Abstract
Tetrahydrobiopterin (BH4) is a naturally occurring cofactor essential for critical metabolic pathways. Studies suggest that BH4 supplementation may ameliorate autism symptoms; the biological mechanism for such an effect is unknown. To help understand the relation between central BH4 concentration and systemic metabolism and to develop a biomarker of central BH4 concentration, the relationship between cerebrospinal fluid BH4 concentration and serum amino acids was studied. BH4 concentration was found to be distributed in two groups, a lower and higher BH4 concentration group. Two serum amino acids, citrulline and methionine, differentiated these groups, and the ratio of serum citrulline-to-methionine was found to correlate with the cerebrospinal fluid BH4 concentration (r = −0.67, p < 0.05). Both citrulline and methionine are substrates in inflammation and oxidative stress pathways – two pathways that utilize BH4 and are abnormally activated in autism. These data suggests that central BH4 concentration may be related to systemic inflammation and oxidative stress pathways.
Keywords: tetrahydrobiopterin, autism, oxidative stress, inflammation, serum amino acids
Introduction
Tetrahydrobiopterin, sapropterin, or BH4 is a naturally occurring essential cofactor for several critical metabolic pathways, including the production of monoamine neurotransmitters, the breakdown of phenylalanine, and the production of nitric oxide. Using BH4 as a cofactor, two aromatic amino acid hydroxylases, tyrosine-3-hydroxylase and tryptophan-5-hydroxylase, catalyze the conversion of tyrosine and tryptophan to l-dopa and 5-hydroxytryptophan, respectively. These products are then further converted into dopamine and serotonin, respectively, and then further metabolized into norepinephrine and melatonin, respectively. BH4 is used as a cofactor with a third aromatic amino acid hydroxylase, phenylalanine-4-hydroxylase, to convert phenylalanine to tyrosine. Additionally, BH4 is essential in the production of nitric oxide, an important second messenger molecule used primarily for communication in vascular and neural tissues. In this reaction, nitric oxide synthase converts l-arginine and oxygen to l-citrulline and nitric oxide.
BH4 is synthesized from guanosine-5′-triphosphate, a purine nucleotide, by three enzymatic reactions (guanosine-5′-triphosphate cyclohydrolase, 6-pyruvoyltetrahydropterin synthase, and sepiapterin reductase; Thony et al., 2000). A deficiency in BH4 production can result in two neurological disorders. Hyperphenylalaninemia, specifically phenylketonuria type IV, presents at birth with elevated phenylalanine in the blood and represents approximately 1–2% of phenylketonuria cases. This condition results from a reduction in phenylalanine-4- hydroxylase activity due to a deficiency in the necessary cofactor for this enzyme, BH4, and should be differentiated from classic phenylketonuria where phenylalanine-4-hydroxylase itself is defective. If phenylalanine levels are not controlled early in life in phenylketonuria, severe mental retardation will develop. BH4 deficiency can also result in dopamine-responsive dystonia, a neurological disorder that demonstrates a wide range of symptoms and age of presentations but invariably patients demonstrate dystonia with diurnal variation (Hyland et al., 1993).
Less well understood is the role of BH4 in neurodevelopmental disorders. Unconjugated 6R-5,6,6,8-tetrahyrobiopterin cerebrospinal fluid concentration in autistic children has been reported to be 42% lower than in neurotypical children. Further analysis of the autistic group found that only autistic children younger than 7 years old, but not older autistic children, demonstrated significantly lower 6R-5,6,6,8-tetrahyrobiopterin levels as compared to the control group. This younger group also demonstrated a reduction in total pterins and biopterin (Tani et al., 1994).
Several investigators have demonstrated a therapeutic effect of BH4 supplementation in children with autism. Over a 7-year period from 1984 to 1990, Japanese researchers demonstrated moderate or marked improvement in 41 to 64% in over 300 mildly to severely affected autistic children with 1–3 mg/kg/day of BH4 in five open-label and one double-blind placebo-controlled studies (Naruse et al., 1984, 1990a,b; Nagahata et al., 1990; Nakane et al., 1990; Takesada et al., 1992). In the double-blind placebo control study, Naruse et al. (1990a) demonstrated that the improvement in autism symptoms was limited to autistic children younger than 5 years of age. While these aforementioned studies treated children with autism without knowing cerebrospinal fluid BH4 concentrations, both Fernell et al. (1997) and Danfors et al. (2005) measured cerebrospinal fluid BH4 concentration and treated children with cerebrospinal fluid BH4 concentrations less than 12 and 30 nM/L, respectively. Treating children with 3 mg/kg/day over a 12-week period in an open-labeled manner, Fernell et al. (1997) found improvements in social responsiveness, communication, and cognitive abilities and demonstrated a decrease in the baseline elevation in D2 receptor binding found by positron emission tomography. In a 26-week double-blind, randomized, placebo-controlled trial Danfors et al. (2005) demonstrated an improvement in social interactions with a dose of 3 mg/kg of BH4 given twice a day. This improvement was found to be greater for autistic children with higher intelligence. Lastly, most recently, Frye et al. (2010) reported that five of eight (63%) patients with autism and low pterin levels demonstrated improvement in social interactions and either verbal or non-verbal communication with treatment of 20 mg/kg/day of BH4 in an open-labeled manner.
Although studies have demonstrated that children with autism, as a group, have a reduced cerebrospinal fluid BH4 concentration and that some children with autism respond positively to BH4 supplementation, the biological mechanism for these findings has not been explained. It is clear from clinical studies that not all children with autism benefit from BH4 supplementation and that some children have side effects of BH4 supplementation, such as increased irritability and sleep disruption (Frye et al., 2010). Thus, understanding the biological mechanisms involved in BH4 supplementation is essential for determining which children may benefit from treatment with BH4 supplementation, understanding which underlying biological pathways are being modified with BH4 supplementation, and understanding which patients may be at risk for side effects of BH4 supplementation. To this end, this study examines whether biochemical markers of common metabolic pathways sampled from the blood are associated with cerebrospinal fluid BH4 concentration. Developing a biomarker of cerebrospinal fluid BH4 concentration would allow individuals with low central BH4 levels to be selected for supplementation without undergoing a lumbar puncture and would allow the effect of BH4 supplementation to be monitored during the treatment period in order to verify an adequate metabolic response to BH4 supplementation and optimize dosing of BH4 supplementation.
In order to consider what biochemical markers might respond to BH4 supplementation, the metabolic pathways that may benefit from BH4 supplementation in autism are considered. Here the focus is on children with autism since this is the population of individuals that has been found to have a positive response to BH4 (Frye et al., 2010). Examination of the metabolism characteristics unique to autism demonstrates that pathways that consume and recycle BH4 may be particularly dysfunctional in autism. For example, several lines of evidence suggest that children with autism manifest excessive inflammation and over activation of the immune system (Pardo et al., 2005; Dietert and Dietert, 2008; Castellani et al., 2009). Nitric oxide, a key mediator of inflammation and immune response, is produced by a BH4 dependent reaction. It is possible that a prolonged over activation of the immune system can result in excessive nitric oxide production and can, over time, deplete BH4. Children with autism might also have an underlying reduction in BH4 recycling for several reasons. Children with autism have been shown to have an impaired methylation capacity and markers of increased oxidative stress (James et al., 2004; Kern and Jones, 2006; Pardo and Eberhart, 2007; Deth et al., 2008). Folate is a key metabolite used to reduce oxidative stress. It is possible that a depletion of folate can result in reduced BH4 recycling through an over activation of the oxidative stress pathway. In addition, BH4 itself can act as an antioxidant, thus being depleted in the setting of high oxidative stress. Empirical evidence exists to support the notion that excess inflammation and oxidative stress can result in an acquired BH4 deficiency. Willoughby et al. (2009) demonstrated that BH4 levels were reduced below normal in three patients with subacute and chronic rabies infection. These investigators suggested that this acquired BH4 deficit was secondary to excess oxidation of BH4 and demonstrated significant neurological improvement in one of the two patients treated with 20 mg/kg of BH4.
Here it is suggested that prolonged excessive consumption and poor recycling of BH4 by non-central nervous system metabolic pathways deplete the available BH4, thereby preventing adequate BH4 levels in the central nervous system. To provide support for this notion, the relation between cerebrospinal fluid BH4 concentrations from a range of children with developmental and neurological disorders, and serum markers of oxidative stress and nitric oxide is examined. In this study serum amino acids are used as serum markers. It is hypothesized that these markers will be associated with cerebrospinal fluid BH4 concentration. Specifically it is hypothesized that a lower cerebrospinal fluid BH4 concentration will be associated with a reduction in methionine due to a reduced availability of folate and activation of the oxidative stress pathway (James et al., 2004), a reduction in arginine and an increase in citrulline due to an increase in the production of nitric oxide from inflammatory processes, and an increase in phenylalanine, tyrosine and tryptophan due to reduced aromatic amino acid hydroxylase activity.
Materials and Methods
The goal of this study was to examine the association of amino acid biochemical markers with cerebrospinal fluid BH4 concentration. This required a population with a wide range of cerebrospinal fluid BH4 concentrations, and the patients reviewed in this study provided a wide range of cerebrospinal fluid BH4 concentrations. Including controls in the current study would not be appropriate due to ethical considerations. Specifically, the risk-benefit ratio of the lumbar puncture procedure is prohibitively high for healthy typically developing individuals who would not otherwise require such a procedure. A chart review found twenty patients who underwent a diagnostic lumbar puncture with measurement of cerebrospinal fluid BH4 concentration. All patients had a neurological diagnosis. Children with autism were diagnosed with developmental delays and encephalopathy and were distinguished from individuals with global developmental delay without autistic features.
Patients provided permission for review of their medical records through an Institutional Review Board approved protocol. Patient characteristics, including positive family history of neurologic or psychiatric disorder, findings from electroencephalogram and magnetic resonance imaging were abstracted from the charts. Cerebrospinal fluid was obtained through a lumbar puncture under general sedation to rule out metabolic and/or infectious disorders. The cerebrospinal fluid of all patients demonstrated a normal number of white and red blood cells, protein, glucose, 5-methyltetrahydrofolate and succinyladenosine concentrations. Cerebrospinal fluid was collected with standardized reagent tubes and frozen at −80°C until BH4 analysis. BH4 content was measured by reversed-phase high performance liquid chromatography with electrochemical detection (Howells and Hyland, 1987). Eleven patients also had plasma amino acids measured within 4 months of the cerebrospinal fluid collection. Since plasma amino acids measured several times for most patients, the average values were calculated and used in further analysis.
Cluster analysis using the Ward technique as implemented by the “CLUSTER” procedure of SAS was used to determine if there were groups with different cerebrospinal fluid BH4 concentrations, and if there were different groups, how many. Groups were analyzed with respect to differences in age, positive family history, spikes or slowing on electroencephalogram, abnormal magnetic resonance imaging and 5-hydroxyindoleacetic acid, homovanillic acid and 3-O-methyldopa concentrations. Defining these groups also allowed the assignment of prior probabilities for each group. These prior probabilities were important for examining the performance of the discriminant functions defined in the next step.
To determine which plasma amino acids were related to cerebrospinal fluid BH4 and could be used to predict the groups identified, a stepwise discriminant analysis as implemented by the “STEPDISC” procedure of SAS was used to select the plasma amino acids that, in combination, could differentiate the BH4 groups found. The specific plasma amino acids entered into the discriminant analysis included tyrosine, tryptophan, phenylalanine, citrulline, arginine, and methionine. In a separate analysis, age was also added to the model since cerebrospinal fluid BH4 concentrations may be age dependent. Once the stepwise discriminant analysis selected the significant plasma amino acids, the selected variables were entered into the “DISCMIN” procedure in SAS, with and without prior probabilities, to determine the performance of the discriminant function.
Results
High and low BH4 groups and their characteristics
Cluster analysis was used to determine if there were distinct groups of patients with a common range of BH4 values. The cluster analysis identified two groups based on BH4 values: a cluster with 14 values with a cerebrospinal fluid BH4 concentration of 31 nM/L and below (mean = 20.14, SE = 0.160) and another cluster with 7 values with a cerebrospinal fluid BH4 concentration of 35 nM/L and above (mean = 39.43, SE = 1.24; see Table 1). From these clusters we formed low and high cerebrospinal fluid BH4 groups. The low BH4 group was significantly older (mean = 66.0 months, SE = 15.10) than the high BH4 group [mean = 24.6, SE = 4.3; t(20) = 2.66, p < 0.05] and age was found to correlate negatively with cerebrospinal fluid BH4 level [r(19) = −0.44, p < 0.05]. There was a significantly greater percentage of patients in the high BH4 group (60%) with discharges on electroencephalogram as compared to the low BH4 group [9%, χ2 (1) = 4.75, p < 0.05]. The two groups were not different in the proportion of patients with a positive family history, slowing on electroencephalogram or abnormal magnetic resonance imaging findings. 5-Hydroxyindoleacetic acid, homovanillic acid and 3-O-methyldopa were not significantly different between groups. The majority of the children with autism were in the low BH4 group (80%) as compared to the high BH4 group. Defining these groups also enabled us to assign prior probabilities of 0.70 for the low group and 0.30 for the high group.
Table 1.
Participant characteristics.
| Presenting diagnosis | FHx | EEG | MRI | Age (m)/gender | BH4 (nM/L) | 5-HIAA (nM/L) | HVA (nM/L) | 3OMD (nM/L) |
|---|---|---|---|---|---|---|---|---|
| GDD | MM | NL | NL | 15/F | 12 | 332 | 669 | 24 |
| GDD | ED, MR | ND | NL | 14/M | 12 | 301 | 479 | 91 |
| Chorea | NS | NL | Punctate lesions | 204/F | 13 | 129 | 189 | 12 |
| Autism | Mom Sz | NL | Posterior fossa cyst | 72/M | 17 | 89 | 342 | 18 |
| Autism | NS | NL | NL | 48/F | 19 | 191 | 636 | 38 |
| GDD | ED | Slow | PVL | 67/M | 19 | 281 | 573 | 16 |
| Autism | NS | NL | NL | 30/M | 19 | 177 | 558 | 83 |
| Refractory seizures | Sz, Psych | NL | Volume loss | 168/M | 20 | 179 | 374 | 18 |
| Autism | NS | NL | DWMA | 65/M | 21 | 230 | 414 | 24 |
| GDD | NS | Slow | Volume loss | 42/M | 21 | 226 | 720 | 35 |
| GDD | NS | NL | PVL | 63/F | 23 | 201 | 318 | 12 |
| Dystonia | NS | ND | NL | 52/M | 27 | 363 | 359 | 61 |
| 28 | 177 | 234 | 25 | |||||
| Autism | Sz, AD | Multifocal spikes | PVL | 32/M | 31 | 195 | 599 | 26 |
| SD | Sz | Focal spikes | NL | 38/F | 35 | 226 | 720 | 35 |
| Autism | PDD | NL | NL | 34/M | 38 | 89 | 342 | 18 |
| GDD | Sz | Slow | Volume loss, PVL | 21/F | 38 | 187 | 500 | 53 |
| GDD | DD | ND | Thin CC | 22/M | 40 | 321 | 575 | 56 |
| GDD | NS | Multifocal spikes | Volume loss | 29/M | 40 | 244 | 622 | 21 |
| Refractory seizures | NS | Multifocal spikes | NL | 6/F | 40 | 336 | 602 | 48 |
| GDD | ADHD | Focal Spikes | DWMA | 22/M | 45 | 201 | 619 | 18 |
Presenting diagnosis: GDD, global developmental delay; Family history (FHX): AD, autism disorder; ADHD, attention deficit hyperactivity disorder; DD, developmental delay; ED, early death; MM, multiple miscarriage; MR, mental retardation; NS, not significant; PDD, pervasive developmental disorder; Psych, psychiatric disorders; Sz, seizure disorder; Electroencephalogram (EEG): ND, not done; NL, normal; Slow, generalized slowing; Magnetic resonance imaging (MRI): CC, corpus callosum; DWMA, diffuse white matter abnormalities; NL, Normal; PVL, periventricular leukomalacia; Age (m)/gender: M, male; F, female; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid; 3-OMD, 3-O-methyldopa.
Serum citrulline and methionine concentrations differentiate the high and low BH4 groups
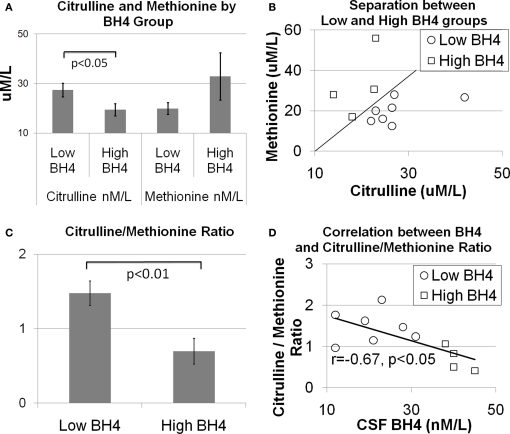
As discussed above, amino acids believed to be related to BH4 metabolism were entered into a discriminant analysis to determine if concentrations of specific amino acids could differentiate individuals in the high and low BH4 groups. The amino acids, citrulline and methionine, significantly contributed to the discriminant function [Wilks’ lambda F(2,8) = 6.66, p < 0.05]. As can be seen in Figure 1A, citrulline was significantly elevated in the low BH4 group [t(10) = 2.34, p < 0.05] while methionine was non-significantly depressed in the low BH4 group [t(10) = 1.32, p > 0.05]. Assuming equal prior group probabilities, the discriminant function did not make any errors, resulting in perfect sensitivity, specificity, and positive and negative predictive values (Table 2). The scatter plot of all citrulline-to-methionine values, along with the criteria that divided the two groups assuming an equal prior group probability, is displayed in Figure 1B. The discriminant function produced using the observed prior group probabilities misclassified one patient from the high BH4 group but none of the patients from the low BH4 group, resulting in a perfect sensitivity and negative predictive value. Since we found an age difference between the two BH4 groups, we added age and interactions of age with amino acid markers into the initial stepwise discriminant analysis. This analysis selected citrulline and the interaction between citrulline and age [Wilks’ lambda F(2,8) = 8.69, p < 0.01]. Assuming equal prior group probabilities, the discriminant function classified one observation in the low BH4 group incorrectly resulting in high sensitivity, specificity, and positive and negative predictive values. Assuming the observed prior group probabilities, the discriminant function classified all observations correctly, resulting in perfect sensitivity, specificity, and positive and negative predictive values.
Figure 1.
The relationship between central tetrahydrobiopterin (BH4) concentration and serum citrulline and methionine concentration. (A) Citrulline was found to be higher and methionine was found to be lower in individuals in the low BH4 group as compared to individuals in the high BH4 group. (B) Scatter plot of citrulline and methionine values illustrating the separation between the low and high BH4 groups. The diagonal line illustrates the group separate. (C) The ratio of citrulline-to-methionine is significantly different between the low and high BH4 groups and (D) correlated with the central BH4 concentration.
Table 2.
Performance statistics for discriminant functions.
| Model variables | Prior group probability | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| Citrulline and methionine | Equal | 100.00 | 100.00 | 100.00 | 100.00 |
| Citrulline and methionine | Observed | 100.00 | 75.00 | 87.50 | 100.00 |
| Citrulline and citrulline × age | Equal | 85.71 | 100.00 | 100.00 | 80.00 |
| Citrulline and citrulline × age | Observed | 100.00 | 100.00 | 100.00 | 100.00 |
| Citrulline/methionine ratio | Equal | 85.71 | 100.00 | 100.00 | 80.00 |
| Citrulline/methionine ratio | Observed | 100.00 | 75.00 | 87.50 | 100.00 |
The citrulline-to-methionine ratio: a simple index that differentiates high and low BH4 groups
To produce a simple, easy to calculate index of cerebrospinal fluid BH4, the ratio of citrulline-to-methionine was calculated. Figure 1C demonstrates that the citrulline-to-methionine ratio was significantly higher in the low BH4 group as compared to the high BH4 group [t(10) = 3.44, p < 0.01]. In addition, the citrulline-to-methionine ratio was found to significantly correlate with cerebrospinal fluid BH4 levels [r(9) = −0.67, p < 0.05; see Figure 1D]. The discriminant function with the citrulline-to-methionine ratio was statistically significantly [Wilks’ lambda F(1,9) = 11.35, p < 0.01]. The discriminant function with equal prior group probabilities classified one observation in the low BH4 group incorrectly but no observations in the high BH4 group incorrectly, resulting in a perfect specificity and positive predictive value but a lower sensitivity and negative predictive value. The discriminant function produced using the observed prior group probabilities misclassified one observation in the high BH4 group and no patients in the low BH4 group, resulting in a perfect sensitivity and negative predictive value. Including age and the interaction of age with the citrulline-to-methionine ratio as predictors in the stepwise analysis did not improve the model.
A practical method for applying the discriminant function results
In order to make the discriminant functions clinically useful, a simple method for calculating group membership is discussed. Since the discriminant function that included methionine and citrulline did not make assumptions about prior group probabilities and provided perfect discrimination between groups, it was used to calculate group membership. Group membership can be calculated by using Eq. 1 with a patient's methionine and citrulline values. If the resulting quantity is greater than 0, then the patient would be considered to be in the low BH4 group. If the quantity resulting from Eq. 1 is less than 0, then the patient would be classified in the high BH4 group.
| (1) |
Alternatively, the citrulline-to-methionine ratio could be used with the caveat that the value does not appear to discriminate among groups as well as the quantity derived from Eq. 1. The dividing point for this ratio, as calculated by the discriminant function, is 1.05 assuming equal group probabilities and 0.98 assuming observed group probabilities.
Discussion
This report examined cerebrospinal fluid BH4 concentrations in 20 children and adolescents with neurological disorders. First, cluster analysis was used to determine if, and how many, BH4 groups were in the patient population. Two groups of patients were found: 70% of the patients had cerebrospinal fluid BH4 concentrations of 31 nM/L and below and 30% had concentrations of 35 nM/L and above. Significantly more patients in the group with higher BH4 concentrations demonstrated epileptiform activity on electroencephalogram, and the majority of the children with the diagnosis of autism were in the low BH4 group. The discriminant analysis revealed that the serum concentration of two amino acids, citrulline and methionine, could discriminate the high and low BH4 groups and that the ratio of citrulline-to-methionine correlated with the cerebrospinal fluid BH4 concentration. The relevance of these specific amino acids to BH4 metabolism is discussed below. The relation identified in the analysis above is significant in that it implies that measurement of these peripheral markers of metabolism may be useful for identifying individuals with low central BH4 levels who might benefit from BH4 supplementation.
The low and high BH4 groups appear to correspond to patients with low-to-normal and normal-to-high cerebrospinal fluid BH4 concentrations, respectively. It is somewhat surprising that a group with markedly low cerebrospinal fluid BH4 levels was not found since some research suggests that cerebrospinal fluid BH4 concentrations in autism might be depressed; however, only a subset of individuals within our population had features of autism. Two studies that measured BH4 concentrations in children with autism have found concentrations, for the most part, below the lowest cerebrospinal fluid BH4 concentration of our patient population (Tani et al., 1994; Fernell et al., 1997). However, another study found cerebrospinal fluid BH4 concentrations in a group of autistic children more comparable with our findings (Danfors et al., 2005). In addition, Danfors et al. (2005) also excluded about one-third of the children they evaluated due to cerebrospinal fluid BH4 concentration greater than 30 μmol/mL, a proportion very close to the proportion of patients in the high BH4 group reported in this study. The great majority of studies that have shown an improvement in autism symptomatology with BH4 supplementation did not measure blood, urine or cerebrospinal fluid BH4 concentrations. Thus, it is difficult to know whether BH4 supplementation affected children with low, normal or high BH4 levels or children with a mixture of these levels. In fact it may be better to think of patient populations that might have over activation of metabolic systems associated with BH4 utilization, such as autism, as having a relative insufficiency in BH4, rather than a BH4 deficiency. In such a case, the central BH4 concentration could be low normal and still be insufficient to meet the metabolic demands of the individual.
One of the important insights that this report highlights, regardless of the exact cerebrospinal fluid BH4 concentrations of our patients, is that central nervous system BH4 levels may very well be associated with peripheral metabolic pathways. As discussed above, we believe that this relationship is a result of consumption and recycling of BH4 by non-central metabolic pathways. Additionally, polymorphisms in one of the several genes that code for BH4 production could be associated with reduced or inefficient BH4 production or recycling. Such a notion would be consistent with the significant nominal association between the 6-pyruvoyl-tetrahydropterin synthase gene and autism (Schnetz-Boutaud et al., 2009). Of course, the population studied does not represent typically developing individuals, and many of the children we examined may be expected to have activation of metabolic pathways that consume BH4. This limits the application of the relationship observed to those children and adolescents with neurodevelopmental disorders. However, this population may be one of the most important populations to which to apply the findings of this study since they may be high consumers of BH4 and have a high need for BH4 supplementation. Indeed, several studies have demonstrated improvement in development and behavior with supplementation of BH4 in children with autism (Naruse et al., 1984, 1990a,b; Nagahata et al., 1990; Nakane et al., 1990; Takesada et al., 1992; Fernell et al., 1997; Danfors et al., 2005; Frye et al., 2010).
Children with autism have symptoms consistent with a central BH4 deficiency. For example, BH4 deficiency can result in low production of monoamine neurotransmitters, including serotonin, dopamine, and norepinephrine. There is a significant amount of evidence that deficits in these monoamine neurotransmitters are present in some children with autism. Dysfunction in the serotonin system in autism has been documented by several investigators (Pardo and Eberhart, 2007). Children with autism have clinical symptoms, such as obsessive-compulsive disorder and anxiety, seen in other disorders where serotonin deficiency has been implicated (McDougle et al., 1995), and there are many studies that suggest that selective-serotonin reuptake inhibitors may be efficacious in the treatment of autistic symptoms (Kolevzon et al., 2006; Posey et al., 2006a). Children with autism have a high incidence of sleep initiation and maintenance disorders, suggesting a deficit in melatonin, a metabolite of serotonin (Richdale and Schreck, 2009). Children with autism have a high rate of executive function, attention regulation and hyperactivity, all symptoms suggesting dopamine and norepinephrine deficits (Bramham et al., 2009; Corbett et al., 2009; Jahromi et al., 2009). Such behaviors are mitigated by psychopharmaceuticals designed to increase levels of dopamine and norepinephrine in children with autism (Posey et al., 2006b; Troost et al., 2006). In addition, positron emission tomography has demonstrated a reduction in the baseline increase in dopamine D2 receptor binding after 12 weeks of BH4 supplementation (Fernell et al., 1997). It is important to state that it is highly likely that not all children with autism have low monoamine neurotransmitters, as the support for this idea across studies in mixed (Kolevzon et al., 2006; Posey et al., 2006b; Soorya et al., 2008). This is most likely the result of the heterogeneity in the biological mechanisms underlying autism and supports the need to develop biochemical markers of central metabolic processes in children with autism and other neurodevelopmental disorders, and might explain why not all children with autism respond to BH4 supplementation.
Low central nervous system BH4 levels can result in several secondary central nervous system consequences. First, BH4 is necessary for the production of nitric oxide, a soluble signaling molecule that appears to be important for cell proliferation, neuronal motility, and synaptic maturation during development (Tegenge and Bicker, 2009) and communication between neurons and both neuronal and non-neuronal cells (Garthwaite, 2008). Second, BH4 is associated with growth factors, including nerve growth factors, in animal models (Anastasiadis et al., 1997). Third, BH4 has been shown to be a protective factor for nitric oxide toxicity and a superoxide radical scavenger (Kojima et al., 1995; Koshimura et al., 1998). Fourth, reduced levels of monoamine neurotransmitters could result in dysfunction of important neural pathways, leading to underdevelopment of such important pathways. Fifth, BH4 is an enhancer of the synaptic release of a wide range of neurotransmitters including the catecholamines, serotonin, acetylcholine, glutamate, and gamma aminobutyric acid (Koshimura et al., 1990; Mataga et al., 1992). Thus, a reduction in BH4 could result in a reduction in neurotransmitter release during a critical time in development. Clearly BH4 is involved in several metabolic and neurotransmitter pathways critical for brain function, and development and low BH4 levels during development could have devastating consequences on brain development.
Two serum amino acids, citrulline and methionine, were found to differentiate high and low cerebrospinal fluid BH4 concentration in individuals with neurological disorders. In addition, the ratio of these two amino acids was correlated with the cerebrospinal fluid BH4 concentration. Since these amino acids are an integral part of metabolic pathway that uses BH4 both systemically and centrally, it is suggested that dysfunction of such metabolic pathways is linked to changes in BH4 levels. Specifically, a reduction in serum methionine is proposed to be linked to a reduction in folate availability and activation of the oxidative stress pathway, and an increase in serum citrulline is proposed to be linked to an increase in nitric oxide production from overactive inflammatory pathways. This notion is supported by the fact that some children with autism demonstrate these metabolic abnormalities and respond to BH4 supplementation.
Several aspects of the hypothesized relation between cerebrospinal fluid BH4 concentration and serum amino acids were not verified by our analysis. For example, arginine was not found to be reduced. This is most likely due to the fact that changes in metabolism, including BH4 metabolism, are due to long-standing chronic effects of disease. In such states, enzymes are regulated to compensate for the abnormal metabolic state. For example, down regulation of arginase, the enzyme that catalyzes the breakdown of arginine to urea and ornithine in the urea cycle results in an increase in citrulline (Rabier and Kamoun, 1995; Tenu et al., 1999). Inhibition of arginase would prevent the decrease in arginine and would result in an increase in citrulline as seen in this study.
Clearly, future studies will need to examine other biomarkers of oxidative stress, nitric oxide, and inflammation to understand whether they correlate with the serum amino acid markers identified in this study and systemic and/or cerebrospinal fluid BH4 concentration. Such information will further our understanding of the biological basis of autism and help validate the ratio of citrulline-to-methionine as a biomarker for central BH4 concentration in other disorders.
Conflict of Interest Statement
Dr Richard Frye has received honoraria from BioMarin, the manufacture of Kuvan (a formulation of tetrahydrobiopterin), for speaking at a workshop on tetrahydrobiopterin in neurodevelopmental disorders.
Acknowledgments
This study was supported by NS046565 to Dr. Richard Frye. The author would like to thank Laura deSouza and Christine Younan for their technical help with this study.
References
- Anastasiadis P. Z., Bezin L., Imerman B. A., Kuhn D. M., Louie M. C., Levine R. A. (1997). Tetrahydrobiopterin as a mediator of PC12 cell proliferation induced by EGF and NGF. Eur. J. Neurosci. 9, 1831–1837 10.1111/j.1460-9568.1997.tb00749.x [DOI] [PubMed] [Google Scholar]
- Bramham J., Ambery F., Young S., Morris R., Russell A., Xenitidis K., Asherson P., Murphy D. (2009). Executive functioning differences between adults with attention deficit hyperactivity disorder and autistic spectrum disorder in initiation, planning and strategy formation. Autism 13, 245–264 10.1177/1362361309103790 [DOI] [PubMed] [Google Scholar]
- Castellani M. L., Conti C. M., Kempuraj D. J., Salini V., Vecchiet J., Tete S., Ciampoli C., Conti F., Cerulli G., Caraffa A., Antinolfi P., Galzio R., Shaik Y., Theoharides T. C., De Amicis D., Perrella A., Cuccurullo C., Boscolo P., Felaco M., Doyle R., Verrocchio C., Fulcheri M. (2009). Autism and immunity: revisited study. Int. J. Immunopathol. Pharmacol. 22, 15–19 [DOI] [PubMed] [Google Scholar]
- Corbett B. A., Constantine L. J., Hendren R., Rocke D., Ozonoff S. (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 166, 210–222 10.1016/j.psychres.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danfors T., von Knorring A. L., Hartvig P., Langstrom B., Moulder R., Stromberg B., Torstenson R., Wester U., Watanabe Y., Eeg-Olofsson O. (2005). Tetrahydrobiopterin in the treatment of children with autistic disorder: a double-blind placebo-controlled crossover study. J. Clin. Psychopharmacol. 25, 485–489 10.1097/01.jcp.0000177667.35016.e9 [DOI] [PubMed] [Google Scholar]
- Deth R., Muratore C., Benzecry J., Power-Charnitsky V. A., Waly M. (2008). How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology 29, 190–201 10.1016/j.neuro.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Dietert R. R., Dietert J. M. (2008). Potential for early-life immune insult including developmental immunotoxicity in autism and autism spectrum disorders: focus on critical windows of immune vulnerability. J. Toxicol. Environ. Health B Crit. Rev. 11, 660–680 10.1080/10937400802370923 [DOI] [PubMed] [Google Scholar]
- Fernell E., Watanabe Y., Adolfsson I., Tani Y., Bergstrom M., Hartvig P., Lilja A., von Knorring A. L., Gillberg C., Langstrom B. (1997). Possible effects of tetrahydrobiopterin treatment in six children with autism – clinical and positron emission tomography data: a pilot study. Dev. Med. Child. Neurol. 39, 313–318 [DOI] [PubMed] [Google Scholar]
- Frye R. E., Huffman L. C., Elliot G. R. (2010). Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurotherapeutics (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. (2008). Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 27, 2783–2802 10.1111/j.1460-9568.2008.06285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells D., Hyland K. (1987). Direct analysis of tetrahydrobiopterin in cerebrospinal fluid by high-performance liquid chromatography with redox electrochemistry: prevention of autoxidation during storage and analysis. Clin. Chim. Acta 167, 23–30 10.1016/0009-8981(87)90081-7 [DOI] [PubMed] [Google Scholar]
- Hyland K., Surtees R. A., Heales S. J., Bowron A., Howells D. W., Smith I. (1993). Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population. Pediatr. Res. 34, 10–14 10.1203/00006450-199307000-00003 [DOI] [PubMed] [Google Scholar]
- Jahromi L. B., Kasari C. L., McCracken J. T., Lee L. S., Aman M. G., McDougle C. J., Scahill L., Tierney E., Arnold L. E., Vitiello B., Ritz L., Witwer A., Kustan E., Ghuman J., Posey D. J. (2009). Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J. Autism Dev. Disord. 39, 395–404 10.1007/s10803-008-0636-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. J., Cutler P., Melnyk S., Jernigan S., Janak L., Gaylor D. W., Neubrander J. A. (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 80, 1611–1617 [DOI] [PubMed] [Google Scholar]
- Kern J. K., Jones A. M. (2006). Evidence of toxicity, oxidative stress, and neuronal insult in autism. J. Toxicol. Environ. Health B Crit. Rev. 9, 485–499 10.1080/10937400600882079 [DOI] [PubMed] [Google Scholar]
- Kojima S., Ona S., Iizuka I., Arai T., Mori H., Kubota K. (1995). Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic. Res. 23, 419–430 10.3109/10715769509065263 [DOI] [PubMed] [Google Scholar]
- Kolevzon A., Mathewson K. A., Hollander E. (2006). Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J. Clin. Psychiatry 67, 407–414 10.4088/JCP.v67n0311 [DOI] [PubMed] [Google Scholar]
- Koshimura K., Miwa S., Lee K., Fujiwara M., Watanabe Y. (1990). Enhancement of dopamine release in vivo from the rat striatum by dialytic perfusion of 6R-L-erythro-5,6,7,8-tetrahydrobiopterin. J. Neurochem. 54, 1391–1397 10.1111/j.1471-4159.1990.tb01974.x [DOI] [PubMed] [Google Scholar]
- Koshimura K., Murakami Y., Tanaka J., Kato Y. (1998). Self-protection of PC12 cells by 6R-tetrahydrobiopterin from nitric oxide toxicity. J. Neurosci. Res. 54, 664–672 [DOI] [PubMed] [Google Scholar]
- Mataga N., Imamura K., Watanabe Y. (1992). L-threo-3,4-dihydroxyphenylserine enhanced ocular dominance plasticity in adult cats. Neurosci. Lett. 142, 115–118 10.1016/0304-3940(92)90352-8 [DOI] [PubMed] [Google Scholar]
- McDougle C. J., Kresch L. E., Goodman W. K., Naylor S. T., Volkmar F. R., Cohen D. J., Price L. H. (1995). A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive-compulsive disorder. Am. J. Psychiatry 152, 772–777 [DOI] [PubMed] [Google Scholar]
- Nagahata M., Kazamatsuri H., Naruse H., Yamazaki K., Takesada M., Nakane Y., Kaihara S., Ohashi T. (1990). Clinical evaluation of aproterin hydrochloride (R-THBP. SUN 0588) on infantile autism – a multicenter cooperative study. Rinsho Iyaku 6, 1877–1899 (in Japanese). [Google Scholar]
- Nakane Y., Asuo T., Shimogawa S., Fujiwara T., Kawabata Y., Kubota J. (1990). Clinical efficacy and effects on physical development of long-term treatment of R-tetrahydrobiopterin (R-THBP, SUN 0588) for autism. Kiso To Rinsho 24, 4579–4598 (in Japanese). [Google Scholar]
- Naruse H., Hayashi T., Takesada M. (1984). “A preliminary study on clinical effect of tetrahydrobiopterin in infantile autism (in Japanese),” in Reports in 1983 for New Drug Development (Tokyo: Ministry of Health and Welfare; ), 71–81 [Google Scholar]
- Naruse H., Takesada M., Nagahata M., Kazamatsuri H., Nakane Y., Yamazaki K. (1990b). An open clinical study of apropterin hydrochloride (R-tetrahydrobiopterin SUN 0588) in infantile autism – clinical study using a Rating Scale for Abnormal Behaviors in Children. Rinsho Iyaku 6, 1859–1875 (in Japanese). [Google Scholar]
- Naruse H., Takesada M., Nakane Y., Yamazaki K., Uchiyama T., Kaihara S., Ohashi T. (1990a). Clinical evaluation of R-tetrahydrobiopterin (SUN 0588) on infantile autism – a double-blind comparative study using placebo as a control. Rinsho Iyaku 6, 1343–1368 (in Japanese). [Google Scholar]
- Pardo C. A., Eberhart C. G. (2007). The neurobiology of autism. Brain Pathol. 17, 434–447 10.1111/j.1750-3639.2007.00102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo C. A., Vargas D. L., Zimmerman A. W. (2005). Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 17, 485–495 10.1080/02646830500381930 [DOI] [PubMed] [Google Scholar]
- Posey D. J., Wiegand R. E., Wilkerson J., Maynard M., Stigler K. A., McDougle C. J. (2006a). Open-label atomoxetine for attention-deficit/hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 16, 599–610 10.1089/cap.2006.16.599 [DOI] [PubMed] [Google Scholar]
- Posey D. J., Erickson C. A., Stigler K. A., McDougle C. J. (2006b). The use of selective serotonin reuptake inhibitors in autism and related disorders. J. Child Adolesc. Psychopharmacol. 16, 181–186 10.1089/cap.2006.16.181 [DOI] [PubMed] [Google Scholar]
- Rabier D., Kamoun P. (1995). Metabolism of citrulline in man. Amino Acids 9, 299–316 10.1007/BF00807268 [DOI] [PubMed] [Google Scholar]
- Richdale A. L., Schreck K. A. (2009). Sleep problems in autism spectrum disorders: prevalence, nature, and possible biopsychosocial aetiologies. Sleep Med. Rev. 13, 403–411 10.1016/j.smrv.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Schnetz-Boutaud N. C., Anderson B. M., Brown K. D., Wright H. H., Abramson R. K., Cuccaro M. L., Gilbert J. R., Pericak-Vance M. A., Haines J. L. (2009). Examination of tetrahydrobiopterin pathway genes in autism. Genes Brain Behav. 8, 753–757 10.1111/j.1601-183X.2009.00521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soorya L., Kiarashi J., Hollander E. (2008). Psychopharmacologic interventions for repetitive behaviors in autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 17, 753–771 10.1016/j.chc.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Takesada M., Naruse H., Nagahata M. (1992). “An open clinical study of sapropterin hydrochloride (R-tetrahydrobiopterin, R-THBP) in infantile autism: clinical effects and long-term follow-up,” in Neurobiology of Infantile Autism: Proceedings of the International Symposium on Neurobiology of Infantile Autism, Tokyo, 10–11 November 1990, eds Naruse H., Ornitz E. (New York: Elsevier Science Publishers; ), 355–358 [Google Scholar]
- Tani Y., Fernell E., Watanabe Y., Kanai T., Langstrom B. (1994). Decrease in 6R-5,6,7,8-tetrahydrobiopterin content in cerebrospinal fluid of autistic patients. Neurosci. Lett. 181, 169–172 10.1016/0304-3940(94)90586-X [DOI] [PubMed] [Google Scholar]
- Tegenge M. A., Bicker G. (2009). Nitric oxide and cyclic GMP signal transduction positively regulates the motility of human neuronal precursor (NT2) cells. J. Neurochem. 110, 1828–1841 10.1111/j.1471-4159.2009.06279.x [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Lepoivre M., Moali C., Brollo M., Mansuy D., Boucher J. L. (1999). Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide 3, 427–438 10.1006/niox.1999.0255 [DOI] [PubMed] [Google Scholar]
- Thony B., Auerbach G., Blau N. (2000). Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347, 1–16 10.1042/0264-6021:3470001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troost P. W., Steenhuis M. P., Tuynman-Qua H. G., Kalverdijk L. J., Buitelaar J. K., Minderaa R. B., Hoekstra P. J. (2006). Atomoxetine for attention-deficit/hyperactivity disorder symptoms in children with pervasive developmental disorders: a pilot study. J. Child Adolesc. Psychopharmacol. 16, 611–619 10.1089/cap.2006.16.611 [DOI] [PubMed] [Google Scholar]
- Willoughby R. E., Opladen T., Maier T., Rhead W., Schmiedel S., Hoyer J., Drosten C., Rupprecht C. E., Hyland K., Hoffmann G. F. (2009). Tetrahydrobiopterin deficiency in human rabies. J. Inherit. Metab. Dis. 32, 65–72 10.1007/s10545-008-0949-z [DOI] [PubMed] [Google Scholar]