Abstract
Objectives
The World Health Organization estimated alcohol consumption in Uganda to be one of the highest in the world. We examined alcohol consumption among Ugandan women prior to and after learning of pregnancy. We developed a screening algorithm using factors that predicted alcohol consumption in this study.
Methods
In 2006, we surveyed 610 women attending antenatal care at the national referral hospital in Kampala, Uganda about consumption of traditional and commercial alcoholic beverages before and after learning of pregnancy. Predictors of alcohol consumption during pregnancy were examined and a practical screening algorithm was developed for use in antenatal clinics.
Results
One hundred eighty women (30%) drank alcohol at least monthly before learning of their pregnancy. Among these women, almost one-third reported usual consumption of at least one beverage type at quantities that equal binging levels for women. Overall, 151 women (25%) consumed alcohol after learning of pregnancy. Commercial beverages, particularly beer, were consumed more often than traditional drinks. A two-stage screening algorithm asking women about their religion, male partner or friends’ drinking, and any lifetime drinking predicted self-reported consumption of alcohol during pregnancy with 97% sensitivity and 89% specificity.
Conclusions
Alcohol consumption among pregnant Ugandan women attending antenatal care is high. A feasible screening algorithm can help providers target education and counseling to women who are likely drinking during pregnancy. Given the preference for commercial alcoholic beverages, it is recommended that labels be placed prominently on bottled alcoholic beverages warning of the adverse effects of consuming alcohol during pregnancy.
Keywords: Alcohol consumption, pregnancy, Uganda, antenatal care
Introduction
Prenatal exposure to ethanol is associated with many adverse pregnancy and birth outcomes(1–4), as well as a spectrum of physical, mental, behavioral, and learning disorders known as fetal alcohol spectrum disorders(FASD) (5–11). Currently, no amount of alcohol is considered safe during pregnancy(12). The prevalence of alcohol consumption during pregnancy is unknown in many developing nations where the overall prevalence of alcohol consumption is on the rise (13).
In its 2004 Global Report on Alcohol, the World Health Organization (WHO) estimated the average adult per capita consumption of pure ethanol in Uganda was 19.7 liters per year, ranking it one of the highest in the world (13). Among the few published studies reporting the prevalence of alcohol consumption among Ugandan adults(14–17), none were solely among pregnant women in whom the effects of alcohol are well documented. Screening for alcohol consumption and binge drinking during pregnancy is not part of routine practice in antenatal care clinics in Uganda. Therefore, in light of the WHO report, we surveyed women attending antenatal care in Kampala, Uganda and examined the prevalence, patterns, and predictors of alcohol consumption during pregnancy.
Methods
Study Design
From June to August 2006, we conducted a cross-sectional study of women attending the antenatal care clinic at Mulago Hospital, a national referral hospital in Kampala, Uganda. The clinic operates three days per week with 150–200 women per day seeking antenatal care on a walk-in basis, for scheduled follow-up visits, or because they were referred to the hospital. Attendees were approached randomly according to hour of arrival so that a representative sample of women was evaluated for eligibility and interest in participation.
To be eligible, women had to be at least 14 years old, be 28–34 weeks gestation as estimated from last normal menstrual period and fundal height using a measuring tape, not having a suspected or confirmed multiple pregnancy, and be able to speak and understand Luganda or English. Interested women provided informed consent or ascent. Women were surveyed about consumption of traditional and commercial alcoholic beverages prior to learning of their pregnancy, change in drinking (increased, decreased, stopped, or newly started) after learning of pregnancy, knowledge of the health effects of alcohol drinking during pregnancy, cigarette use, and reproductive and medical history. HIV status was ascertained by self-report and standard opt-out testing at the clinic. We assessed potential problem drinking using the CAGE questionnaire(18). Questions were ordered in a manner to reduce the chance of “primed” responses and reporting bias, culturally adapted when necessary, translated and back translated. The instrument was piloted prior to the study and administered by trained health workers. Women were counseled about the harmful effects of alcohol and smoking during pregnancy in a question and answer period following the interview.
Women were classified as “never drinkers” if they never had a drink containing alcohol, as “regular drinkers” if they consumed alcohol at least monthly prior to learning of their pregnancy, and as “non-regular” if they drank less frequently. Women were considered to have an alcohol-exposed pregnancy (AEP) if they reported regular consumption of alcohol prior to recognition of pregnancy (19)and we re considered to have drunk during pregnancy if they reported not to have stopped drinking or newly started after learning of their pregnancy.
For each beverage and for regular drinkers only, monthly consumption was calculated as the product of the frequency of drinking per month and the mean number of drinks consumed during a typical episode. Servings typical in Uganda for various drinks were converted to U.S. standard drinks (12 grams ethanol per serving), if alcohol by volume (ABV) was known, to report mean number of drinks consumed in an episode(20). Muslims, born-again Christians, Seventh Day Adventists, and Jehovah Witnesses were considered religions that explicitly restrict drinking alcohol.
This study was approved by the Institutional Review Boards of University Hospitals of Cleveland/Case Western Reserve University and Makerere University School of Medicine and by the Uganda National Council of Science and Technology.
Data Analysis
Never, non-regular, and regular drinkers were compared using Pearson’s chi-square or Fisher’s exact test for categorical variables and Student’s t-test or Kruskall-Wallis test for continuous variables. We used log-binomial regression with robust variance estimation to model predictors of regular drinking prior to learning of pregnancy. This approach is preferred over logistic regression because it yields the prevalence ratio (PR)with 95% confidence intervals (CI), which is a better estimate of the relative risk when the outcome of interest is common(21,22). Factors for which the unadjusted associations with regular drinking were at least suggestive (p<0.20) were examined for possible retention in the adjusted model.
We calculated sensitivity and specificity of individual factors associated with self-reported drinking alcohol during pregnancy. Using Boolean terms, we combined factors simultaneously and sequentially to develop a prediction rule based on the data collected in the cross-sectional study that would be both sensitive and specific to screen for alcohol consumption during pregnancy. Antenatal care providers at Mulago Hospital evaluated the overall algorithm for face validity, appropriateness, and feasibility. Using Monte Carlo simulations of 1000 repetitions, we conducted sensitivity analyses to quantify the effects of 5%, 10%, and 15% underreporting of alcohol consumption during pregnancy on the net sensitivity, net specificity, positive and negative predictive value (PPV and NPV, respectively), and area under the curve ( AUC)of the screening algorithm.
Results
Overall, 618 eligible women attending antenatal clinic were approached over eighteen clinic days, of whom 610 (98.7%) agreed to participate. Almost all women lived in either Kampala District (N=395; 66.2%) or encircling Wakiso District (N=192; 32.2%). The median (interquartile range; IQR) age was 22(18,27) years with 20.2% of women between 14 and 17 years of age. Overall, 71.6% were formally or customarily married, about half attended secondary school or higher, and almost one -third had a job that generated income. Fifty-seven percent of women reported having one or more previous pregnancies and 8.9%. of women were HIV-infected.
Alcohol consumption prior to recognition of pregnancy
Prior to learning of pregnancy, 29.5% of women reported regular consumption of alcoholic beverages and were assumed to have an AEP, 4.3% reported non-regular drinking, and 66.2% never drank alcohol(Table 1). Alcohol consumption was associated with older age (p<0.001), being married (p=0.005), having a job that generated income ( p=0.023), belonging to a religion that does not restrict alcohol (p<0.001), having a previous pregnancy or live birth (p<0.001) and being HIV-infected (p=0.006).
Table 1.
Prevalence of alcohol consumption before recognition of pregnancy by sociodemographic, clinical, and reproductive factors and substance use, among 610 women seeking antenatal at Mulago Hospital, Kampala, Uganda, June – August 2006.
| Sociodemographic characteristic | Drank alcohol regularly n (%) | Drank alcohol non-regularly n (%) | Never drank alcohol n (%) | p1 |
|---|---|---|---|---|
| All women | 180 (29.5) | 26 (4.3) | 404 (66.2) | |
| Marital status | ||||
| Married or living as married | 140 (32.1) | 21 (4.8) | 275 (63.1) | 0.038 |
| Unmarried, separated, or widowed | 40 (23.1) | 5 (2.9) | 128 (74.0) | |
| Able to read and write Luganda or English | ||||
| Yes | 156 (29.3) | 22 (4.1) | 355 (66.6) | 0.845 |
| No | 22 (30.1) | 4 (5.5) | 47 (64.4) | |
| Highest level of education attended | ||||
| None | 10 (43.5) | 0 (0.0) | 13 (56.5) | 0.358 |
| Primary school | 91 (31.3) | 11 (3.8) | 189 (65.0) | |
| Secondary school | 75 (27.5) | 15 (5.5) | 183 (67.0) | |
| Degree or post-graduate | 4 (17.4) | 0 (0.0) | 19 (82.6) | |
| Has a job that generates income | ||||
| Yes | 69 (36.3) | 10 (5.3) | 111 (58.4) | 0.023 |
| No | 111 (26.4) | 16 (3.8) | 293 (69.8) | |
| Partner has a job that generates income | ||||
| Yes | 168 (29.4) | 22 (3.9) | 381 (66.7) | 0.185 |
| No | 8 (25.8) | 4 (12.9) | 19 (61.3) | |
| Does not know | 3 (42.9) | 0 (0.0) | 4 (57.1) | |
| Religion | ||||
| Catholicism | 80 (41.2) | 7 (3.6) | 107 (55.2) | <0.001 |
| Protestantism | 57 (31.7) | 11 (6.1) | 112 (62.2) | 0.212 |
| Islam2 | 22 (13.5) | 5 (3.1) | 136 (83.4) | <0.001 |
| Born-again Christianity2 | 17 (30.9) | 2 (3.6) | 36 (65.5) | 0.966 |
| Seventh Day Adventistism2 | 2 (12.5) | 1 (6.3) | 13 (81.3) | 0.256 |
| Other3 | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0.170 |
| Clinical and reproductive factors | ||||
| HIV status | ||||
| Infected | 23 (42.6) | 5 (9.3) | 26 (48.2) | 0.006 |
| Uninfected | 157 (28.2) | 21 (3.8) | 378 (68.0) | |
| Previous pregnancies | ||||
| None | 51 (19.5) | 12 (4.6) | 198 (75.9) | <0.001 |
| One | 36 (37.9) | 3 (3.2) | 56 (59.0) | |
| Two to three | 56 (37.6) | 8 (5.4) | 85 (57.1) | |
| Four or more | 37 (35.6) | 3 (2.9) | 64 (61.5) | |
| Previous live births | ||||
| None | 60 (20.4) | 13 (4.4) | 221 (75.2) | <0.001 |
| One | 42 (40.4) | 3 (2.9) | 59 (56.7) | |
| Two to three | 52 (38.5) | 8 (5.9) | 75 (55.6) | |
| Four or more | 26 (34.2) | 2 (2.6) | 48 (63.2) | |
| Current pregnancy was planned | ||||
| Yes | 78 (29.7) | 11 (4.2) | 174 (66.2) | 0.995 |
| No | 102 (29.5) | 15 (4.3) | 229 (66.2) | |
| Substance use | ||||
| Ever smoked a cigarette | ||||
| Yes | 12 (63.2) | 0 (0.0) | 7 (36.8) | 0.009 |
| No | 168 (28.4) | 26 (4.4) | 397 (67.2) | |
| Currently smokes cigarettes | ||||
| Yes | 4 (66.7) | 0 (0.0) | 2 (33.2) | 0.151 |
| No | 176 (29.1) | 26 (4.3) | 402 (66.6) | |
| Male partner drinks alcohol | ||||
| Yes | 90 (51.1) | 11 (6.3) | 75 (42.6) | <0.001 |
| No | 90 (20.7) | 15 (3.5) | 329 (75.8) | |
| Has friends who drink alcohol | ||||
| Yes | 133 (46.2) | 12 (4.2) | 143 (49.7) | <0.001 |
| No | 47 (14.6) | 14 (4.4) | 261 (81.1) | |
| Believes that alcohol consumed during pregnancy affects baby’s health | ||||
| Yes | 145 (26.9) | 23 (4.3) | 371 (68.8) | 0.001 |
| No | 35 (49.3) | 3 (4.2) | 33 (46.5) | |
Three-way comparisons between regular, non-regular, and never drinkers
Religions that prohibit alcohol consumption
Other religions reported were Jehovah Witness (N=1) and no religious affiliation (N=1), the former restricting alcohol consumption
Few women (3.1%) had ever smoked cigarettes and only 1.0% currently smoked; nevertheless, these women tended to drink regularly prior to pregnancy. Women who drank regularly were more likely to have male partners (p<0.001) and friends ( p<0.001) who also drank alcohol. Women who reported that alcohol consumption during pregnancy was harmful to their baby’s health were less likely to regularly consume alcohol (26.9%) compared to women who believed otherwise (49.3%; p=0.001).
Among regular drinkers, most (92.8%) drank commercial alcoholic beverages (Table 2)with beer (4–6% ABV) consumed most commonly (98.2%). Twelve women drank wine (12.5% ABV), 7 drank fortified wine (20% ABV), and 11 drank spirits (40% ABV). Although 94 women (52.2%) drank traditional alcoholic beverages, only 7.2% drank them solely. Fifty-eight women drank tonto (banana wine; 6–11% ABV) (23), 50 drank local waragi (banana gin; 35–45% ABV; unpublished data), 36 drank malwa (fermented millet; 6–8% ABV) (13), 15 drank kweete (fermented maize and millet; ABV unknown), 15 drank munanansi (fermented pineapple; ABV unknown), 3 drank omuramba (fermented sorghum; ABV unknown), and 6 drank other varieties. Overall, 52.2% of regular drinkers reported drinking two or more beverage types.
Table 2.
Types and frequency of alcoholic beverages consumed by 180 women who drank regularly before learning of pregnancy, Mulago Hospital, Kampala, Uganda, June to August 2006.
| Alcoholic beverage | Women (N=180) | Frequency of consumption before learning of pregnancy, n (%) | Mean (SD) drinks per occassion1 | ||||
|---|---|---|---|---|---|---|---|
| Once per month | 2–4 times per month | 2–3 times per week | >4 times per week | Uganda | Estimated standard U.S. drinks | ||
| Commercial | 167 | ||||||
| Beer | 164 | 79 (48.2) | 49 (29.9) | 16 (9.8) | 20 (12.2) | 2.5 (1.9) | 3.5 (2.6) |
| Wine | 12 | 7 (58.3) | 5 (41.7) | 0 (0.0) | 0 (0.0) | 2.7 (1.8) | 2.7 (1.8) |
| Fortified wine | 7 | 3 (42.9) | 3 (42.9) | 1 (14.3) | 0 (0.0) | 2.1 (1.5) | 2.1 (1.5) |
| Spirits2 | 11 | 4 (40.0) | 3 (30.0) | 3 (30.0) | 0 (0.0) | 2.3 (1.9) | 2.5 (2.2) |
| Traditional | 94 | ||||||
| Tonto | 58 | 31 (53.1) | 18 (31.0) | 4 (6.9) | 5 (8.6) | 2.1 (1.8) | 5.0 (4.3) |
| Waragi | 50 | 26 (52.0) | 12 (24.0) | 4 (8.0) | 8 (16.0) | 2.0 (1.6) | 2.3 (1.8) |
| Mialwa | 36 | 19 (55.9) | 10 (29.4) | 4 (11.8) | 1 (2.9) | NE | NE |
| Kweete | 15 | 12 (80.0) | 1 (6.7) | 2 (13.3) | 0 (0.0) | 1.9 (0.8) | NE |
| Munanansi | 15 | 4 (26.7) | 6 (40.0) | 2 (13.3) | 3 (20.0) | 4.1 (3.0) | NE |
| Omuramba | 3 | 0 (0.0) | 2 (66.6) | 0 (0.0) | 1 (33.3) | 4.2 (1.2) | NE |
| Others | 6 | 3 (50.0) | 0 (0.0) | 2 (33.3) | 1 (16.7) | 2.2 (1.0) | NE |
NE=Not estimated
Drink-specific typical serving in Uganda and U.S. standard drink equivalent if alcohol content was known; malwa usually consumed by multiple individuals sitting around a common pot.
Frequency of consuming commercial spirits not reported by one participant
Women reported drinking most alcoholic beverages weekly or less (Table 2). However, almost one-quarter of women who drank commercial beer or local waragi reported consuming these beverages on two or more days per week. Women who drank beer and tonto, both of which are usually 0.5 liters per serving, reported drinking on average an equivalent of 3.5 and 5.0 standard U.S. drinks during an episode, respectively. Overall, 55 women (9.0%) reported drinking usual quantities of a single beverage that equaled levels considered binging for women(4 or more standard U.S. drinks in one occasion )(24), and 48 women (7.9%) averaged more than one drink per day, considered to be a “risky” level of consumption for women by the U.S. Department of Agriculture (USDA) (25). Sixteen women (2.6%) reported consuming an average of at least 7 drinks of a given beverage in a single occasion, a quantity which the USDA recommends women not to exceed in an entire week(25).
Alcohol consumption after recognition of pregnancy
Among the 180 women who drank regularly prior to pregnancy, 33 (18.3%)stopped drinking alcohol altogether, 123 (68.3%)decreased consumption of all alcoholic beverages, 8 (4.4%) reported no change in drinking, and 20 (11.1%) reported an increase in consumption of at least one alcoholic beverage type after recognition of pregnancy.
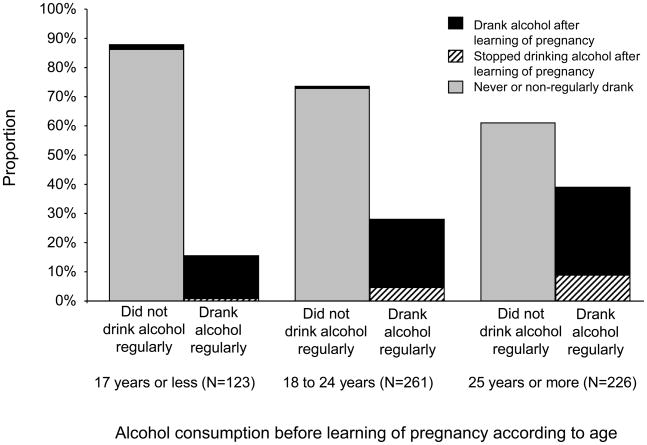
Pattern of alcohol consumption was strongly associated with age (Figure 1). Regular drinking prior to learning of pregnancy increased with age (15.5%, 28.0%, 38.9% among women ≤17, 18–24, and ≥25 years, respectively; p<0.001, χ2test for linear trend). However, older women were more likely to stop drinking after learning of pregnancy as compared to younger women (5.3%, 16.4%, and 22.7% among women ≤17, 18–24, and ≥25 years, respectively; p<0.001, χ2test for linear trend).
Figure 1.
Alcohol before and after learning of pregnancy according to age among 610 women attending antenatal care, Mulago Hospital, Kampala, Uganda, June to August 2006.
Among the 404 women who had not previously consumed alcohol, 4 (1.0%) initiated drinking after learning of pregnancy. These women ranged from 16 to 20 year sin age, all had friends who drank, two had male partners who drank, one was married, none were previously pregnant, and one had previously attended antenatal care. Two women reported not knowing that alcohol consumption was harmful during pregnancy. When probed for reasons why they began drinking for the first time during pregnancy, one woman reported being told that “drinking waragi relieves heartburn” and “drinking beer will make [the] baby grow big.” Two women believed that drinking waragi would make their baby lighter in reference to their preference to deliver vaginally. The fourth woman began drinking half a bottle of vodka per week, but did not reveal why.
CAGE scores of women who stopped drinking (n=33) were similar to those of women who continued to drink (n=147) after learning of their pregnancy: 81.8% and82.3% had a CAGE. ≥1 (p=0.946); 48.5% and 42.2% had a CAGE. ≥2 (p=0.509 ), respectively. Continued drinkers were more likely to answer in the affirmative to the fourth item of the CAGE (“Do you ever feel the need to have a drink first thing in the morning?”) compared to women who stopped (19.7% vs. 9.1%), but the difference was not statistically significant ( p=0.149).
Predictors of alcohol consumption
In unadjusted analysis, per year increase in age was associated with 4% higher prevalence of regular drinking before learning of pregnancy (PR=1.04; 95% CI:1.02, 1.05; Table 3). Higher prevalence of regular drinking was also significantly associated with several characteristics correlated with older age: 1) being married; 2) having a job that generated income; 3) previously being pregnant; and 4) previously having a live birth. HIV-infected women had a 51% higher prevalence of regular drinking before learning of pregnancy compared to uninfected women (PR=1.51; 95% CI:1.08, 2.11).
Table 3.
Unadjusted log-binomial regression of factors associated with alcohol consumption before and after learning of pregnancy among 610 women attending antenatal care, Mulago Hospital, Kampala, Uganda, June to August 2006.
| Characteristic | Regular alcohol consumption before learning of pregnancy (180/610 or 29.5%) PR (95% CI) | Any alcohol consumption after learning of pregnancy (147/180 or 81.7%) PR (95% CI) |
|---|---|---|
| Age (per year) | 1.04 (1.02, 1.05) | 0.99 (0.97, 1.00) |
| Married or living as married | 1.62 (1.15, 2.26) | 0.88 (0.77, 1.01) |
| Has a job that generates income | 1.37 (1.07, 1.76) | 0.91 (0.78, 1.06) |
| Attended secondary school or higher | 0.83 (0.65, 1.06) | 0.91 (0.78, 1.05) |
| Believes that alcohol consumed during pregnancy affects baby’s health | 0.55 (0.42, 0.72) | 1.07 (0.88, 1.30) |
| Previous pregnancy | 1.90 (1.42, 2.51) | 0.96 (0.83, 1.11) |
| Previous live birth | 1.87 (1.43, 2.44) | 0.91 (0.80, 1.05) |
| HIV-infected | 1.51 (1.08, 2.11) | 1.14 (0.98, 1.32) |
| Religion prohibits alcohol | 0.49 (0.36, 0.66) | 0.81 (0.65, 1.00) |
| Currently smokes cigarettes | 2.29 (1.28, 4.09) | 1.23 (1.15, 1.32) |
| Male partner drinks alcohol | 2.47 (1.95, 3.12) | 1.33 (1.15, 1.54) |
| Has friends who drink | 3.16 (2.36, 4.24) | 1.22 (1.00, 1.49) |
| Reported binge drinking levels prior to learning of pregnancy | NA | 1.04 (0.73, 1.47) |
PR=Prevalence ratio
Women smokers were over twice as likely to drink regularly before learning of their pregnancy (PR=2.29; 95% CI: 1.28, 4.09)and 23% more likely to continue after learning of their pregnancy (PR=1.23; 95% CI: 1.15, 1.32)compared to non -smokers. Women whose male partners or friends drank alcohol were over twice as likely (PR=2.47; 95% CI: 1.95, 3.12) or three times as likely (PR=3.16; 95% CI: 2.36, 4.24), respectively, to drink regularly before pregnancy compared to those whose partners and friends did not drink. These women were also more likely to continue to drink after learning of pregnancy.
Women whose religion explicitly prohibited alcohol were 50% less likely to drink regularly before learning of pregnancy compared to women whose religion did not have an explicit restriction (PR=0.49; 95% CI: 0.36, 0.66). On average, these women were 19% less likely to continue drinking after learning of their pregnancy (PR=0.81; 95% CI: 0.65, 1.00). Women who believed that alcohol consumed during pregnancy was harmful to the baby’s health were 45% less likely to report regular drinking before learning of pregnancy (PR=0.55; 95% CI: 0.42, 0.72). Binge drinking prior to learning of pregnancy was not predictive of continued drinking after learning of pregnancy (PR=1.04; 95% CI: 0.73, 1.47).
Several factors remained associated with regular drinking before learning of pregnancy in the adjusted model: having a male partner who drinks alcohol (PR=1.56; 95% CI: 1.21, 2.02), having friends who drink alcohol (PR=2.36; 95% CI:1.72, 3.23), belonging to a religion that prohibits alcohol (PR=0.61; 95% CI:0.45, 0.81), and per year increase in age (PR=1.03; 95% CI: 1.01, 1.04).
Screening algorithm
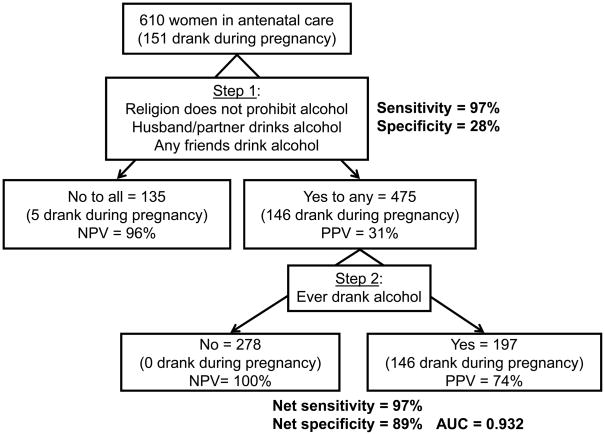
Overall, 151 of 610 women (24.8%) consumed alcoholic beverages after knowing they had become pregnant. This included 147 women who drank regularly before and 4 who newly started after learning of their pregnancy. We developed a two-step algorithm to screen for alcohol consumption during pregnancy using questions that would not elicit socially desirable responses (Figure 2).
Figure 2.
Proposed algorithm to screen for drinking alcohol during pregnancy based on consumption reported among 610 women attending antenatal care, Mulago Hospital, Kampala, Uganda, June to August 2006.
In the first step, a provider asks a woman about her religion and whether any of her friends or male partner drinks alcohol. Based on our observed data, 135 women (22.1%) belonged to a religion that prohibits alcohol consumption and did not have friends and male partners who drank alcohol, of whom only 5 drank during pregnancy (NPV=96.3%). There were 475 women (77.9%) who belonged to religions that do not prohibit alcohol or had friends or male partners who drank alcohol, of whom 146 drank during pregnancy (PPV=30.7%). If the woman was predicted by the first step to be drinking during pregnancy, then the provider would ask whether she has ever had a drink containing alcohol. Based on the observed data, 278 women (58.5%) never and 197 women (41.5%) previously drank alcohol. Of the former, none drank during pregnancy as expected (NPV=100%); of the latter, 146 drank during pregnancy (PPV=74.1%).
Step 1 of the algorithm had 96.7% sensitivity but only 28.3% specificity in correctly identifying women who drank and did not drink during pregnancy, respectively. Step 2 maintained the overall sensitivity at 96.7% but improved the overall specificity to 88.9%. The AUC for the algorithm was 0.928, and drinking during pregnancy was correctly classified for 90.2% of women. If at random 5% (n=23), 10% (n=46), or 15% (n=69) of the 459 women who reported not drinking during pregnancy (404 never, 26 non-regular, and 19 regular drinkers before learning of pregnancy) actually did drink, Monte Carlo simulations demonstrated that the algorithm was still able to classify correctly drinking during pregnancy for 87.9%, 85.0%, and 82.1% of women, respectively.
Discussion
In this cross-sectional study of 610 women attending antenatal care at a national referral hospital in Kampala, Uganda, 29.5% had an alcohol-exposed pregnancy of which 18.3% reported abstinence of drinking alcohol and 81.7% continued to drink after recognition of pregnancy. To contrast, in a representative sample of U.S. women, consumption of alcohol prior to pregnancy recognition was 55.5% and pregnancy-related abstinence from alcohol was 74% (26).
Women consumed commercial beverages more than traditional drinks with over 90% of all regular drinkers drinking beer. Thirty-one percent of women who drank regularly prior to learning of their pregnancy reported consuming quantities that equaled or exceeded binging levels for at least one beverage type, and 83.6% of these women continued to drink after learning of their pregnancy. In the 2001 Uganda Demographic Health Survey, 24% of women aged 15–54 years reported alcohol consumption the previous 30 days (17), as compared to 30% among 15–45 year olds in our study. Our study was also consistent with others that reported prevalence of alcohol consumption among Ugandan women (14–17).
While studies examining the effects of low to moderate alcohol consumption during pregnancy have been inconsistent(27), it is concerning that almost one-third of women reported drinking at levels associated with fetal alcohol syndrome prior to their pregnancy(28) and most reported continued consumption after learning of their pregnancy. While we did not collect information on quantity and frequency of drinking after learning of pregnancy, and were unable to estimate actual ethanol exposure to the fetus, it is likely our pre-pregnancy estimates represent exposures experienced early in gestation. In the absence of routine pregnancy testing in Uganda, most women become aware of their pregnancy between 6 to 12 weeks gestation (Byamugisha J, personal communication, July 30, 2008). This coincides with critical points in embryonic development associated with FASD. Furthermore, the prevalence of regular drinking prior to learning of pregnancy did not differ between women who planned and did not plan their pregnancies suggesting that women in our study were unaware of the hazards of consuming alcohol early in pregnancy or were unwilling to stop.
The Uganda Ministry of Health antenatal care package includes health education and counseling on pregnancy and emergency preparedness, nutrition, hygiene, birth plan, postpartum care, breastfeeding, sexually transmitted infection prevention and family planning. It also involves antenatal examinations, disease treatment, syphilis screening, opt-out HIV testing, prevention of mother-to-child transmission of HIV, periodic de-worming, nutrition supplementation and tetanus immunization(29). Absent in this package is screening for and education about alcohol consumption.
Other alcohol screening instruments have been proposed for use in pregnant women including the T-ACE, Short Michigan Alcoholism Screening Test, CAGE, AUDIT, and Prenatal Alcohol Use Interview. However, most are designed to identify problem drinking and all directly ask about alcohol consumption which may be stigmatizing and/or illicit false answers(30,31). We proposed a practical, two-step algorithm to screen for alcohol consumption among women attending an antenatal care clinic. The algorithm is not intrusive and is considered appropriate by providers at Mulago Hospital. There is no gold standard to ascertain alcohol consumption apart from very recent use; thus, we relied on self-reported data. We believe our screening algorithm is robust as simulations demonstrated that drinking during pregnancy would still be correctly classified for 8 out of10 women screened if underreporting in our study was as high as 15%.
We recommend antenatal, as well as general obstetrics and gynecology clinics to incorporate alcohol education into group counseling for all women of reproductive age regardless of pregnancy status. Providers should carry out the screening algorithm during a short conversation with both pregnant women and women who may become pregnant and target individual counseling and education towards those predicted to drink during pregnancy.
Our study demonstrates a need for a comprehensive alcohol policy in Uganda. At the time of this study, commercial beverages bottled and sold in Uganda have warning labels regarding their effect on health, but do not specifically warn about the harmful effects of alcohol to the fetus. Given the preference for commercial alcoholic beverages among women, particularly beer, we recommend this specific warning be placed prominently on bottles. Point of sale warnings and social marketing campaigns in English and local languages, largely absent at the time we conducted this study, are also needed. While our cross -sectional survey prompts further epidemiologic and clinical studies in Uganda and in other African nations, promoting awareness and education of the adverse effects of alcohol consumption during pregnancy are warranted now.
Acknowledgments
This work was supported in part by grants from the National Institutes of Alcohol Abuse and Alcoholism (AA015488); the Fogarty International Center International Clinical, Operational, and Health Services Research and Training Award (TW0006900); and the Case/University Hospitals of Cleveland Center For AIDS Research (AI36219).
Reference List
- 1.Windham GC, Von BJ, Fenster L, Schaefer C, Swan SH. Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology. 1997;8(5):509–514. doi: 10.1097/00001648-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Aliyu MH, Wilson RE, Zoorob R, Chakrabarty S, Alio AP, Kirby RS, et al. Alcohol consumption during pregnancy and the risk of early stillbirth among singletons. Alcohol. 2008;42(5):369–374. doi: 10.1016/j.alcohol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Passaro KT, Little RE, Savitz DA, Noss J. The effect of maternal drinking before conception and in early pregnancy on infant birthweight. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Epidemiology. 1996;7(4):377–383. doi: 10.1097/00001648-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Parazzini F, Chatenoud L, Surace M, Tozzi L, Salerio B, Bettoni G, et al. Moderate alcohol drinking and risk of preterm birth. Eur J Clin Nutr. 2003;57(10):1345–1349. doi: 10.1038/sj.ejcn.1601690. [DOI] [PubMed] [Google Scholar]
- 5.West JR, Blake CA. Fetal alcohol syndrome: an assessment of the field. Exp Biol Med (Maywood ) 2005;230(6):354–356. doi: 10.1177/15353702-0323006-02. [DOI] [PubMed] [Google Scholar]
- 6.Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21(1):150–161. [PubMed] [Google Scholar]
- 7.Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 8.Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265(15):1961–1967. [PubMed] [Google Scholar]
- 9.Abel EL, Sokol RJ. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet. 1986;2(8517):1222. doi: 10.1016/s0140-6736(86)92234-8. [DOI] [PubMed] [Google Scholar]
- 10.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 11.Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- 12.US Surgeon General releases advisory on alcohol use in pregnancy [press release] Washington, D.C: U.S. Department of Health and Human Services; Feb 21, 2005. [Google Scholar]
- 13.World Health Organization. Global status report on alcohol 2004. World Health Organization; 2004. pp. 1–67. 4-16-2008. [Google Scholar]
- 14.Koenig MA, Lutalo T, Zhao F, Nalugoda F, Kiwanuka N, Wabwire-Mangen F, et al. Coercive sex in rural Uganda: prevalence and associated risk factors. Soc Sci Med. 2004;58(4):787–798. doi: 10.1016/s0277-9536(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 15.Mbulaiteye SM, Ruberantwari A, Nakiyingi JS, Carpenter LM, Kamali A, Whitworth JA. Alcohol and HIV: a study among sexually active adults in rural southwest Uganda. Int J Epidemiol. 2000;29(5):911–915. doi: 10.1093/ije/29.5.911. [DOI] [PubMed] [Google Scholar]
- 16.Tumwesigye NM, Kasirye R. Gender and the major consequences of alcohol consumption in Uganda. In: Obot IS, Room R, editors. Alcohol, gender and drinking problems: perspectives from low and middle income countries. Geneva: World Health Organization; 2005. pp. 189–208. [Google Scholar]
- 17.Uganda Bureau of Statistics (UBOS) and ORC Macro. Uganda Demographic and Health Survey 2000–2001. UBOS and ORC Macro; Calverton, Maryland, USA: 2001. [Google Scholar]
- 18.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 19.Floyd RL, Ebrahim SH, Boyle CA. Observations from the CDC -Preventing alcohol -exposed pregnancies among women of childbearing age: The necessity of a preconceptional approach. Journal of Womens Health & Gender-Based Medicine. 1999;8(6):733–736. doi: 10.1089/152460999319048. [DOI] [PubMed] [Google Scholar]
- 20.Dawson DA. Methodological issues in measuring alcohol use. Alcohol Res Health. 2003;27(1):18–29. [PMC free article] [PubMed] [Google Scholar]
- 21.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Mwesigye PK, Okurut TO. A survey of the production and consumption of traditional alcoholic beverages in Uganda. Process Biochemistry. 1995;30(6):497–501. [Google Scholar]
- 24.NIAAA Newsletter. NIAAA Council Approves Definition of Binge Drinking. 2004. [Google Scholar]
- 25.U.S.Department of Health and Human Services and U.S.Department of Agriculture. Dietary Guidelines for Americans. 6. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 26.Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996–1998. Obstetrics and Gynecology. 2003;101(2):374–379. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 27.Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low -moderate prenatal alcohol exposure on pregnancy outcome. BJOG. 2007;114(3):243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 28.Henderson J, Kesmodel U, Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J Epidemiol Community Health. 2007;61(12):1069–1073. doi: 10.1136/jech.2006.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ministry of Health Uganda. Health Sector Strategic Plan II, 2005/06 – 2009/2010. Vol. 1. Kampala: Government of Uganda; 2005. [Google Scholar]
- 30.Chang G. Alcohol-screening instruments for pregnant women. Alcohol Res Health. 2001;25(3):204–209. [PMC free article] [PubMed] [Google Scholar]
- 31.Bhuvaneswar CG, Chang G, Epstein LA, Stern TA. Alcohol use during pregnancy: prevalence and impact. Prim Care Companion. J Clin Psychiatry. 2007;9(6):455–460. doi: 10.4088/pcc.v09n0608. [DOI] [PMC free article] [PubMed] [Google Scholar]