Abstract
Background
The Mirasol Pathogen Reduction Technology system for platelets and plasma uses riboflavin and UV light to introduce irreparable lesions into nucleic acids thereby inhibiting pathogen and white blood cell replication and reducing the load of infectious pathogens. The aim of the present study was to evaluate low plasma buffy coat platelet concentrates obtained from the OrbiSac System and to examine the effects on the development of platelet storage lesion during storage in platelet additive solution.
Material and Methods
Twenty buffy coat platelet concentrates were generated by pooling five individual units using the OrbiSac System. Riboflavin was added during the final pooling step, and the units were exposed to UV light. The bag was removed after the target energy of 6.24 J/mL had been delivered and 150 mL of platelet additive solution were added prior to storage. Platelet quality was assessed by pH, swirl, CD62P expression, lactate dehydrogenase, lactate production and glucose consumption rates over 7 days of storage.
Results
Buffy coat platelet concentrates generated on the OrbiSac contained an average 3.5 ± 0.6 x 1011 platelets at a concentration of 2976± 406 x 106/mL. After addition of 150 mL platelet additive solution the storage concentration was 1043 ± 148x 106/mL. Values obtained for pH, lactate production and glucose consumption rates were all within the limits of previously established correlations between in vitro cell quality and in vivo performance of Pathogen Reduction Technology-treated platelets in plasma.
Discussion
In vitro studies show that OrbiSac-derived platelets treated with the Mirasol Pathogen Reduction Technology system preserve adequate function, which would indicate acceptable in vitro viability.
Keywords: Pathogen inactivation, platelet storage, Mirasol, buffy coat platelet concentrates
Introduction
New approaches to blood safety have dramatically reduced the risk of infectious contamination of blood components. Currently most of the residual transfusion-transmitted infections occur as the result of the interval between the time the donor is infected and the time at which tests are capable of detecting the agent, the so-called “window period” which has been considerably reduced by the increased sensitivity of nucleic acid testing1. However, the safety of blood transfusions is still compromised by contamination of blood products with a variety of pathogens such as viruses, bacteria and parasites, including emerging pathogens2,3. Bacterial contamination of platelet concentrates is estimated to occur in between 1 per 900 and 1 per 100,000 units4. The most promising approaches so far to reduce the risk of contamination are different pathogen reduction systems5. Two main systems have been developed for platelet concentrates; psoralen light treatment and riboflavin (vitamin B2) light treatment. Among the different psoralen compounds, atmotosalen (S-59) is the most used and has demonstrated promising results for pathogen inactivation while preserving platelet function6. Among riboflavin-based systems that target pathogen nucleic acids, the Mirasol Pathogen Reduction Technology (Mirasol PRT; CaridianBCT, Lakewood, CO, USA) system has been well described7–11. The Mirasol PRT System for platelets and plasma uses riboflavin and UV light to introduce irreparable lesions into nucleic acids thereby inhibiting pathogen and white blood cell (WBC) replication.
This procedure has been the object of numerous in vitro studies to ensure safety margins in terms of toxicity to the cells and to the recipients11. Although PRT-treated platelets had significantly poorer post-transfusion recovery and survival than control, untreated platelets, the PRT-treated platelets retained sufficient in vivo efficacy for clinical use12,13.
A clinical evaluation in a multicentre patient trial followed, which established that PRT treatment is safe and platelet functionality is maintained14. In order to allow future predictions of in vivo platelet performance based on in vitro cell quality measurements, analyses were performed to evaluate the relationship between in vitro and in vivo parameters of platelet function12,13. In these studies, pH (at 22° C) and lactate production were found to be most strongly correlated with the in vivo recovery and survival of PRT-treated platelets12,13. Glucose consumption rate and swirl also showed some correlation with these in vivo parameters, though to a lesser extent. pO2, pCO2 and P-selectin expression in PRT-treated platelets were poorly correlated with in vivo platelet recovery and survival12,13.
During the last few decades, the development and use of platelet additive solutions have improved both the quality of platelet storage and the results in transfusion medicine15. Storing platelets in a platelet additive solution has become an attractive alternative to their storage in plasma because the former strategy increases the amount of plasma available for other purposes and may reduce the incidence of non-haemolytic transfusion reactions and transfusion-related acute lung injury (TRALI)15.
The aim of this study was to examine the effects of the PRT process on the development of platelet storage lesion during the storage of platelets in a platelet additive solution.
Material and methods
Twenty plasma buffy coat platelet concentrates (BCPC) were generated by pooling five individual units, using the Gambro BCT OrbiSac Buffy Coat System (OrbiSac System, Gambro Bct, Lund, Sweden). The buffy coat method involves a hard-spin of the original whole blood collection, separating the whole blood into three distinct layers: red cells, white cells and platelets (buffy coat) and plasma. Plasma and red cells were removed and used as transfusable components. Buffy coats of approximately 50 mL and a 30–40% haematocrit were generated. The OrbiSac BC system then pooled five buffy coats with suspension medium (30–35 mL Riboflavin), centrifuged the pooled buffy coats and passed the platelet concentrate through an in-process leucoreduction filter. The platelet concentrate was transferred into an Illumination/Storage Bag. The bag was placed in the Illuminator and exposed to UV light with a linear agitation of 120 cpm and a product temperature of = 37°C. After the target energy of 6.24 J/mL had been delivered, the bag was removed and 150 mL of platelet additive solution were added prior to storage.
The in vitro cell quality of the platelet products was evaluated over 7 days. This study was intended to verify the compatibility of platelet concentrates obtained using the OrbiSac System with the PRT System. Five ABO-identical whole blood units were collected for each single BCPC product made. The BCPC was treated in a small volume of plasma (approximately 30–45 mL plasma/1x1011 cells) and stored in platelet additive solution at a final storage concentration of 980–1920x106/mL.
All platelet concentrates were stored for 7 days under standard blood bank conditions. A small sample was removed from the storage bag on days 0, 1, 3, 5 and 7 for cell quality analysis of the following parameters; cell counts, pH (days 1, 3, 5 and 7), glucose (days 5 and 7), lactate concentrations (days 5 and 7), swirl (days 0, 1, 5 and 7), P-selectin (CD62) (days 5 and 7) and lactate dehydrogenase (LDH)(days 3, 5 and 7).
Glucose and LDH were measured using a Modular (Hitachi High-Technologies corp., Tokyo, Japan), lactate was measured with an ABL 725 (Radiometer, Copenhagen, Denmark), and cells were counted with an Advia 2120 machine (Bayer HealthCare, Tarrytown, NY, USA). P-selectin was measured by flow cytometry (FACS-Alibur and CellQuest, Becton-Dickinson, Franklin Lakes, NJ, USA). The swirling phenomenon was recorded by visual inspection and graded 0 (no swirl) to 3 (excellent swirl) by trained technicians. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation (SD).
Results
BCPC generated from the OrbiSac contained an average 3.5 ± 0.6 x 1011 platelets at a concentration of 2,976± 406 x 106/mL. After addition of 150 mL platelet additive solution the storage concentration was, on average, 1,043 ± 148x 106/mL. The final platelet volume after addition of platelet additive solution was 290.3 ± 13.72 mL.
The results of the main cellular and biochemical measurements performed in the study are summarised in table I.
Table I.
The results from different parameters for in vitro cell quality of the platelet products evaluated over 7 days
| Day 1 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|
| pH | 6.89 ± 0.057 | 7.02 ± 0.043 | 6.90 ± 0.047 | 6.90 ± 0.052 |
| Glucose consumption rate; mmol/1012 platelets/hour | 0.041 ± 0.007 | 0.035 ±0.006 | ||
| Lactate; mmol/L) | 18.43 ± 1.108 | 11.35 ± 1.089 | 15.84 ± 1.455 | 17.81 ± 1.384 |
| Swirl | 3.00 ± 0 | 2.75 ± 0.380 | 2.20 ± 0.523 | 1.30 ± 0.523 |
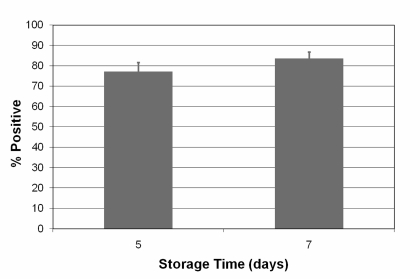
| P-selectin/CD62; percent positive cells | 77.27 ± 4.316 | 53.57 ± 3.181 | ||
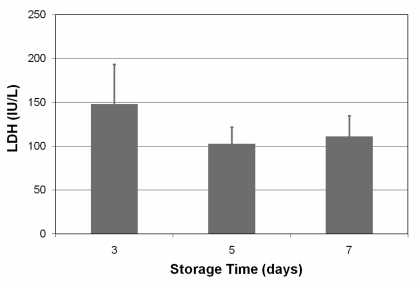
| LDH (IU/L) | 147.85 ± 45.297 | 102.90 ± 18.767 | 111.2 ± 23.410 |
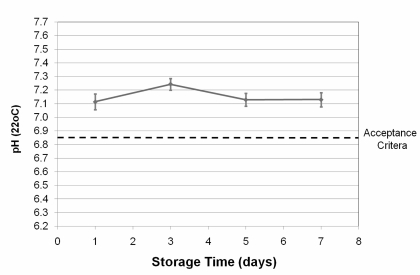
pH values, corrected for storage temperature, are shown in figure 1. The prediction for clinically acceptable platelet recovery and survival requires pH values greater than 6.8512,13. All pH values measured during the entire storage period were above 7.0 (Figure 1).
Figure 1.
pH (at 22°C) as a function of time (error bars indicate one standard deviation). The lowest pH value predicting acceptable platelet recovery and survival is represented by the dotted line.
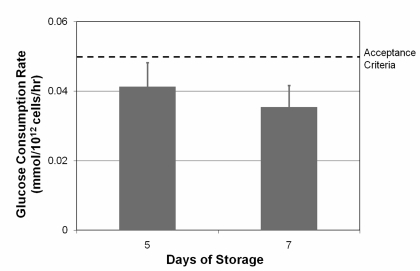
The average glucose consumption rate measured on days 5 and 7 was below the previously established acceptance criteria of 0.050 mmol/1012 platelets/hour (Figure 2).
Figure 2.
Glucose consumption rate as a function of time (error bars indicate one standard deviation). The highest glucose consumption rate to predict acceptable platelet recovery and survival is represented by the dotted line.
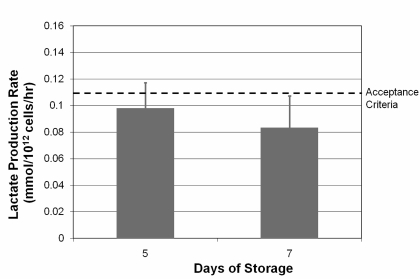
The results on lactate production rate are shown in figure 3. The average lactate production rate was within the limits of acceptable according to the predefined criterion (<0.110 mmol/1012 platelets/hour).
Figure 3.
Lactate production rate as a function of time (error bars indicate one standard deviation). The highest lactate production rate predicting acceptable platelet recovery and survival is represented by a dotted line.
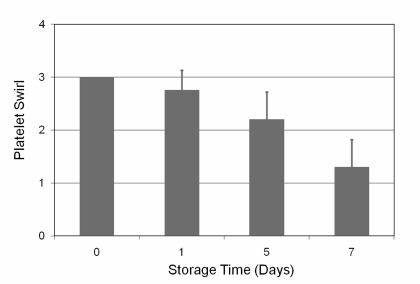
The swirl values decreased during the storage period, but swirl was detected for all units evaluated on days 0, 1, 5 and 7. The results are illustrated in figure 4.
Figure 4.
Swirl as a function of time (error bars indicate one standard deviation).
Surface expression of P-selectin (CD62P) was elevated, but within previously reported ranges (Figure 5).
Figure 5.
P-selectin (CD62P) expression as a function of time (error bars indicate one standard deviation).
Results of the measurement of LDH on days 3, 5 and 7 are presented in figure 6.
Figure 6.
LDH as a function of time (error bars indicate one standard deviation).
Discussion
Ever since platelet transfusions were shown to reduce mortality from haemorrhage in patients with acute leukaemia in the 1950s, the use of this therapy has grown steadily, such that it has become an essential part of the treatment of cancer, haematological malignancies, marrow failure, and haematopoietic stem cell transplantation19. However, platelet transfusions can transmit bacterial infections, which still threaten patients’ safety2,3. There are several types of platelet components; leucoreduced platelets can be used to reduce platelet alloimmunisation, transmission of infections, and febrile transfusion reactions, while gamma irradiation prevents transfusion-associated graft-versus-host disease. Although, significant progress has been made in reducing the risk of pathogen transmission by transfusions, there remains a continuing risk of transmission of microbiological agents to recipients. Pathogen inactivation technologies provide additional protection against both known and as-yet-unidentified agents. However, the impact of pathogen inactivation on a product’s functional quality and performance in the clinical setting has not yet been fully examined 20. The Mirasol PRT System for Platelets and Plasma is a new technology utilising riboflavin as a photosensitiser in combination with UV light. Riboflavin is a dietary nutrient with known pharmacokinetic and toxicology profiles11. Riboflavin can rapidly traverse lipid membranes where it non-specifically associates with nucleic acids. Upon exposure to light, riboflavin causes modification of the nucleic acid present in pathogens through oxidation of guanine residues and generation of reactive oxygen species. As a result of these photochemical processes, the nucleic acids of pathogens in blood products undergoing this treatment are rendered unable to replicate21. The aim of our study was to collect information about the effects of this PRT process on the development of storage lesion in OrbiSac-derived BCPC during storage in platelet additive solution.
We demonstrated that BCPC contained an average of 3.5 ± 0.6 x 1011 platelets at a concentration of 2976± 406 x 106/mL. After addition of 150 mL platelet addition solution the storage concentration was, on average, 1043 ± 148x 106/mL. These conditions are similar to those described by other authors22. During normal storage, platelets show activation of caspase 3 and consequent cleavage of gelsolin, which lead to apoptosis23. Therefore, independently of platelet activation, a loss of total platelets counts during storage is acceptable. Evaluation of the mechanism of apoptosis in platelets might be the basis for further development of novel strategies to enhance platelet viability during storage23. In our study platelet function was assessed by pH, swirl, CD62P expression, lactate production and glucose consumption rates over 7 days of storage. We observed elevated lactate production and glucose consumption and reduced pH rates after PRT treatment. These findings confirm previously reported observations, and are a result of cellular activation and increased metabolism10,13 22. Consistent with previously established correlations between in vitro cell quality and in vivo performance of PRT-treated platelets in plasma, these in vitro values predict acceptable platelet recovery and survival after storage in platelet additive solution12,13.
Platelet quality was assessed by CD62P expression on the platelet surface, which the level of expression is known to be a predictor of platelet viability24. CD62P expression was elevated in our study and increased as the period of storage became longer, reflecting increased platelet activation after PRT treatment. This has also been demonstrated for another pathogen inactivation method that utilises amotosalen and UVA light25,26. Measurements of CD62P expression are highly variable and values on day 5 of storage for PRT-treated platelets can range from 18% to 70%10,16. Similar variability can be observed in untreated control platelets, in which CD62P expression ranges from 8% to 48%17,18. However, the measured CD62P levels after PRT treatment remain within ranges that often are seen at the time of transfusion, and P-selectin positive platelets have been shown to continue to circulate and function in vivo27. It should also be emphasised that previous studies involving PRT-treated platelets failed to demonstrate a statistically significant correlation between P-selectin expression and in vivo platelet recovery and survival of platelets16.
The visual inspection of swirling is performed before the issue of a platelet concentrate and a swirling score can be recorded. The swirling effect can be seen when a fresh platelet unit is held up to a light source and slightly agitated. When normal discoid platelets are gently rocked, they scatter the incident light in different directions and a moving opalescence is caused by the changing orientation of platelets relative to the incident light. When platelets have undergone shape change (a disk-to-sphere transformation), they lose the ability to change their orientation. As a consequence, all activated (spherical) platelets scatter light in the same direction, resulting in the sample having a dull, unchanging appearance. The discoid shape of platelets can also be determined photometrically by the extent of shape change and the hypotonic shock response (HSR). The extent of shape change and the hypotonic shock response have been demonstrated to correlate well with in vivo viability28, and extent of shape change correlates strongly with morphology scores. In our study swirling remained clearly visible in all PRT-treated units, predicting acceptable in vivo viability. Platelet membrane integrity can also be assessed by measuring the amount the of the LDH enzyme that leaks from the cell. LDH levels in the supernatant of the stored products remained low and stable over time, indicating little or no lysis of platelets after treatment and during storage.
This study lack a potential control group, e.g. untreated platelets or platelets stored in other solutions such as plasma. However, we believe it is important to characterise the changes of platelet function related to activation during the PRT process, and our results suggest that despite the increases in different parameters of platelet activation, the quality of the platelet product is still in line with the criteria for acceptability.
Conclusion
To conclude, the quality of OrbiSac-derived BCPC treated with the Mirasol PRT system and stored in platelet additive solution for 7 days remains with the defined criteria for acceptability of stored platelets.
However, the PRT-treated platelets show signs of increased cellular activation and increased metabolism. The platelets had elevated CD62P expression, lactate production and glucose consumption rates after the PRT treatment. Swirling was detected in all units, reflecting viable platelets. These observations suggest that despite the general activation, platelets treated by this PRT method are suitable for storage.
Acknowledgments
We thank the staff at the Blood Bank at Haukeland University Hospital for helping with the sampling and analyses included in this study.
References
- 1.Cumming PD, Wallace EL, Schorr JB, Dodd RY. Exposure of patients to human immunodeficiency virus through the transfusion of blood components that test antibody-negative. N Engl J Med. 1989;321:941–6. doi: 10.1056/NEJM198910053211405. [DOI] [PubMed] [Google Scholar]
- 2.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–26. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–62. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 4.Walther-Wenke G. Incidence of bacterial transmission and transfusion reactions by blood components. Clin Chem Lab Med. 2008;46:919–25. doi: 10.1515/CCLM.2008.151. [DOI] [PubMed] [Google Scholar]
- 5.Solheim BG. Pathogen reduction of blood components. Transfus Apher Sci. 2008;39:75–82. doi: 10.1016/j.transci.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Wagner SJ, Skripchenko A, Myrup A, et al. Evaluation of in vitro storage properties of prestorage pooled whole blood-derived platelets suspended in 100 percent plasma and treated with amotosalen and long-wavelength ultraviolet light. Transfusion. 2009;49:704–10. doi: 10.1111/j.1537-2995.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodrich RP, Edrich RA, Li J, Seghatchian J. The Mirasol PRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus Apher Sci. 2006;35:5–17. doi: 10.1016/j.transci.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Asano H, Lee CY, Fox-Talbot K, et al. Treatment with riboflavin and ultraviolet light prevents alloimmunization to platelet transfusions and cardiac transplants. Transplantation. 2007;84:1174–82. doi: 10.1097/01.tp.0000287318.94088.d7. [DOI] [PubMed] [Google Scholar]
- 9.Cardo LJ, Salata J, Mendez J, et al. Pathogen inactivation of Trypanosoma cruzi in plasma and platelet concentrates using riboflavin and ultraviolet light. Transfus Apher Sci. 2007;37:131–7. doi: 10.1016/j.transci.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Picker SM, Steisel A, Gathof BS. Effects of Mirasol PRT treatment on storage lesion development in plasma-stored apheresis-derived platelets compared to untreated and irradiated units. Transfusion. 2008;48:1685–92. doi: 10.1111/j.1537-2995.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 11.Reddy HL, Dayan AD, Cavagnaro J, et al. Toxicity testing of a novel riboflavin-based technology for pathogen reduction and white blood cell inactivation. Transfus Med Rev. 2008;22:133–53. doi: 10.1016/j.tmrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Goodrich RP, Li J, Pieters H, et al. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–85. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 13.AuBuchon JP, Herschel L, Roger J, et al. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion. 2005;45:1335–41. doi: 10.1111/j.1537-2995.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Goodrich RP, Roberts T, Folléa G.Clinical evaluation of Mirasol PRT treated apheresis platelets in thrombocytopenic patients Transfusion 20084820A17944798 [Google Scholar]
- 15.Gulliksson H. Additive solutions for the storage of platelets for transfusion. Transfus Med. 2000;10:257–64. doi: 10.1046/j.1365-3148.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- 16.Ruane PH, Edrich R, Gampp D, et al. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44:877–85. doi: 10.1111/j.1537-2995.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Pujol S, Tonda R, Lozano M, et al. Effects of a new pathogen-reduction technology (Mirasol PRT) on functional aspects of platelet concentrates. Transfusion. 2005;45:911–9. doi: 10.1111/j.1537-2995.2005.04350.x. [DOI] [PubMed] [Google Scholar]
- 18.Apelseth TO, Hervig TA, Wentzel-Larsen T, Bruserud O. Cytokine accumulation in photochemically treated and gamma-irradiated platelet concentrates during storage. Transfusion. 2006;46:800–10. doi: 10.1111/j.1537-2995.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 19.Stroncek DF, Rebulla P. Platelet transfusions. Lancet. 2007;370:427–38. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- 20.Webert KE, Cserti CM, Hannon J, et al. Proceedings of a Consensus Conference: pathogen inactivation-making decisions about new technologies. Transfus Med Rev. 2008;22:1–34. doi: 10.1016/j.tmrv.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodrich RP. The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang. 2000;78(Suppl 2):211–5. [PubMed] [Google Scholar]
- 22.Li J, de Korte D, Woolum MD, et al. Pathogen reduction of buffy coat platelet concentrates using riboflavin and light: comparisons with pathogen-reduction technology-treated apheresis platelet products. Vox Sang. 2004;87:82–90. doi: 10.1111/j.1423-0410.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Xia Y, Bertino AM, et al. The mechanism of apoptosis in human platelets during storage. Transfusion. 2000;40:1320–9. doi: 10.1046/j.1537-2995.2000.40111320.x. [DOI] [PubMed] [Google Scholar]
- 24.Holme S, Sweeney JD, Sawyer S, Elfath MD. The expression of P-selectin during collection, processing, and storage of platelet concentrates: relationship to loss of in vivo viability. Transfusion. 1997;37:12–7. doi: 10.1046/j.1537-2995.1997.37197176945.x. [DOI] [PubMed] [Google Scholar]
- 25.van Rhenen DJ, Vermeij J, Mayaudon V, et al. Functional characteristics of S-59 photochemically treated platelet concentrates derived from buffy coats. Vox Sang. 2000;79:206–14. doi: 10.1159/000056732. [DOI] [PubMed] [Google Scholar]
- 26.Picker SM, Speer R, Gathof BS. Functional characteristics of buffy-coat PLTs photochemically treated with amotosalen-HCl for pathogen inactivation. Transfusion. 2004;44:320–9. doi: 10.1111/j.1537-2995.2003.00590.x. [DOI] [PubMed] [Google Scholar]
- 27.Michelson AD, Barnard MR, Hechtman HB, et al. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877–82. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holme S, Moroff G, Murphy S. A multi-laboratory evaluation of in vitro platelet assays: the tests for extent of shape change and response to hypotonic shock. Biomedical Excellence for Safer Transfusion Working Party of the International Society of Blood Transfusion. Transfusion. 1998;38:31–40. doi: 10.1046/j.1537-2995.1998.38198141495.x. [DOI] [PubMed] [Google Scholar]