Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare disorder characterized by thrombocytopenia, microangiopathic haemolytic anaemia, neurological and renal abnormalities and fever1, with a mortality rate, in the absence of treatment, of almost 90%. Since such criteria do not distinguish TTP from haemolytic uraemic syndrome (HUS), the comprehensive term TTP-HUS is more approriate2. The standard therapy is urgent plasma exchange (PE)1, which reduces mortality to 10% or less3–9. Because of its dramatic effect on short and long-term outcome, it is now accepted that PE can be begun, in the absence of an alternative diagnosis, even when not all of the above criteria are fulfilled3,4,6,9,10. The evident advantage of early initiation of therapy along with the decreased diagnostic threshold has resulted in a 7-fold increase of patients treated with PE for TTP-HUS from 1981 to 199711.
The symptoms of TTP are related to the presence of von Willebrand factor (VWF)-rich platelet thrombi in arterioles and capillaries. VWF is a multimeric plasma glycoprotein crucial for both platelet adhesion and aggregation, especially at the high shear rates present in the microvasculature. The size of VWF multimers is physiologically regulated in vivo by a specific metalloprotease, ADAMTS-13 (a disintegrin-like and metalloprotease with thrombospondin type 1 repeats)12.
A severe deficiency of ADAMTS-13 (< 5% of normal activity) may be specific for TTP13 and it has been proposed that severe ADAMTS-13 deficiency now defines TTP14,15. Because ADAMTS-13 deficiency, whether idiopathic or caused by an autoantibody, provides a possible explanation for the effectiveness of PE (removal of the autoantibody by apheresis; supply of ADAMTS-13 by plasma replacement), it has been suggested that the levels of this metalloprotease can be used to guide treatment decisions14,16–19. At present it is not possible to establish the sensitivity of ADAMTS-13 deficiency for identifying patients who may respond to PE. In seven reports, 45% to 100% of patients with TTP were reported to have severe deficiency of ADAMTS-13 activity19–25 while such a high rate has not been described in those with HUS19,20,23. However, the interpretation of these studies is limited by the absence of explicit criteria for distinguishing patients with TTP from patients with HUS. PE has been proven effective even in patients without deficiency of ADAMTS-13 activity, which makes it difficult to understand how PE is benificial2. In conclusion, the role of ADAMTS-13 activity in the diagnosis and treatment decisions in patients with TTP or HUS remains unknown.
Therapy with PE should be implemented in all patients with TTP-HUS and continued until the resolution of signs and/or symptoms and normalisation of laboratory tests; this can require long-term therapy. PE has some other disadvantages: first of all, it is not a risk-free procedure since a substantial number of major complications have been reported26,27. Furthermore, about 10% to 20% of TTP-HUS patients do not respond or have only an incomplete response2. Various different types of immunosuppressive treatment have been proposed for refractory patients14,29,30,32, including steroids and immunosuppressive or immune-modulating agents; however, the lack of robust data does not allow proper suggestion of such agents in the setting of acute refractory or chronic relapsing TTP28,32. Splenectomy has been proposed for patients with refractory or relapsing TTP, with reported remission rates of 50–100%29, but relapses have occurred in a considerable proportion of patients, most of them with severe ADAMTS-13 deficiency2,29,33,35. It has recently been shown that splenectomy can cause the disappearance of antibodies, normalisation of ADAMTS-13 activity and clinical remission in cases of refractory/relapsing TTP associated with anti-ADAMTS-13 autoantibodies. Other authors reported a low frequency of relapses in a large cohort of patients who underwent splenectomy30.
Rituximab, a chimaeric monoclonal antibody directed against the CD20 antigen present on B lymphocytes, is used in lymphoma patients and those with rheumatoid arthritis33. Its action relies on clearance of the B lymphocytes responsible for antibody production by complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity or directly by inducing apoptosis31,33. The understanding that ADAMTS-13 deficiency could be antibody-mediated first provided the rationale for the use of rituximab in TTP-HUS12, but its reported effectiveness even in TTP-HUS patients without antibody-mediated ADAMTS-13 deficiency as well as in cases of refractory/relapsing cases makes this monoclonal antibody a very attractive therapeutic agent33–35. The data suggest that the drug may not simply decrease ADAMTS-13 autoantibody production by depleting B cells, but that it may have additional mechanisms of action. Kameda et al.34 suggested that B-cell depletion by rituximab reduces excessive cytokine production in patients with secondary TTP, thus containing the level of VWF multimers within the normal range. At present, only data from case series have been published and many questions remain open regarding the target population, timing of initiation, duration of treatment and concomitant PE34–49. Here we describe four patients with refractory/relapsing idiopathic TTP-HUS who were successfully treated with rituximab (Table I).
Table I.
Patients’ characteristics
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age/gender | 28 male | 53 male | 52 male | 16 female |
| Relapsing/Refractory TTP | Relapsing TTP # | Refractory TTP | Refractory TTP | Refractory TTP |
| Neurological symptoms | None | Yes | Yes | None |
| Laboratory values at diagnosis | ||||
| Haemoglobin g/dL | 8 | 7.9 | 10.9 | 12.6 |
| Platelet count x109/L | 20 | 30 | 50 | 9 |
| Lactate dehydrogenase U/L | 1900 | 1500 | 629 | 2341 |
| Creatinine mg/dL | 1.14 | 2.5 | 1.3 | 0.71 |
| Schistocytes | ++ | ++ | ++ | ++++ |
| ADAMTS-13 activity^ | < 5% | 100% | 40%* | < 5% |
| ADAMTS-13 inhibitor¤ | >120 U/mL | No detectable | 65 U/mL* | >120 U/mL |
| PE volume | 1 | 1 | 1 | 1 |
| PE plasma | Solvent/detergent | Solvent/detergent | Solvent/detergent | Solvent/detergent |
| FFP (Octaplas®) | FFP (Octaplas®) | FFP (Octaplas®) | FFP (Octaplas®) | |
| N. of PE before R | None | 9 | 9 | 13 |
| Indication for R | Relapsing TTP | Refractory TTP | Refractory TTP | Refractory TTP |
| Laboratory values prior to R | ||||
| Haemoglobin g/dL | 11.7 | 9 | 7.6 | 8.7 |
| Platelet count x109/L | 54 | 80 | 80 | 89 |
| Lactate dehydrogenase U/L | 1870 | 850 | 568 | 404 |
| Creatinine mg/dL | 1.1 | 2.3 | 1.2 | 0.8 |
| Schistocytes | ++ | ++ | ++ | +++ |
| Complete remission after R | Yes | Yes | Yes | Yes |
| Days to CR from first R infusion | 14 | 7 | 30 | 14 |
| Toxicity | None | None | None | None |
| Duration of CR | 4+ months | 11+ months | 10+ months | 3+ months |
| Relapse | No | No | No | No |
| Status at last follow-up | Alive in CR | Alive in CR | Alive in CR | Alive in CR |
| ADAMTS-13 activity after R | 35% | NA | 90% | 25% |
| ADAMTS13 inhibitor after R | No detectable | NA | 8 | 16 |
Legend:
Initial episode of TTP diagnosed elsewhere 39 months – achie ved complete remission (CR) with 20 plasma exchange (PE) procedures and steroids prior to presenting in relapse; FFP = fresh-frozen plasma; CR= complete remission; R= rituximab; NA= not available; NV= normal value;
ADAMTS-13 activity (normal value: 50–150%);
ADAMTS-13 inhibitor (normal value: <17 U/mL); lactate dehydrogenase (normal value: 240–480 U/L);
Had undergone PE prior to initial ADAMTS-13 testing .
Case reports
Case 1
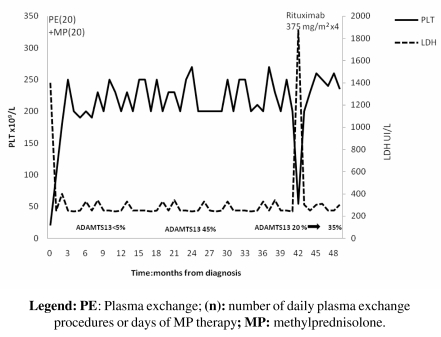
In October 2005 a 28-year-old male was diagnosed with idiopathic TTP-HUS. At presentation, the patient had anaemia (haemoglobin 8 g/dL), an abnormal platelet count of 20x109/L and a low-grade fever; he did not have nausea, vomiting or any focal neurological deficiency. A direct antiglobulin test (DAT) was negative; his lactate dehydrogenase (LDH) concentration was 1900 UI/L (normal value, n.v. 240–480), and that of the indirect bilirubin was 2.5 mg/dL. Schistocytes, helmet forms, nucleated red blood cells (RBC) and marked thrombocytopenia were noted on a peripheral blood smear. ADAMTS-13 activity was <5% (n.v. 50–150%) due to the presence of inhibitory antibodies against ADAMTS-13 (titre >120 U/mL; n.v. <17 U/mL). ADAMTS-13 activity was determined by a fluorimetric method and IgG anti-ADAMTS-13 by an enzyme-linked immunosorbent assay (ELISA). The patient was initially treated with daily PE and methylprednisolone i.v. 1 mg/kg/die and achieved a complete remission after 20 sessions of PE. At that time, ADAMTS-13 activity was 45% and antibodies were not detectable. The complete remission lasted for 39 months. In October 2008 the patient relapsed, and ADAMTS-13 activity was 20%. Rituximab was initiated, given as a single agent once a week for 4 weeks, at a dose of 375 mg/m2 for each single infusion (Figure 1). Laboratory values normalised after the first two infusions (platelet count increased to 133 x 109/L and LDH decreased to 455 UI/L) and antibodies against ADAMTS-13 were not detectable; ADAMTS-13 activity was 35%. At the present, the patient is in complete remission.
Figure 1.
Changes in LDH and platelet count during treatment and in the follow-up period (October 2005– February 2009) for patient n. 1
Case 2
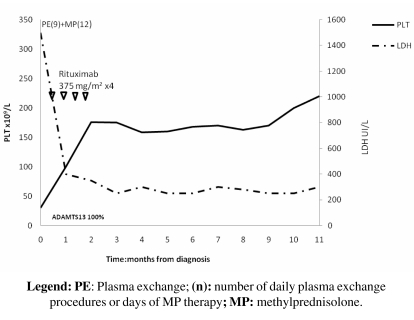
In March 2008 a 53-year-old male with suspected TTP-HUS was admitted to our division because of the onset of behavioural changes, fainting, loss of bladder sphincter control, anaemia and thrombocytopenia. His platelet count was 30x109/L and haemoglobin concentration 7.9 g/dL; LDH was increased to 1500 UI/L and serum creatinine was 2.5 mg/dL. The patient’s coagulation profile was normal. A peripheral blood smear showed 2+ schistocytes and the DAT was negative. ADAMTS-13 activity was 100% and antibodies against ADAMTS-13 were not detectable. ADAMTS-13 activity was determined by fluorimetry and IgG anti-ADAMTS-13 by ELISA. PE and methylprednisolone i.v 1 mg/Kg/die were initiated promptly. Because the response was only partial (platelets: 80x109/L) after nine sessions of PE, we started, in accordance with current literature33–51,54,55, treatment with rituximab (at a standard dosage of 375mg/m2 once a week for 4 weeks) while the methylprednisolone therapy was reduced (from 1 mg/kg during the first week to 0.5 mg/kg during the second week). Laboratory values recovered quickly after the first dose of rituximab and remained stable during all the courses of the therapy. After a follow-up of 11 months, the patient has achieved a complete remission (Figure 2).
Figure 2.
Changes in LDH and platelet count during rituximab treatment and in the follow-up period (March 2008– February 2009) for patient n. 2
Case 3
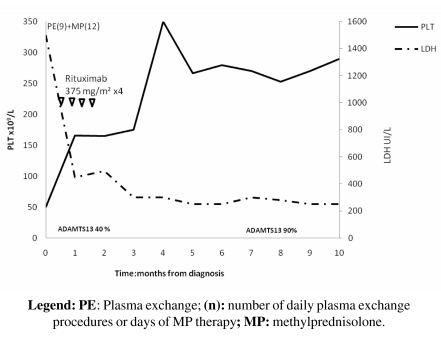
In April 2008 a 52-year-old male was admitted to an intensive care ward for dizziness, bilious vomiting, anaemia and thrombocytopenia. He was diagnosed as having idiopathic TTP-HUS and PE and methylprednisolone 1mg/kg/die were initiated promptly. ADAMTS-13 activity was assayed after only six PE procedures and found to be 40%; the anti-ADAMTS-13 antibody (IgG) titre was 65 U/mL. As for the other cases, ADAMTS13 activity was determined by a fluorimetric method and IgG anti-ADAMTS-13 by ELISA. PE was continued up to nine procedures; however, although there was modest recovery of platelet count from 50 x 109/L to 80 x 109/L, the presence of anaemia (haemoglobin, 7.6 g/dL), still high LDH (568 UI/L) and 2+ schistocytes on a peripheral blood smear led us to consider the patient as refractory to PE treatment. Therapy with rituximab (4 weekly doses of 375mg/m2) was, therefore, started. ADAMTS-13 activity increased promptly to 90% and the IgG anti-ADAMTS-13 titre decreased to 8 U/mL after the fourth infusion of rituximab. After a follow-up of 10 months, the patient is clinically stable and in complete remission (Figure 3).
Figure 3.
Changes in LDH and platelet count during rituximab treatment and during the follow-up period (April 2008 – February 2009) for patient n. 3
Case 4
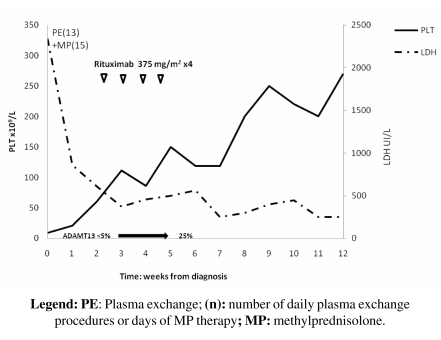
In November 2008 a 16-year-old female was admitted to our Division of Haematology because of jaundice, severe thrombocytopenia and increased LDH. Her platelet count was 9x109/L, haemoglobin 12.6 g/dL, LDH 2341 UI/L and serum indirect bilirubin 16.6 mg/dL; the fibrinogen concentration and coagulation profile were normal. A peripheral blood smear showed 4+ schistocytes; the DAT was negative. ADAMTS-13 activity was <5% because of the presence of specific antibodies (titre, >120 U/mL). The patient was diagnosed as having idiopathic TTP-HUS and was initially treated with PE and methylprednisolone i.v. 1 mg/kg/die for 13 days. The patient did not recover completely (platelet count 89 x 109/L, hemoglobin 8.7 g/dL, LDH 404 UI/L) and she was considered refractory to PE treatment. Rituximab was initiated at the standard dose (Figure 4). Laboratory values normalised after two rituximab infusions and ADAMTS-13 activity increased to 25%. Three months after the end of therapy the patient is in complete remission.
Figure 4.
Changes in LDH and platelet count during treatment with rituximab and in the follow-up period (November 2008–February 2009) for patient n. 4
Discussion
Idiopathic TTP/HUS is a life-threatening disease that is still difficult to manage properly. The current standard therapy is PE performed daily until resolution of symptoms and/or normalisation of laboratory values (recovery of platelet count, increase of haemoglobin, decrease of LDH and absence of
The discovery that deficiency of ADAMTS-13 may be related to the severity and prognosis of idiopathic TTP-HUS has raised many questions about the need to test this marker at diagnosis and/or during remission with the aim of identifying patients at high risk of recurrent TTP/HUS15–18. Some uncertainties remain since ADAMTS-13 deficiency is not detected in all patients who may be appropriately diagnosed as having TTP-HUS2,34,35. At present, there are insufficient data to manage TTP patients safely based on levels of ADAMTS-13.
PE may be an effective treatment for TTP because of clearance of antibodies against ADAMTS-13, but it poorly effective in cases of secondary TTP. Consequently, it has been postulated that PE may not be effective in patients without ADAMTS-13 deficiency, but some reports have been proven that it can be2,33,34. For these reasons, PE remains the standard treatment for idiopathic TTP-HUS independently of the detection of ADAMTS-13 antibodies2.
Despite the considerable improvement in survival associated with prompt initiation of daily PE in patients with idiopathic TTP-HUS, some patients have an incomplete or delayed response or do not respond at all to this standard treatment2,10–33,51. About 10% to 20% of TTP-HUS patients are refractory (even after several procedures) or relapse a few years after treatment46. Although there is no generally accepted definition of refractory disease, escalation of therapy should be considered after 7 to 14 days of treatment with daily PE and steroids, when the clinical picture deteriorates or laboratory findings do not improve2,52,53. In these cases, additional therapy with immunosuppressive drugs (such as steroids, cyclophosphamide or cyclosporine) has not been proven to be beneficial in all investigations14,29,30.
There is increasing evidence that rituximab has a role to play in the treatment of acute refractory/relapsing idiopathic TTP/HUS, particularly in those cases in which ADAMTS-13 inhibitory antibodies are present36–49,56. In our experience, three out of four patients with severe, antibody-mediated deficiency of ADAMTS-13 had a complete remission after treatment with rituximab. We, and others34,35, found that this drug was effective even in a patient with refractory TTP-HUS without ADAMTS-13 deficiency. The mechanism of action of rituximab in such patients is unclear. Kameda et al. suggested that B-cell depletion by the anti-CD20 monoclonal antibody may reduce excessive cytokine production in patients with secondary TTP and thus contain the level of VWF multimers within the normal range33,34. This mechanism may also explain the success of PE in patients without ADAMT-13 deficiency or in those with secondary TTP-HUS.
All our patients achieved clinical remission and normalisation of laboratory values after a few infusions of rituximab without experiencing any adverse reaction. The median follow-up was 7 months (range, 3 to 11); this is similar to the follow-up in previous reports34–49.
Our results and those of others suggest that rituximab could be a useful treatment in TTP patients but there is still a lack of reliable data on the optimal time schedule and dose, duration of therapy, long-term maintenance of remission and side effects in patients with acute refractory and relapsing TTP. When determining the role of rituximab in this setting, the risks and benefits of different treatments must be balanced. PE is expensive and often associated with complications related to venous access lines, including infection, catheter thrombosis, hypoxaemia, hypotension and transfusion reactions50. Rituximab induces rapid remissions in a high proportion of patients with acute refractory TTP treated early after 7–14 PE sessions and seems to have few side effects33,45. Clinical trials are now ongoing and will probably provide answers to some of the most debated questions about the clinical use of ADAMTS-13 measurements as well as the appropriate setting for therapy with rituximab. In the meantime, the use of rituximab fairly early (within 7–14 days) in patients with refractory/relapsing TTP-HUS can be beneficial. Although the optimal dose of rituximab in this setting has not been defined, the standard treatment of 375 mg/m2 once weekly for 4 weeks seems appropriate. The effectiveness of different schedules of therapy, such as only one or two doses of 375 mg/m2 or a reduction of each dose administered, needs further study.
In conclusion, rituximab has a role to play in the treatment of patients with a first episode of acute refractory autoantibody-induced TTP/HUS. Induction of remission by rituximab is associated with the disappearance of ADAMTS-13 inhibitors and normalisation of ADAMTS-13 activity. Rituximab may also be effective for treating patients with relapsed/refractory TTP-HUS without ADAMTS-13 deficiency. In our opinion and based on the available data, rituximab may be a therapeutic option in patients with idiopathic TTP-HUS, with or without ADAMTS-13 deficiency, who fail to respond after 7–14 days of standard treatment.
References
- 1.Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45:139–59. [Google Scholar]
- 2.Vesely SK, George JN, Lämmle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–8. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 3.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325:393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 4.Rock G, Shumak K, Kelton J, et al. Thrombotic thrombocytopenic purpura: outcome in 24 patients with renal impairment treated with plasma exchange. Transfusion. 1992;32:710–4. doi: 10.1046/j.1537-2995.1992.32893032096.x. [DOI] [PubMed] [Google Scholar]
- 5.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. N Engl J Med. 1991;325:398–403. doi: 10.1056/NEJM199108083250605. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CE, Damon LE, Ries CA, Linker CA. Thrombotic microangiopathies in the 1980s: clinical features, response to treatment, and the impact of the human immunodeficiency virus epidemic. Blood. 1992;80:1890–5. [PubMed] [Google Scholar]
- 7.Hayward CPM, Sutton DMC, Carter WH, Jr, et al. Treatment outcomes in patients with adult thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Arch Intern Med. 1994;154:982–7. [PubMed] [Google Scholar]
- 8.Elkins SL, Wilson PP, Files JC, Morrison FS. Thrombotic thrombocytopenic purpura: evolution across 15 years. J Clin Apheresis. 1996;11:173–5. doi: 10.1002/(SICI)1098-1101(1996)11:4<173::AID-JCA1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Lara PN, Jr, Coe TL, Zhou H, et al. Improved survival with plasma exchange in patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Am J Med. 1999;107:573–9. doi: 10.1016/s0002-9343(99)00286-7. [DOI] [PubMed] [Google Scholar]
- 10.George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood. 2000;96:1223–9. [PubMed] [Google Scholar]
- 11.Clark WF, Rock GA, Buskard N, et al. Therapeutic plasma exchange: an update from the Canadian Apheresis Group. Ann Int Med. 1999;131:453–62. doi: 10.7326/0003-4819-131-6-199909210-00011. [DOI] [PubMed] [Google Scholar]
- 12.Fankouri F, Vernant JP, Veyradier A, et al. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13 deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood. 2005;106:1932–7. doi: 10.1182/blood-2005-03-0848. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi V, Robles R, Alberio L, et al. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–3. doi: 10.1182/blood-2002-02-0344. [DOI] [PubMed] [Google Scholar]
- 14.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 15.Tsai H-M. Deficiency of ADAMTS13 and thrombotic thrombocytopenic purpura. Blood. 2002;100:3839–40. doi: 10.1182/blood-2002-07-2241. [DOI] [PubMed] [Google Scholar]
- 16.Mannucci PM. Thrombotic thrombocytopenic purpura: a simpler diagnosis at last? Thromb Haemost. 1999;82:1380–1. [PubMed] [Google Scholar]
- 17.Cines DB, Konkle BA, Furlan M. Thrombotic thrombocytopenic purpura: a paradigm shift? Thromb Haemost. 2000;84:528–35. [PubMed] [Google Scholar]
- 18.Lankford KV, Hillyer CD. Thrombotic thrombocytopenic purpura: new insights in disease pathogenesis and therapy. Transfus Med Rev. 2000;14:244–57. doi: 10.1053/tm.2000.7394. [DOI] [PubMed] [Google Scholar]
- 19.Mori Y, Wada H, Gabazza EC, et al. Predicting response to plasma exchange in patients with thrombotic thrombocytopenic purpura with measurement of vWF-cleaving protease activity. Transfusion. 2002;42:572–80. doi: 10.1046/j.1537-2995.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 20.Furlan M, Robles R, Galbusera M, et al. Von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the haemolytic uremic syndrome. N Engl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 21.Tsai H-M, Lian ECY. Antibodies to von-Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore JC, Hayward CPM, Warkentin TE, Kelton JG. Decreased von Willebrand factor protease activity associated with thrombocytopenic disorders. Blood. 2001;98:1842–6. doi: 10.1182/blood.v98.6.1842. [DOI] [PubMed] [Google Scholar]
- 23.Veyradier A, Obert B, Houllier A, et al. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98:1765–72. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 24.Raife TJ, Lentz SR, Atkinson BS, et al. Factor V Leiden: a genetic risk factor for thrombotic microangiopathy in patients with normal von Willebrand factor-cleaving protease activity. Blood. 2002;99:437–42. doi: 10.1182/blood.v99.2.437. [DOI] [PubMed] [Google Scholar]
- 25.Rick ME, Moll S, Taylor MA, et al. Clinical use of a rapid collagen-binding assay for von Willebrand factor cleaving protease in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2002;88:598–604. [PubMed] [Google Scholar]
- 26.Rizvi MA, Vesely SK, George JN, et al. Complications of plasma exchange in 71 consecutive patients treated for clinically suspected thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Transfusion. 2000;40:896–901. doi: 10.1046/j.1537-2995.2000.40080896.x. [DOI] [PubMed] [Google Scholar]
- 27.McMinn JR, Thomas IA, Terrell DR, et al. Complications of plasma exchange in patients treated for clinically suspected thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: an additional study of 78 consecutive patients. Transfusion. 2003;43:415–6. doi: 10.1046/j.1537-2995.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 28.Allford SL, Hunt BJ, Rose P, Machin S. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anemia. Br J Haematol. 2003;120:556–73. doi: 10.1046/j.1365-2141.2003.04049.x. [DOI] [PubMed] [Google Scholar]
- 29.Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology 2004. Washington DC: American Society of Hematology; 2004. pp. 407–23. [DOI] [PubMed] [Google Scholar]
- 30.Kappers-Klunne MC, Wijermans P, Fijnheer R, et al. Splenectomy for the treatment of thrombotic thrombocytopenic purpura. Br J Haematol. 2005;130:768–76. doi: 10.1111/j.1365-2141.2005.05681.x. [DOI] [PubMed] [Google Scholar]
- 31.Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 32.Quintini G, Barbera V, Iannitto E, Mariani G. Potential strategies for the treatment of plasma exchange-resistant thrombotic thrombocytopenic purpura. Br J Haematol. 2001;113:560–2. doi: 10.1046/j.1365-2141.2001.02782-3.x. [DOI] [PubMed] [Google Scholar]
- 33.Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol Rev. 2008;141:149–69. doi: 10.1111/j.1365-2141.2008.07054.x. [DOI] [PubMed] [Google Scholar]
- 34.Kameda T, Dobashi H, Kittaka K, et al. Two cases of refractory thrombotic thrombocytopenic purpura associated with collagen vascular disease were significantly improved by rituximab treatment. Clin Rheumatol. 2007;26:2159–62. doi: 10.1007/s10067-007-0631-0. [DOI] [PubMed] [Google Scholar]
- 35.Reddy PS, Deauna-Limayo D, Cook JD, et al. Rituximab in the treatment of relapsed thrombotic thrombocytopenic purpura. Ann Hematol. 2005;84:232–5. doi: 10.1007/s00277-004-0964-6. [DOI] [PubMed] [Google Scholar]
- 36.Yomtovian R, Niklinski W, Silver B, et al. Rituximab for chronic recurring thrombotic thrombocytopenic purpura: a case report and review of the literature. Br J Haematol. 2004;124:787–95. doi: 10.1111/j.1365-2141.2004.04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chemnitz J, Draube A, Scheid C, et al. Successful treatment of severe thrombotic thrombocytopenic purpura with the monoclonal antibody rituximab. Am J Hematol. 2002;71:105–8. doi: 10.1002/ajh.10204. [DOI] [PubMed] [Google Scholar]
- 38.Sallah S, Husain A, Wan JY, Nguyen NP. Rituximab in patients with refractory thrombotic thrombocytopenic purpura. J Thromb Haemost. 2004;2:834–6. doi: 10.1111/j.1538-7836.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad A, Aggarwal A, Sharma D, et al. Rituximab for treatment of refractory/relapsing thrombotic thrombocytopenic purpura (TTP) Am J Hematol. 2004;77:171–6. doi: 10.1002/ajh.20166. [DOI] [PubMed] [Google Scholar]
- 40.Koulova L, Alexandrescu D, Dutcher JP, et al. Rituximab for the treatment of refractory idiopathic thrombocytopenic purpura (ITP) and thrombotic thrombocytopenic purpura (TTP): report of three cases. Am J Hematol. 2005;78:49–54. doi: 10.1002/ajh.20243. [DOI] [PubMed] [Google Scholar]
- 41.Millward PM, Bandarenko N, Chang PP, et al. Cardiogenic shock complicates successful treatment of refractory thrombotic thrombocytopenic purpura with rituximab. Transfusion. 2005;45:1481–6. doi: 10.1111/j.1537-2995.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 42.Scott SM, Szczepieorkowski ZM. Rituximab for TTP. Am J Hematol. 2005;80:878. doi: 10.1002/ajh.20364. [DOI] [PubMed] [Google Scholar]
- 43.Yassa SK, Blessios G, Marinides G, Venuto RC. Anti-CD20 monoclonal antibody (Rituximab) for life-threatening hemolytic–uremic syndrome. Clin Transplant. 2005;19:423–6. doi: 10.1111/j.1399-0012.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 44.Daraibi K, Berg AH. Rituximab can be combined with daily plasma exchange to achieve effective B-cell depletion and clinical improvement in acute autoimmune TTP. Am J Clin Pathol. 2006;125:592–7. doi: 10.1309/RLNM-J01W-BJRN-LH03. [DOI] [PubMed] [Google Scholar]
- 45.Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol. 2006;136:451–61. doi: 10.1111/j.1365-2141.2006.06448.x. [DOI] [PubMed] [Google Scholar]
- 46.Rüfer A, Brodmann D, Gregor M, et al. Rituximab for acute plasma-refractory thrombotic thrombocytopenic purpura. A case report and concise review of the literature. Swiss Med Wkly. 2007;137:518–24. doi: 10.4414/smw.2007.11908. [DOI] [PubMed] [Google Scholar]
- 47.Chow KV, Carroll R, Branley P, et al. Anti-CD20 antibody in thrombotic thrombocytopenic purpura refractory to plasma exchange. Intern Med J. 2007;37:329–32. doi: 10.1111/j.1445-5994.2007.01338.x. [DOI] [PubMed] [Google Scholar]
- 48.Jasti S, Coyle T, Gentile T, et al. Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J Clin Apher. 2008;23:151–6. doi: 10.1002/jca.20172. [DOI] [PubMed] [Google Scholar]
- 49.Arici B, Trendelenburg M. Successful treatment of thrombotic thrombocytopenic purpura (TTP) with rituximab. Ther Umsch. 2008;65:710–2. doi: 10.1024/0040-5930.65.12.710. [DOI] [PubMed] [Google Scholar]
- 50.Lalmuanpuii J, Yalamanchili K, Fircanis S, Nelson JC. Hypersensitivity to plasma exchange in a patient with thrombotic thrombocytopenic purpura. J Clin Apher. 2009;24:18–20. doi: 10.1002/jca.20183. [DOI] [PubMed] [Google Scholar]
- 51.Bresin E, Gastoldi S, Daina E, et al. Rituximab as preemptive treatment in patients with thrombotic thrombocytopenic purpura and evidence of anti-ADAMTS13 autoantibodies. Thromb Haemost. 2009;101:233–8. [PubMed] [Google Scholar]
- 52.Pereira A, Mazzara R, Monteagudo J, et al. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: a multivariate analysis of factors predicting the response to plasma exchange. Ann Hematol. 1995;70:319–23. doi: 10.1007/BF01696619. [DOI] [PubMed] [Google Scholar]
- 53.Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. British Committee for Standards in Haematology, Blood Transfusion Task Force. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 54.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112:11–8. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mannucci PM, Lavoretano S, Peyvandi F. The thrombotic microangiopathies. Blood Transfus. 2005;3:120–35. [Google Scholar]
- 56.Franchini M, Veneri D, Lippi G, Stenner R. The efficacy of rituximab in the treatment of inhibitor-associated hemostatic disorders. Thromb Haemost. 2006;96:119–25. [PubMed] [Google Scholar]