversion to AD, whereas p-tau181p/Aβ1-42 and, marginally, FDG-PET predict longitudinal cognitive decline. Complementary information provided by these biomarkers may aid in future selection of patients for clinical trials or identification of patients likely to benefit from a therapeutic intervention.
GLOSSARY
- AD
= Alzheimer disease;
- ADAS-Cog
= Alzheimer's Disease Assessment Scale–Cognitive Subscale;
- ADNI
= Alzheimer's Disease Neuroimaging Initiative;
- AVLT
= Auditory Verbal Learning Test;
- CDR
= Clinical Dementia Rating;
- CI
= confidence interval;
- FDG
= [18F]fluorodeoxyglucose;
- MCI
= mild cognitive impairment;
- MNI
= Montreal Neurological Institute;
- p-tau181p
= hyperphosphorylated tau;
- ROC
= receiver operating characteristic;
- t-tau
= total tau.
Individuals with mild cognitive impairment (MCI) are a target population for evaluating very early treatment interventions for Alzheimer disease (AD) since they represent an intermediate stage between normal function and AD, and are at higher risk for decline than healthy older individuals. Because individuals with MCI decline at different rates and some never develop AD, there is a need for tools to select patients with MCI who would benefit most from treatment. Existing research has implicated a number of biomarkers that predict cognitive decline or conversion to AD in this population, including glucose metabolism reductions, measured by [18F]fluorodeoxyglucose uptake (FDG-PET) in parietal, posterior cingulate, and temporal brain regions1,2; MRI evidence of medial temporal lobe and hippocampal atrophy3–6; increased CSF total tau (t-tau) and hyperphosphorylated tau (p-tau181p), indicating neurofibrillary tangle pathology, and decreased Aβ1-42, indicating amyloid (Aβ) plaque pathology7–9; and presence of the apolipoprotein E (APOE) ɛ4 allele.10 While each of these measures has independently shown promise for predicting disease progression,11 they have not yet been compared to one another in the same patient population. Furthermore, the relative value of biomarkers compared to neuropsychological tests is not well-understood. Word list learning ability, a form of episodic memory, is a particularly well-studied and strong predictor of conversion.12–14 A number of studies have compared the predictive value of 2 or more biomarkers at a time, such as MRI and CSF,15,16 MRI and cognitive testing,17–19 FDG-PET and CSF,8 FDG-PET and cognitive testing,20 and MRI, CSF, and FDG-PET,21 but findings have been inconsistent, likely due to small sample sizes and a variety of methodologic factors.
In this study, we used MCI participant data from the Alzheimer's Disease Neuroimaging Initiative (ADNI), a multicenter project with approximately 50 medical center and university sites across the United States and Canada. ADNI is supported by the NIH, private pharmaceutical companies, and nonprofit organizations, and has the primary goal of evaluating MRI, PET, CSF, and clinical measures acquired serially over 2–3 years.
We compared the prognostic ability of a number of candidate biomarkers that were obtained at baseline to determine which marker or combination of markers is optimal for predicting both conversion to AD and cognitive decline. Determination of sensitive and specific markers of very early AD progression is intended to help develop new treatments and to decrease the time and cost of clinical trials.
METHODS
Subjects.
A total of approximately 200 cognitively normal older subjects, 400 subjects with MCI, and 200 patients with early AD are enrolled in ADNI, all of whom have had MRI scanning, approximately 50% have had PET scanning, and approximately 50% also agreed to lumbar puncture. As of April 2009, a subset of 85 subjects with MCI had baseline data available for all measures of interest and were used for this study. Serial clinical diagnostic assessments were carried out at 6, 12, 18, 24, and 36 months. Approximately 8% of subjects completed 3 visits (12 mo), 15% completed 4 visits, the majority, 72%, completed 5 visits (24 mo), and the remaining 5% completed 6 visits (36 mo). Conversion to AD was established at individual recruitment sites, with review centrally, and none of the variables used in the prediction of outcome were used as indicators of conversion. An examination of each measure for outliers revealed that 3 subjects had abnormally high t-tau, p-tau181p, or both (Z score >3), so they were excluded from tests that involved these measurements.
Full inclusion/exclusion criteria are described in detail at www.adni-info.org. Briefly, all subjects were between ages 55 and 90 years, had completed at least 6 years of education, were fluent in Spanish or English, and were free of any other significant neurologic diseases. Subjects with MCI were classified as single-domain or multidomain amnestic MCI,22 normal subjects had Clinical Dementia Rating (CDR) scores of 0, and patients with AD met standard diagnostic criteria.23
The Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog)24 and diagnostic status (remaining stable as MCI or converting to AD) were the outcome variables of interest. The ADAS-Cog contains 11 items assessing fundamental cognitive functions (language, memory, praxis, comprehension), and the total score ranges from 0 to 70, with a higher score indicating poorer cognitive function.
Candidate predictors included presence of an APOE ε4 allele, neuroimaging measurements (FDG-PET, hippocampal volume), CSF biomarkers (Aβ1-42, t-tau, p-tau181p), and episodic memory performance on the Auditory Verbal Learning Test (AVLT), all obtained at baseline.
Standard protocol approvals, registrations, and patient consents.
The procedures for this study were approved by institutional review boards of all participating institutions. All subjects gave written, informed consent to blood sampling, lumbar puncture, cognitive testing, and neuroimaging prior to participation.
Biomarker predictors.
Episodic memory.
The total number of words correctly recalled on all 5 immediate recall trials of the AVLT25 was assessed at baseline and used as a predictor variable in our analysis because recent studies have shown that word list learning in particular is a predictor of conversion compared to other neuropsychological tests.12–14
Genetic.
APOE genotypes were determined for all ADNI subjects through analysis of blood samples that was carried out at the University of Pennsylvania Alzheimer's Disease Biomarker Laboratory.
Hippocampal volume.
Structural magnetic resonance scans (1.5-T) were acquired at multiple ADNI sites using a standardized MRI protocol described elsewhere.26 Bilateral hippocampal volumes were obtained using Freesurfer software (http://surfer.nmr.mgh.harvard.edu), an atlas-based approach that has been validated for use in subjects with a great deal of morphologic variability.27 More information is provided in appendix e–1 on the Neurology® Web site at www.neurology.org.
CSF biomarkers.
CSF biomarker variables included Aβ1-42, t-tau, and p-tau, phosphorylated at threonine 181, in pg/mL (p-tau181p), as well as ratios (t-tau/Aβ1-42, p-tau181p/Aβ1-42). Methods for analysis have been previously described28 and are provided online.
FDG-PET.
ADNI PET data were acquired at sites nationwide using a protocol described elsewhere (http://www.loni.ucla.edu/ADNI/Data/ADNI_Data.shtml). Briefly, PET images were acquired 30–60 minutes postinjection. Images were averaged, spatially aligned, interpolated to a standard voxel size, intensity normalized, and smoothed to a common resolution of 8-mm full width at half maximum. Spatial normalization of each individual's PET volume to the standard 15O-H2O PET template was conducted using SPM529 (template voxel dimensions: 91 × 109 × 91; voxel size: 2 mm × 2 mm × 2 mm). PET volumes were intensity normalized to a single region made up of the cerebellar vermis, defined by the AAL region within the Montreal Neurological Institute (MNI) atlas, and the pons, defined by manual tracing on the MNI template. Methods for analysis have been previously described30 and are provided in appendix e–1 on the Neurology® Web site at www.neurology.org.
Cutoffs for subject classification.
Dichotomous forms of all independent variables (defined as AD+/AD−) with the exception of APOE were established using receiver operating characteristic (ROC) analyses with AD and cognitively normal ADNI participants to determine optimal cutoffs for each measure. Cutoffs for each variable were selected by choosing the threshold that optimized both sensitivity and specificity, and were subsequently used to categorize subjects with MCI as AD+ or AD− on each measure. ROC analyses were carried out using all available ADNI data for each measure.
For APOE, subjects were divided based on the presence (AD+) or absence (AD−) of at least one APOE ε4 allele.
Statistical analyses.
Statistical analyses were carried out using SPSS 16.0. Independent samples t, Mann-Whitney U, and χ2 tests were used to assess differences between converter and nonconverter groups on each measure and associations between dichotomized variables. Positive predictive value was calculated as the number of MCI converters correctly classified as AD+ divided by all MCI converters, and negative predictive value was the number of MCI nonconverters correctly classified as AD− divided by all MCI nonconverters.
Both univariate and multivariate models were examined to assess the degree to which each baseline predictor was associated with the outcome measure independently or in conjunction with the other variables. However, due to overlap in AD+/AD− status among CSF measures (table e–2 in appendix e–1), only one CSF measure could be included in multivariate models. p-tau181p/Aβ1-42 was selected because it showed the strongest prediction of conversion at the univariate level. Thus, 5 variables were included in the multivariate analyses: APOE status, FDG-PET, hippocampal volume, p-tau181p/Aβ1-42, and AVLT recall.
For the Cox proportional hazards models predicting conversion, the time variable was amount of time (in years) from baseline to the visit in which AD was diagnosed, or to the most recent visit for censored cases. In the mixed effects models, the outcome measure consisted of all available serial ADAS-Cog measurements, which incorporated individual variability in the number of completed visits, missing data, and individual variability in between-scan intervals.31 Each model included a random intercept to account for variability in individual starting point, and time between visits (in years since the initial visit) was computed separately for each individual. The interaction term for each independent variable of interest × time represents the degree to which that variable was associated with change in the ADAS-Cog over time.
Assumptions of linearity were verified for each model. Age, education, and sex were included as covariates in all models, and all statistical tests were evaluated for statistical significance at α = 0.05, 2-sided, and trends at 0.05 < α < 0.10.
RESULTS
Differences between converters and nonconverters.
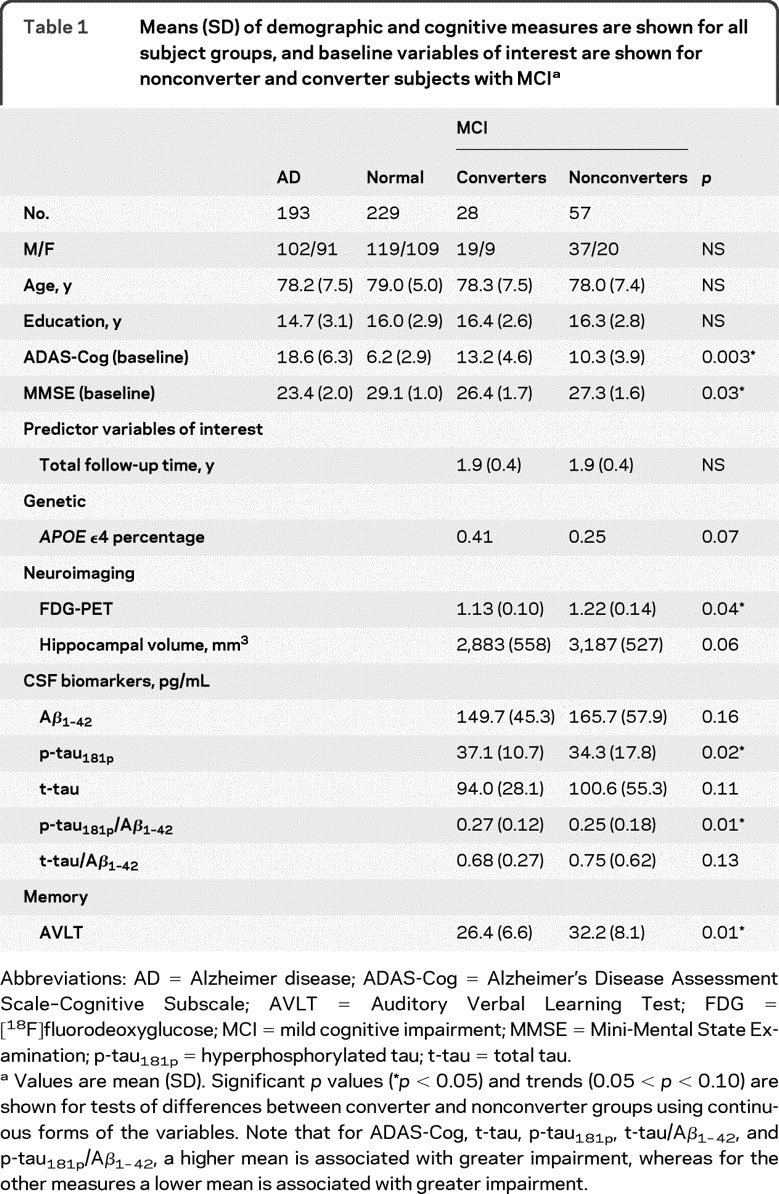
Table 1 summarizes demographic information for all groups (AD, MCI, normal), baseline measurements for converter and nonconverter MCI groups, and statistical differences between converter and nonconverter groups. Of the 85 MCI participants, 28 (32.9% total, or an annual rate of 17.2%) converted to AD. None of the subjects with MCI reverted to a normal diagnosis or converted to a non-Alzheimer dementia. Converters and nonconverters did not differ on demographic characteristics, but were either significantly or marginally different on cognitive variables and predictor variables of interest. Agreement between AD+ and AD− categorizations across variables is reported online.
Table 1 Means (SD) of demographic and cognitive measures are shown for all subject groups, and baseline variables of interest are shown for nonconverter and converter subjects with MCI
Classification of AD+ and AD− subjects.
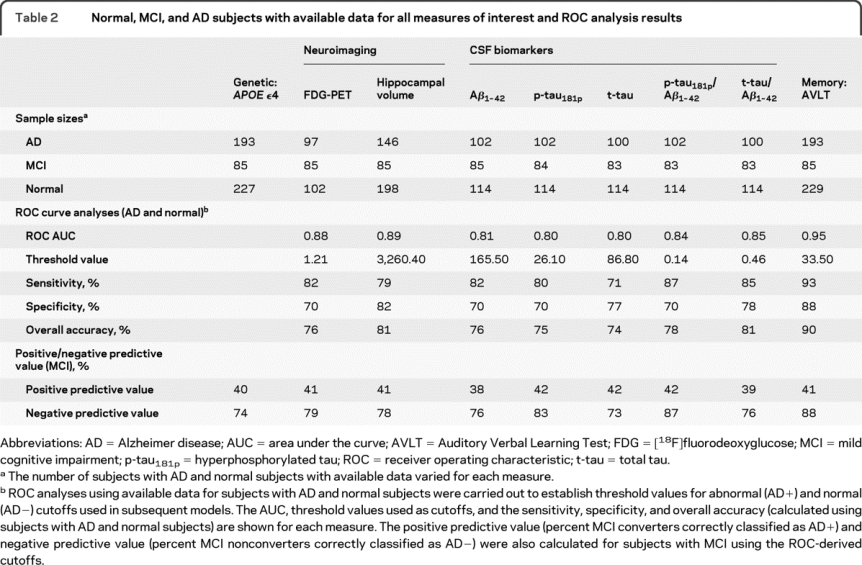
Table 2 summarizes the numbers of normal subjects, subjects with MCI, and subjects with AD with available data for all measures of interest, and the ROC analysis results, which include the area under the curve (range 0.80–0.95), threshold value, sensitivity (range 71%–93%), specificity (range 70%–88%), and overall accuracy (range 74%–90%) for classification of normal subjects and subjects with AD with each measure. Finally, the thresholds for each measure were applied to the MCI participants to determine positive and negative predictive values for conversion.
Table 2 Normal, MCI, and AD subjects with available data for all measures of interest and ROC analysis results
Cox proportional hazards models: Predicting conversion.
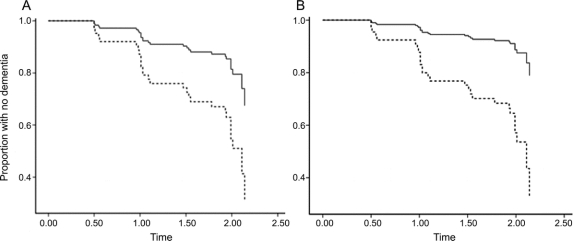
In univariate models, several variables were associated with increased risk of conversion to AD. As shown in table 3, subjects categorized as AD+ on FDG-PET, hippocampal volume, p-tau181p, p-tau181p/Aβ1-42, AVLT, and, marginally, APOE had a higher risk of converting than AD− subjects on each measure (hazard ratio range 2.94–4.68). In the multivariate model, only FDG-PET and AVLT remained significant predictors. Specifically, subjects who were AD+ on FDG-PET had a 2.72 (p = 0.05) greater risk of converting to AD than subjects who were AD− on FDG-PET (figure, A), assuming equal AD+ status on the other variables, and this risk was 4.30 (p = 0.02) for AVLT (figure, B). Taken together, these findings indicate that the 36 subjects (42%) who were AD+ for both FDG-PET and AVLT had an 11.7-fold (95% confidence interval [CI] 2.22–61.75) greater risk of converting to AD than subjects categorized as AD− on both measures.
Table 3 Results of Cox proportional hazards models, with potential conversion to AD as the outcome measure, and mixed effects models, with ADAS-Cog change as the outcome measure
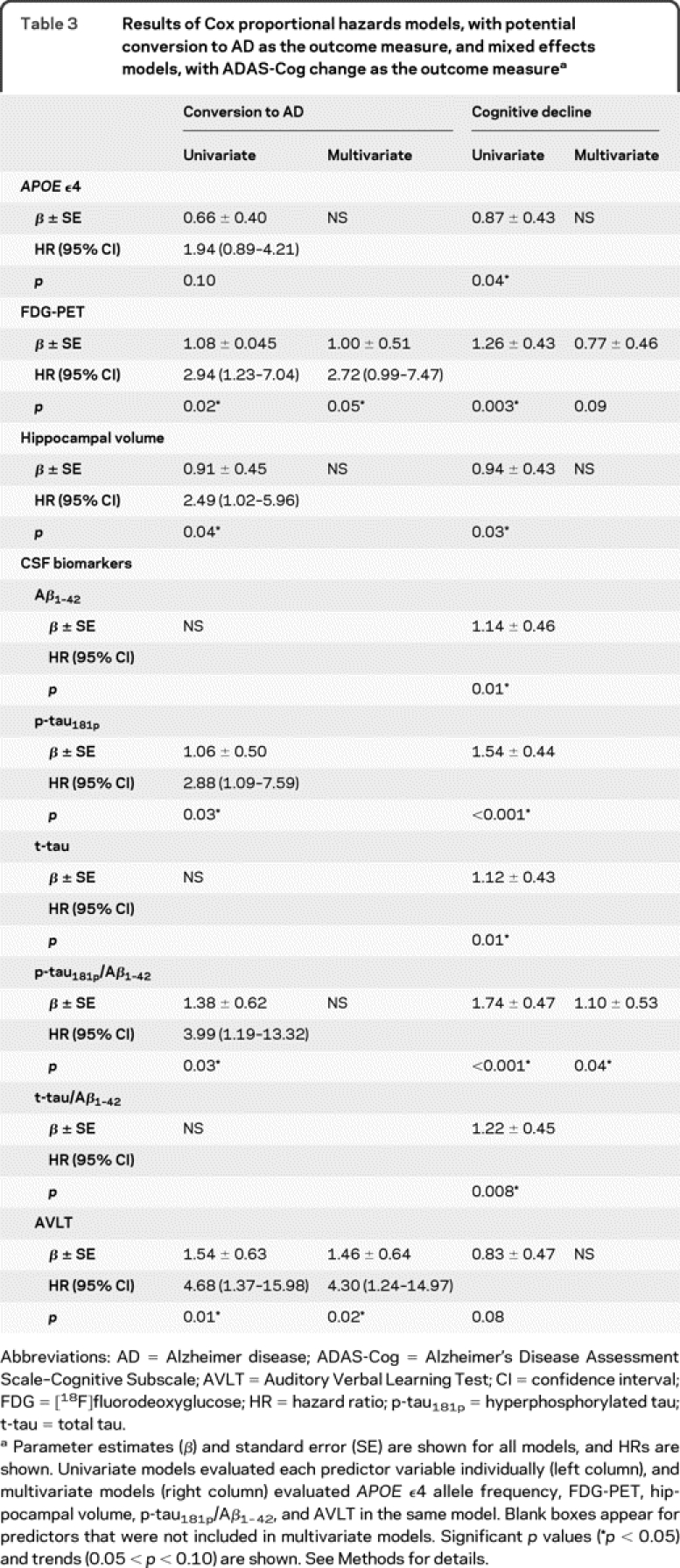
Figure FDG-PET and AVLT survival curves show increased conversion over time for abnormal relative to normal subjects
Predicted survival curves based on Cox proportional hazards models (table 3) illustrate the univariate results for (A) FDG-PET and (B) AVLT, which were the 2 variables that remained significant in the multivariate model. Both curves show that for each variable, a higher proportion of AD− subjects (solid black line) remained dementia-free over time compared to AD+ subjects (dotted line). Age, education, and sex were included as control covariates. Proportion of subjects remaining dementia-free is shown on the y-axis.
Mixed effects models: Predicting cognitive decline.
Average decline on the ADAS-Cog was 1.11 ADAS-Cog points/year (95% CI 0.69–1.53). All baseline variables predicted subsequent ADAS-Cog decline in univariate models, although AVLT was marginally significant (table 3). In the multivariate model, high p-tau181p/Aβ1-42 remained a significant predictor (p = 0.04), low FDG-PET was a marginally significant (p = 0.09) predictor, and no other variables were significant (p > 0.33). The 43 subjects (51%) who were AD+ on p-tau181p/Aβ1-42 had an increased ADAS-Cog annual decline rate of 1.10 units/year compared with AD− subjects, accounting for all other variables (0.77 units/year for FDG-PET).
DISCUSSION
The goal of this multicenter longitudinal study was to compare a variety of candidate predictors of decline in MCI over a follow-up period of approximately 2 years. All of these biomarkers were significant predictors of conversion or decline in univariate models. In multivariate models predicting conversion, glucose metabolism and episodic memory function were significant. Individuals who were abnormal (AD+) on both FDG-PET and AVLT were 11.7 times more likely to convert to AD than individuals who were normal on these measures. In multivariate models predicting decline, the CSF ratio p-tau181p/Aβ1-42 (and, marginally, FDG-PET) were significant when accounting for all the other variables.
There were several noteworthy features of our approach. First, cutoffs used to classify subjects with MCI as abnormal or normal on each predictor variable were derived from an independent sample, and are therefore potentially applicable outside of this population. Second, all predictors were defined a priori, as opposed to frequently used exploratory methods, such as voxel-wise analyses for PET and MRI data, that are optimized for a study-specific dataset. The availability of data through ADNI made it possible to directly compare all of these predictors in the same individuals for the first time. Finally, the use of dichotomous predictors, as opposed to a continuous variable, provides a precise way of selecting individual subjects with MCI for a clinical trial or potential treatment.
The fact that different combinations of markers predict conversion status and cognitive decline suggests that these markers may track different aspects of disease progression. Predictors associated with conversion (AVLT and FDG-PET) likely reflect disease severity, i.e., how close an individual is to a significant clinical transition. On the other hand, predictors associated with cognitive decline (primarily CSF p-tau181p/Aβ1-42, and secondarily FDG-PET) likely reflect rate of change, independent of absolute disease severity.
Studies comparing the predictive value of multiple biomarkers have been variable, although several useful meta-analyses or reviews have summarized recent findings.11,32–34 Our data are consistent with reports of the predictive value of CSF biomarkers,35,36 and a recent large, multicenter study that is one of the few to use externally derived cutoffs.7 Our results are consistent with previous reports of the predictive value of FDG-PET,1 studies that have examined both FDG-PET and APOE,37,38 and a report that FDG-PET was superior to the Mattis dementia scale.20 Our findings are not in agreement, however, with recent data indicating that MRI and PET were superior to CSF in predicting cognitive decline.21 Additionally, although we found that FDG-PET and AVLT performance were the best predictors of conversion, our univariate findings are consistent with studies showing that MRI and CSF,15,16,39 MRI and cognition,17 and MRI and APOE40 measures are useful for predicting conversion to AD, although others have suggested that MRI measures are superior to cognitive measures in predicting decline.18,19 Finally, although studies of AD conversion frequently do not account for rates of conversion or time-dependent change as we have done here, our results are also consistent with one study that did account for time and compared p-tau181p, t-tau, and FDG-PET and found that p-tau181p optimally predicted cognitive decline, while FDG-PET optimally predicted conversion.8
Inconsistencies in recent findings are likely due to a variety of methodologic issues such as differences in neuroimaging processing techniques and variable selection, CSF protein immunoassay techniques, criteria for setting cutoff points for subject categorization, study design, and statistical analysis. Importantly, few studies on AD prediction define predictor variables before evaluating their performance; instead, a “predictive” measure is developed based on differences between MCI converters and nonconverters and retrospectively applied to the initial population without validating it in an independent population (often due to sample size constraints).
An important limitation of our findings is that the hippocampal and FDG-PET ROIs may not be optimized for this sample. Whole-brain, data-driven, voxel-based, or other approaches to the analysis of PET or magnetic resonance data might have resulted in stronger prediction outcomes for the imaging variables. Hippocampal volume was a significant predictor of decline at the univariate but not multivariate level, suggesting that the association between hippocampal volume and decline may be mediated in part by the other measures in our analysis. In contrast, other studies have reported a stronger role for hippocampal or other structural measurements in predicting decline,3 even when examined in conjunction with CSF measurements.16,41 However, the regions of interest that we selected were study independent, frequently associated with decline in AD and MCI, and obtained through an automated and standardized processing stream and are therefore strong candidate measurements for clinical trials of therapeutic treatments.
There are several other limitations of this study that deserve comment. First, these models are based on the subset of MCI participants who agreed to participate in all biomarker testing, and future studies will be needed to address the question of whether our findings generalize to a broader sample, and whether differences exist for single-domain and multidomain MCI or for patients with amnestic MCI who convert to non-Alzheimer dementias. Second, our results may depend on the number and precise combination of predictor variables included in the model. A longer follow-up period may also result in model changes, since the value of predictors is likely modulated by the phase of disease. Clinical diagnosis of AD involves factors that are difficult to standardize and involves some degree of subjectivity and uncertainty. Furthermore, there are other cognitive measures and biomarkers that may play important roles in prediction that were not addressed here (such as the CDR–sum of boxes, Mini-Mental State Examination, genetic markers, and diffusion tensor imaging measures). Importantly, forthcoming ADNI data using PET tracers for Aβ deposition will help elucidate the role of brain amyloid load in patient outcomes.
The current clinical role of these, and other, biomarkers in dementia care is relatively limited largely because of the lack of effective treatment. However, our results suggest that these biomarkers could be effective in identifying patients with MCI who are more likely to progress to AD over relatively brief time periods. This approach could be useful for identifying patients who would benefit from treatment when it becomes available and for selecting subjects in clinical trials of therapeutic agents for MCI.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Landau and Dr. Harvey.
STUDY FUNDING
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904). ADNI is funded by the National Institute on Aging, by the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, Inc., GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc., F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as nonprofit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514 and the Dana Foundation.
DISCLOSURE
Dr. Landau receives research support from the NIH (U01 AG024904 [Research Specialist]). Dr. Harvey serves as an Associate Editor of Statistics for Alzheimer Disease and Associated Disorders; and receives research support from the NIH (NIA 2P30AG10129 [Biostatistician], NIA R01AG029672 [Biostatistician], NIA 1U01AG24904 [Member of Biostatistics Core], NRCC RL1NS062412 [Biostatistician], NIA R01AG031252 [Biostatistician], 1RC2AG036535-01 [Member of Biostatistics Team]) and from the Hillblom Foundation. Dr. Madison reports no disclosures. Dr. Reiman serves on scientific advisory boards for Accera, Inc., AstraZeneca, Elan Corporation, Eli Lilly and Company, and GlaxoSmithKline; serves as a consultant to Amnestix/Sygnis; serves as Deputy Editor of the Journal of Clinical Psychiatry; holds a patent re: Methods for Tracking the Progression of Alzheimer's Disease Identifying Treatment Using Transgenic Mice and has patents pending re: Evaluation Of Treatment To Decrease The Risk Of Progressive Brain Disorder Or Slow Brain Aging, Methodologies Linking Patterns From Multi-Modality Datasets, and Methods of Diagnosing Alzheimer's Disease and Associated Genetic Markers; and receives research support from Kronos Life Sciences, GlaxoSmithKline, AstraZeneca, Avid Radiopharmaceuticals, Inc., the NIH (NIA 9 R01 AG031581-10 [PI]), and from the State of Arizona. Dr. Foster serves/has served on scientific advisory boards for Myriad Pharmaceuticals, GE Healthcare, Wyeth/Elan Corporation, the National Alliance for Caregiving, the University of Texas Southwestern Alzheimer's Disease Research Center, the University of Alabama at Birmingham Alzheimer's Disease Research Center, and the NIH Alzheimer's Disease Neuroimaging Initiative (ADNI); has received speaker honoraria from Myriad Pharmaceuticals and GE Healthcare; receives research support from Eli Lilly and Company, Baxter Bioscience/ADCS, Elan Corporation, Merck & Co., Inc., Myriad Genetics, Eisai Inc./ICON Medical Research; the NIH (NIA U01 AG024904 [Site PI], R01 EB00768 [Co-I], U01 AG10483 [Site Co-I], NIA R01 AG022394 [PI], NINDS T32 NS07222 [Co-I]), CMS, the University of Pennsylvania, the Donald W. Reynolds Foundation, and from the Ben B. and Iris M. Margolis Foundation. Dr. Aisen serves on a scientific advisory board for NeuroPhage; serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Neurochem Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, and Medivation, Inc.; receives research support from Pfizer Inc, Baxter International Inc., Neuro-Hitech, Abbott, Martek, and the NIH (NIA U01-AG10483 [PI], NIA U01-AG024904 [Coordinating Center Director], NIA R01-AG030048 [PI], and R01-AG16381 [Co-I]); and has received stock options from Medivation, Inc. and NeuroPhage. Dr. Petersen serves on scientific advisory boards for Elan Corporation, Wyeth, and GE Healthcare; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA (U01 AG 06786 [PI], P50 AG 16574 [PI], U01 AG 024904 [Subcontract PI], and R01 AG11378 [Co-I]). Dr. Shaw has received funding for travel and speaker honoraria from Pfizer Inc; serves on the editorial board of Therapeutic Drug Monitoring; may potentially receive revenue for patent pending (application number 10/192,193): O-methylated rapamycin derivatives for alleviation and inhibition of lymphoproliferative disorders, licensed by the University of Pennsylvania to Novartis; receives royalties from publication of Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring (Wolters Kluwer/Lippincott Williams & Wilkins, 2005); receives research support from the NIH (AG024904 [Co-PI Biomarker Core Laboratory]); and receives board of directors' compensation and holds stock options in Saladax Biomedical. Dr. Trojanowski has received funding for travel and honoraria from Takeda Pharmaceutical Company Ltd. and to attend numerous conferences not funded by industry; serves as an Associate Editor of Alzheimer's & Dementia; may accrue revenue on patents re: Modified Avidin-Biotin Technique, Method of Stabilizing Microtubules to Treat Alzheimer's Disease, Method of Detecting Abnormally Phosphorylated Tau, Method of Screening for Alzheimer's Disease or Disease Associated with the Accumulation of Paired Helical Filaments, Compositions and Methods for Producing and Using Homogeneous Neuronal Cell Transplants, Rat Comprising Straight Filaments in Its Brain, Compositions and Methods for Producing and Using Homogeneous Neuronal Cell Transplants to Treat Neurodegenerative Disorders and Brain and Spinal Cord Injuries, Diagnostic Methods for Alzheimer's Disease by Detection of Multiple MRNAs, Methods and Compositions for Determining Lipid Peroxidation Levels in Oxidant Stress Syndromes and Diseases, Compositions and Methods for Producing and Using Homogenous Neuronal Cell Transplants, Method of Identifying, Diagnosing and Treating Alpha-synuclein Positive Neurodegenerative Disorders, Mutation-specific Functional Impairments in Distinct Tau Isoforms of Hereditary Frontotemporal Dementia and Parkinsonism Linked to Chromosome-17: Genotype Predicts Phenotype, Microtubule Stabilizing Therapies for Neurodegenerative Disorders; and Treatment of Alzheimer's and Related Diseases with an Antibody; and receives research support from the NIH (NIA P01 AG 09215-20 [PI], NIA P30 AG 10124-18 [PI], NIA PO1 AG 17586-10 [Project 4 Leader], NIA 1PO1 AG-19724-07 [Core C Leader], NIA 1 U01 AG 024904-05 [Co-PI Biomarker Core Laboratory], NINDS P50 NS053488-02 [PI], NIA UO1 AG029213-01 (Co-I); RC2NS069368 (PI), RC1AG035427 (PI), and NIA P30AG036468 [PI]), and from the Marian S. Ware Alzheimer Program. Dr. Jack serves as a consultant for Eli Lilly and Company and Elan Corporation; is an investigator in clinical trials sponsored by Baxter International Inc., Pfizer Inc, the NIH/NIA (AG11378 [PI], P50-AG16574 [Co-I], and U01 AG024904-01 [Co-I], and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock in GE Healthcare. Dr. Weiner serves on scientific advisory boards for Bayer Schering Pharma, Eli Lilly and Company, CoMentis, Inc., Neurochem Inc, Eisai Inc., Avid Radiopharmaceuticals Inc., Aegis Therapies, Genentech, Inc., Allergan, Inc., Lippincott Williams & Wilkins, Bristol-Myers Squibb, Forest Laboratories, Inc., Pfizer Inc, McKinsey & Company, Mitsubishi Tanabe Pharma Corporation, and Novartis; has received funding for travel from Nestlé and Kenes International and to attend conferences not funded by industry; serves on the editorial board of Alzheimer's & Dementia; has received honoraria from the Rotman Research Institute and BOLT International; serves as a consultant for Elan Corporation; receives research support from Merck & Co., Radiopharmaceuticals Inc., the NIH (U01AG024904 [PI], P41 RR023953 [PI], R01 AG10897 [PI], P01AG19724 [Co-I], P50AG23501 [Co-I], R24 RR021992 [Co-I], R01 NS031966 [Co-I], and P01AG012435 [Co-I]), the US Department of Defense (DAMD17-01-1-0764 [PI]), the Veterans Administration (MIRECC VISN 21 [Core PI]), and from the State of California; and holds stock in Synarc and Elan Corporation. Dr. Jagust has served on a scientific advisory board for Genentech, Inc.; has served as a consultant for Synarc, Elan Corporation, Genentech, Inc., Ceregene, Schering-Plough Corp., and Merck & Co; and receives research support from the NIH (AG027859 [PI], AG027984 [PI], and AG 024904 [Co-I]) and from the Alzheimer's Association.
Supplementary Material
Address correspondence and reprint requests to Dr. Susan M. Landau, 118 Barker Hall MC #3190, UC Berkeley, Berkeley, CA 94720-3190 slandau@berkeley.edu
Editorial, page 204
Supplemental data at www.neurology.org
e–Pub ahead of print on June 30, 2010, at www.neurology.org.
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators is available in appendix e–2 at www.neurology.org.
Study funding: Funding information is provided at the end of the article.
Disclosure: Author disclosures are provided at the end of the article.
Received December 7, 2009. Accepted in final form March 9, 2010.
REFERENCES
- 1.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 2003;30:1104–1113. [DOI] [PubMed] [Google Scholar]
- 2.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci USA 2001;98:10966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr., Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundman M, Sencakova D, Jack CR, Jr., et al. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci 2002;19:23–27. [DOI] [PubMed] [Google Scholar]
- 5.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol 2000;47:430–439. [PubMed] [Google Scholar]
- 6.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology 2005;64:1520–1524. [DOI] [PubMed] [Google Scholar]
- 7.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009;302:385–393. [DOI] [PubMed] [Google Scholar]
- 8.Fellgiebel A, Scheurich A, Bartenstein P, Muller MJ. FDG-PET and CSF phospho-tau for prediction of cognitive decline in mild cognitive impairment. Psychiatry Res 2007;155:167–171. [DOI] [PubMed] [Google Scholar]
- 9.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228–234. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Berry-Kravis E, Bennett DA. The apolipoprotein E epsilon4 allele and incident Alzheimer's disease in persons with mild cognitive impairment. Neurocase 2005;11:3–7. [DOI] [PubMed] [Google Scholar]
- 11.de Leon MJ, Mosconi L, Blennow K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann NY Acad Sci 2007;1097:114–145. [DOI] [PubMed] [Google Scholar]
- 12.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology 2005;64:1853–1859. [DOI] [PubMed] [Google Scholar]
- 13.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol 2007;64:862–871. [DOI] [PubMed] [Google Scholar]
- 14.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry 2006;63:916–924. [DOI] [PubMed] [Google Scholar]
- 15.Bouwman FH, Schoonenboom SN, van der Flier WM, et al. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging 2007;28:1070–1074. [DOI] [PubMed] [Google Scholar]
- 16.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology 2009;73:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 2007;68:828–836. [DOI] [PubMed] [Google Scholar]
- 18.Visser PJ, Verhey FR, Hofman PA, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry 2002;72:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geroldi C, Rossi R, Calvagna C, et al. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry 2006;77:1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chetelat G, Eustache F, Viader F, et al. FDG-PET measurement is more accurate than neuropsychological assessments to predict global cognitive deterioration in patients with mild cognitive impairment. Neurocase 2005;11:14–25. [DOI] [PubMed] [Google Scholar]
- 21.Walhovd KB, Fjell AM, Brewer J, et al. Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. AJNR Am J Neuroradiol 2010;31:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen RC. Conceptual overview. In: Petersen RC, ed. Mild Cognitive Impairment: Aging to Alzheimer's Disease. New York: Oxford University Press; 2003:1–14. [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 24.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 25.Rey A. l'Examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 26.Jack CR, Jr., Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 28.Shaw LM. PENN biomarker core of the Alzheimer's Disease Neuroimaging Initiative. Neurosignals 2008;16:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 30.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging Epub 2009 Aug 4. [DOI] [PMC free article] [PubMed]
- 31.Gould R, Abramson I, Galasko D, Salmon D. Rate of cognitive change in Alzheimer's disease: methodological approaches using random effects models. J Int Neuropsychol Soc 2001;7:813–824. [PubMed] [Google Scholar]
- 32.Petersen RC. Alzheimer's disease: progress in prediction. Lancet Neurol 2010;9:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modrego PJ. Predictors of conversion to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr Alzheimer Res 2006;3:161–170. [DOI] [PubMed] [Google Scholar]
- 34.Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement 2008;4:38–48. [DOI] [PubMed] [Google Scholar]
- 35.Diniz BS, Pinto Junior JA, Forlenza OV. Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer's disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 2008;9:172–182. [DOI] [PubMed]
- 36.Brys M, Pirraglia E, Rich K, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging 2009;30:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med 2005;46:1625–1632. [PubMed] [Google Scholar]
- 38.Mosconi L, Sorbi S, Nacmias B, et al. Age and ApoE genotype interaction in Alzheimer's disease: an FDG-PET study. Psychiatry Res 2004;130:141–151. [DOI] [PubMed] [Google Scholar]
- 39.Brys M, Glodzik L, Mosconi L, et al. Magnetic resonance imaging improves cerebrospinal fluid biomarkers in the early detection of Alzheimer's disease. J Alzheimers Dis 2009;16:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuff N, Woerner N, Boreta L, et al. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain 2009;132:1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Leon MJ, DeSanti S, Zinkowski R, et al. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Intern Med 2004;256:205–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.