Introduction
Haemoglobin S (HbS) is caused by a point mutation in the β-globin chain (located on chromosome 11), which leads to the substitution of the hydrophilic amino acid glutamic acid by the hydrophobic amino acid valine in position six. The association of two wild-type α-globin subunits with two mutant β-globin subunits forms HbS. Under low-oxygen conditions, the lack of a polar amino acid at position six of the β-globin chain promotes the non-covalent polymerisation of deoxygenated HbS, reducing the elasticity of erythrocytes and producing the typical phenomenon of “sickling”. Sickle cells are relatively inelastic and can, therefore, occlude the microvasculature and lead to tissue infarction. Furthermore, the permanently deformed cells are removed from the circulation much earlier than normal red blood cell, which characteristically have a life span of 90 to 120 days1. Sickle cell anaemia is the disease that characterises homozygous patients with HbSS; however subjects with double heterozygosity for HbS and HbC or β-thalassaemia can have similar clinical manifestations, including moderate to severe haemolytic anaemia, increased severity of certain infections, tissue infarction with organ damage and failure, and recurrent pain episodes. This is often called sickle trait and is not associated with significant haematological abnormalities. Carriers may have episodes of haematuria and may have more urinary tract infections. Rarely, pain episodes or splenic infarctions have been observed with extreme hypoxaemia. Sudden death may be slightly more common at the extremes of human endurance2. Of the many genetic haemoglobin variants and thalassaemia syndromes identified so far, only a few have substantial clinical significance. Among these, HbS is the most common (being present in 8% of the African-American population). According to the National Institutes of Health, the overall prevalence of this inherited disorder is approximately 1 in 5,000 in the USA, with most cases being Americans of Sub-Saharan African descent, among whom the prevalence is about 1 in 500. The prevalence is also increasing in western countries and in Europe. In a recent epidemiological investigation, we showed that HbS represents the most prevalent hemoglobin variant in Northern Italy (2.8%), followed by hemoglobin C (0.7%) and E (0.4%)3. A steady increase of case notifications and hospital admissions (95% of patients are immigrants with HbS/HbS sickle cell disease) have also been observed in the same geographical area over the past 5 years4. Given these estimates, it is conceivable that some asymptomatic HbS carriers might be enrolled for regular blood donations, with the risk of causing transfusion-acquired HbS.
Case report
An 88-year old diabetic woman was admitted to the local diabetic ward for the treatment of a diabetic ulcer on the left foot. She underwent laboratory testing to assess the effectiveness of the management plan on her glycaemic control. Her main laboratory data assessed on admission are shown in Table I.
Table I.
Main haematological and biochemical parameters on the day of admission, and on the following day, after blood transfusion
| Day of admission | Day after admission | |
|---|---|---|
| White blood cell count (109/L) | 7.26 | 8.25 |
| Red blood cell count (1012/L) | 2.50 | 3.35 |
| Haemoglobin (g/dL) | 6.8 | 9.6 |
| Haematocrit (%) | 21.8 | 29.9 |
| Mean corpuscular volume (fL) | 87.5 | 89.1 |
| Mean cell haemoglobin (pg) | 27.3 | 28.6 |
| Red blood cell distribution width (%) | 13.5 | 14.4 |
| Serum glucose (mg/dL) | 119 | 112 |
| Glycosylated haemoglobin (%) | 7.1 | 6.5 |
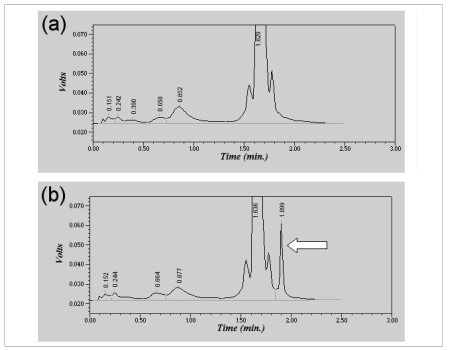
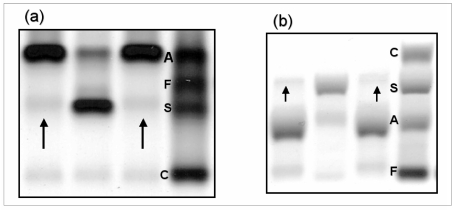
Given her severe anaemia (haemoglobin: 6.8 g/dL, haematocrit 21.8%), she was transfused with 6 units of red blood cells (1980 mL). The analysis of the chromatogram of glycosylated haemoglobin (HbA1c), performed by high pressure liquid chromatography (HPLC) with a VARIANT II HbA1c instrument (Bio-Rad Laboratories S.r.l., Milan, Italy), did not reveal the presence of abnormal peaks (Figure 1a). The day after the blood transfusion, the patient’s blood was tested again, and showed partial correction of anaemia (Table I). However, while reassessing HbA1c on this second sample using a Bio-Rad Turbo analyser, which is specifically designed to identify abnormal haemoglobins, a small peak (3.9%), suggestive of the presence of a haemoglobin variant, was noted on the chromatogram (Figure 1b). Subsequent haemoglobin analysis using ion-exchange HPLC (Bio-Rad Classic Variant, Bio-Rad) confirmed the presence of the hemoglobin variant, with a retention time corresponding to that of HbS. The sample was, therefore, separated on agarose gels and the haemoglobin type was analysed by measuring the migration distance of various haemoglobin fractions and comparing them to known haemoglobin standards, as currently recommended 5. Haemoglobin electrophoresis showed a pattern suggestive of sickle cell trait. In particular, alkaline gel electrophoresis revealed three bands migrating as haemoglobins A1, S and A2 (Figure 2a). Acid gel electrophoresis revealed bands migrating as haemoglobins A and S (Figure 2b). The positive sickling test, that is, a positive sickle cell dex (in which a hemolysate is exposed to a reducing agent), confirmed the presence of HbS in the sample. The 26-year old male donor of the blood was confirmed to have sickle cell trait. At the time of the donation, the subject was unaware of his state of HbS carrier (HbS: 35%), and in apparently good health. All the main haematological parameters assessed at the time of donation were also within the normal reference range of our laboratory (red blood cell count, 5.16 x 1012/L; haemoglobin, 16.1 g/dL; haematocrit, 47%; mean corpuscular volume, 90.5 fL; mean cell haemoglobin, 31.2 pg; red blood cell distribution width, 12.5%).
Figure 1.
Chromatogram of haemoglobins assessed using VARIANT II HbA1c the day of admission (a) and on the following day (b), after blood transfusion. The arrow indicates the presence of a small peak (3.9%), which is suggestive of a hemoglobin variant.
Figure 2.
Agarose gel alkaline (pH 8.6) (a) and acid (pH 6.0) (b) electrophoretogram of haemoglobins. The arrows indicate the presence of a haemoglobin variant, displaying migration patterns suggestive of HbS.
Lanes 1 and 3: haemolysate from the patient described in this case report; lane 2: haemolysate from another patient carrying HbS; lane 4: standard containing HbA, HbF, HbS and HbC.
Discussion
This case report raise the significant clinical problem of whether it might be advisable to screen blood donors for haemoglobinopathies in order to prevent transfusion of dysfunctional, if not pathological, red blood cells. Pragmatically, systematic screening of blood donors might be viable, considering that testing for sickle cell trait, for example, can be performed in several ways besides ion-exchange HPLC (e.g., sickle dex, also called sickle quick or sickle prep). To the best of our knowledge there are only six case reports (three on HbC and three on HbS) 6–11, and one systematic study describing the occurrence of apparent haemoglobinopathies caused by blood transfusion 12. In this last investigation, 52 episodes of apparent haemoglobinopathy were detected in 32 recipients (46 HbC, 4 HbS, and 2 HbO-Arab). When first detected, the abnormal haemoglobins in recipients accounted for between 0.8% to 14% (median, 5.6%) of the total haemoglobin. Multiple transfusions with abnormal haemoglobins occurred in 11 patients with two patients receiving HbC blood on five separate occasions. As such, it seems reasonable to hypothesise that blood transfusion-acquired haemoglobinipathies might occur at a relatively high frequency, so that this apparently unusual case of a blood transfusion from a blood donor carrier of HbS might simply represent the tip of an iceberg.
The current recommendations of the World Health Organisation suggest that blood should be collected only from voluntary unpaid blood donors at low risk of acquiring transfusion-transmissible infections, and complying with stringent blood donor selection criteria. All donated blood should also be tested for transfusion-transmissible infections, for the presence of red cell antibodies before transfusion, and for blood groups and compatibility, but there is no mention of screening donors or donated blood for haemoglobinopathies 13. Likewise, the Italian regulations do not impose screening candidate blood donors for haemoglobinopathies during their first access at the Transfusion Centre 14. Sickle cell trait and other clinically modest haemoglobinopathies might be relatively frequent in the general population, as well as in blood donors, especially in certain geographical areas. The results of a large, systematic screening for haemoglobinopathies among 8,961 blood donors aged 18 to 60 years in Guadeloupe (French West Indies) revealed that 7.75% were sickle cell trait carriers, 2.36% were heterozygous for HbC and 0.2% had a significant elevation of HbF 15. It should also be noted that a broad spectrum of pathologies, such as sickle cell disease, which are relatively uncommon in certain countries (e.g. those of central and northern Europe) are more prevalent among immigrant populations, and might become more frequent due to increasing migratory fluxes 4,5,16. Finally, carriers of sickle cell trait and other clinically modest haemoglobinopathies cannot be easily identified by routine clinical and laboratory assessments at the time of blood donation, because they are frequently asymptomatic, and most haematological parameters (i.e., red blood cell count, haemoglobin, haematocrit, mean corpuscular volume, mean cell haemoglobin and red blood cell distribution width) are within the normal range 17.
Although only a minority of subjects experience adverse consequences attributable to the beta S gene, so that sickle cell trait has been long considered a benign carrier state, this trait has occasionally been associated with significant mortality and morbidity, especially with haematuria, renal papillary necrosis, hyposthenuria, splenic infarction, exertional rhabdomyolysis, exercise-related sudden death, complicated hyphema, venous thromboembolic events, foetal loss, neonatal deaths, pre-eclampsia, acute chest syndrome, asymptomatic bacteriuria, and anaemia in pregnancy 18. Therefore, given the large number of people carrying sickle cell trait who might not be aware of their condition before enrolling to be blood donors, it seems reasonable to consider the possibility of implementing a practice of routine screening for haemoglobinopathies in blood donors or blood bags, both to prevent the transfusion of pathological erythrocytes to a recipient and to establish a useful diagnosis of sickle cell trait in the blood donor.
References
- 1.Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151:839–45. doi: 10.1093/oxfordjournals.aje.a010288. [DOI] [PubMed] [Google Scholar]
- 2.Schnog JB, Duits AJ, Muskiet FA, et al. Sickle cell disease; a general overview. Neth J Med. 2004;62:364–74. [PubMed] [Google Scholar]
- 3.Lippi G, Montagnana M, Danese E, et al. Frequency and type of newly diagnosed haemoglobin variants in Northern Italy. Blood Transfus. doi: 10.2450/2009.0103-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombatti R, Dalla Pozza LV, Mazzucato M, et al. Hospitalization of children with sickle cell disease in a region with increasing immigration rates. Haematologica. 2008;93:463–4. doi: 10.3324/haematol.11766. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty JD, Waye JS, Chui DH, et al. Quality Management Program-Laboratory Services Hematology Committee Good practice guidelines for laboratory investigation of hemoglobinopathies. Lab Hematol. 2003;9:237–45. [PubMed] [Google Scholar]
- 6.Suarez AA, Polski JM, Grossman BJ, Johnston FM. Blood transfusion-acquired hemoglobin C. Arch Pathol Lab Med. 1999;123:642–3. doi: 10.5858/1999-123-0642-BTAHC. [DOI] [PubMed] [Google Scholar]
- 7.Rechavi G, Brok-Simoni F, Ben-Bassat I, Ramot B. Spurious hemoglobinopathy. Lancet. 1986;1:1035. doi: 10.1016/s0140-6736(86)91306-1. [DOI] [PubMed] [Google Scholar]
- 8.Strobel SL, Panke TW, Zelenski K. Hemoglobin C acquired via a blood transfusion: detection by automated blood counter. Arch Pathol Lab Med. 1987;111:565–8. [PubMed] [Google Scholar]
- 9.Gibaud A, Braconnier F, Garel MC, et al. Surune hemoglobin “S” acquise de l’adult. Nouv Presse Med. 1974;28:2013–4. [PubMed] [Google Scholar]
- 10.Robertson PB, Danielson CFM, McCarthy LJ. Unexpected hemoglobin electrophoresis results following red cell exchange in a sickle cell anemia patient with acute chest syndrome. Transfus Sci. 1997;18:195–8. doi: 10.1016/s0955-3886(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Palermo GJ, Granville N. Factitious sickle-cell trait. N Engl J Med. 1982;306:1487. doi: 10.1056/NEJM198206173062415. [DOI] [PubMed] [Google Scholar]
- 12.Kozarski TB, Howanitz PJ, Howanitz JH, et al. Blood transfusions leading to apparent hemoglobin C, S, and O-Arab hemoglobinopathies. Arch Pathol Lab Med. 2006;130:1830–3. doi: 10.5858/2006-130-1830-BTLTAH. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Universal access to safe blood and blood products for transfusion. Available at: http://www.who.int/bloodsafety/en/index.html. Last accessed: 20 November 2009.
- 14.Ministero del Lavoro, della Salute e delle Politiche Sociali. Decreto Legislativo 20 dicembre 2007, n. 261. Available at: http://www.normativasanitaria.it Last accessed: 20 November 2009.
- 15.Fabritius H, Millan J, Le Corroller Y. Systematic screening of hemoglobinopathies in blood donors in Guadeloupe (French West Indies) Rev Fr Transfus Immunohematol. 1978;21:937–50. doi: 10.1016/s0338-4535(78)80051-8. [DOI] [PubMed] [Google Scholar]
- 16.Roberts I, de Montalembert M. Sickle cell disease as a paradigm of immigration hematology: new challenges for hematologists in Europe. Haematologica. 2007;92:865–71. doi: 10.3324/haematol.11474. [DOI] [PubMed] [Google Scholar]
- 17.Schilirò G, Comisi FF, Testa R, et al. Hematological findings in 375 Sicilians with Hb S trait. Haematologica. 1990;75:113–6. [PubMed] [Google Scholar]
- 18.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009;122:507–12. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]