Summary
Fungal vacuoles are acidic organelles with degradative and storage capabilities that have many similarities to mammalian lysosomes and plant vacuoles. In the past several years, well-developed genetic, genomic, biochemical and cell biological tools in S. cerevisiae have provided fresh insights into vacuolar protein sorting, organelle acidification, ion homeostasis, autophagy, and stress-related functions of the vacuole, and these insights have often found parallels in mammalian lysosomes. This review provides a broad overview of the defining features and functions of S. cerevisiae vacuoles and compares these features to mammalian lysosomes. Recent research challenges the traditional view of vacuoles and lysosomes as simply the terminal compartment of biosynthetic and endocytic pathways (i.e. the “garbage dump” of the cell), and suggests instead that these compartments are unexpectedly dynamic and highly regulated.
Keywords: yeast, vacuole, V-ATPase, acidification, autophagy, trafficking, morphology, stress
1. Introduction
Actively growing yeast cells have several prominent vacuoles that are functionally similar to mammalian lysosomes and plant vacuoles. Vacuoles are the most acidic compartment of the cell and have a defined lipid and protein composition that supports roles in protein degradation, ion and metabolite storage, and detoxification (reviewed in [1]). General insights into membrane traffic and organelle biogenesis have been obtained from vacuolar protein sorting mutants, which have provided a molecular description of many features in the biosynthetic and endocytic pathways of all eukaryotes. Studies of the transfer of vacuoles from mother to daughter cells provided some of the first insights into mechanisms underlying organelle inheritance. More recent studies have highlighted changes in vacuolar morphology in response to extracellular conditions, and the importance of the vacuole in responses to stresses ranging from acute osmotic and ionic shock to long-term nutrient deprivation. The emerging picture of both yeast vacuoles and mammalian lysosomes suggests that they are not only “endpoints” (i.e. terminal compartments in the biosynthetic and endocytic pathways), but also “crossroads” that are acutely sensitive and responsive to changing extracellular environment. In this review, we will first highlight the defining features of yeast vacuoles, including protein and lipid composition, morphological features, and mechanisms of acidification. We will then discuss how this repertoire of features is used by the vacuole to perform the wide variety of constitutive and stress-related functions. The vacuole continues to be a very informative model for mammalian lysosomes; we will highlight similarities and differences between vacuoles and lysosomes throughout the review.
2. Identifying features of yeast vacuoles
2.1 Protein content of the yeast vacuole
The yeast vacuole has a defined set of resident proteins, a distinctive ionic milieu, and a characteristic membrane lipid composition, all of which combine to provide its “compartment identity”. For many years, research focused primarily on specific protein content responsible for major vacuolar functions such as proteolysis or transport.. More recently, genomic and proteomic approaches are also providing comprehensive views of the vacuole. A search of the yeast genome database (www.yeastgenome.org) for all open reading frames characterized by the gene ontology (GO) term “vacuolar localization” [2] revealed that two hundred of the approximately 6000 yeast ORFs are currently annotated as having vacuolar localization, at least under some conditions. Not all of the proteins currently listed are likely to be functionally contributing vacuolar residents; some GO terms are attributed based solely on the localization of GFP-tagged ORFs [3] whose functionality has not been confirmed, and some proteins targeted from other locations to the vacuole for degradation may reside there long enough to be visualized. Nevertheless, scanning the list of proteins generated provides global insights into vacuolar function. 27% of the total proteins that are identified with vacuolar GO terms are annotated as having transporter function, representing a signficant enrichment of the total cellular population of transporters in the vacuole. These transporters have a wide variety of substrates, ranging from amino acids to transition metals to glutathione conjugates, and include both importers and exporters, highlighting the importance of the vacuole as a storage compartment and cellular buffer in multiple homeostatic mechanisms. As expected, there is also an enrichment of proteases. Other hydrolases are also present, along with proteins implicated in autophagy, vacuolar protein targeting, or vacuole-vacuole fusion.
Proteomic approaches are also contributing to a comprehensive picture of the protein composition of the vacuole. An analysis of the yeast vacuolar luminal proteome from yeast was published recently, and the results highlight the proteolytic functions of the vacuole [4],. Vacuoles were highly purified under conditions that preserve vacuolar content, and proteinase K-resistant (lumenal) soluble proteins were identified by 2D gel electrophoresis and mass spectrometry [4]. Hydrolytic enzymes represented a major class of lumenal proteins, but a number of “non-canonical” vacuolar proteins were also identified. These proteins were suggested to have entered the vacuole for eventual degradation and to have been detected because of the sensitivity of the methods. The yeast vacuolar membrane proteome has not yet been reported, but there have been several proteomic studies of the plant tonoplast (plant vacuolar membrane) [5, 6]. Consistent with the genomic analysis in yeast, these studies indicate a rich variety of transporters in the plant tonoplast membrane. Together, these data support the view of the vacuole as a degradative and highly versatile storage compartment in yeast and plants.
2.2 The vacuolar membrane has a distinctive lipid composition
Aside from having a distinct luminal and membrane proteome, the yeast vacuole also possesses a unique lipid profile. While the plasma membrane is known to be populated by sphingolipid-rich lipid rafts which are implicated in signaling and membrane traffic [7], these have not been found in yeast vacuoles. Furthermore, the vacuole has a drastically low ergosterol to phospholipid ratio, and low levels of sphingolipids. [8–10]. As a result, while most plasma membrane proteins appear to reside in “detergent resistant membranes”, vacuolar membrane proteins are generally detergent soluble [11]. Note-worthy lipids involved in vacuolar function are discussed below.
Fusion between vacuolar membranes (homotypic vacuole fusion, discussed below) requires the presence of regulatory lipids such as ergosterol, diacylglycerol, phosphatidyl inositol 3-phosphate (PI3P) and phosphatidyl inositol 4-phosphate (PI4P) [12, 13]. Cells without these lipids (except PtdIns[3]P) have vacuoles. These “regulatory lipids” interdependently congregate at the vertices of fusing membranes and are necessary for the enrichment of other fusion factors such as SNAREs, Ypt7p1 and HOPS [13]. The autophagy and cvt (cytosol to vacuole targeting) pathways are both protein delivery pathways to the (discussed below). It has recently been shown that these processes require phosphatidylethanolamine, due to covalent binding of the phospholipids to Atg8p, an autophagy protein. This lipid modification is necessary for the proper localization of Atg8p to the pre-autophagosomal structure, leading to vesicle formation during Cvt and autophagy [14]. Though phosphorylated derivatives of myo-inositol make up a tiny fraction of total cellular lipid (<1%) [15], phosphoinositide signaling is crucial for trafficking membranous vesicles and their cargo to appropriate compartments. The fidelity of this process must be maintained to ensure proper identity. The major phosphoinositide species on vacuolar membranes is phosphatidylinositol 3,5-bisphosphate (PI3,5P2), which is generated from PI3P by the Fab1 kinase [16]. Fab1p is activated by two other peripheral membrane proteins, Vac14p and Vac7p, to generate a pool of PtdIns[3,5]P2 on late membranes [17–19] [20]. Cells that cannot produce this lipid have severe defects in vacuolar morphology, characterized by a grossly enlarged vacuole that is incapable of fission. This grossly enlarged vacuole appears to be a result of defects in vacuolar fission as well as membrane recycling from the do not have a mislocalized V-ATPase [21–23] The presence of PtdIns[3,5]P2 is necessary for maintaining vacuolar identity, as specific PtdIns[3,5]P2 effectors are required for protein sorting from multivesicular bodies (MVBs) to the vacuole, as well as protein retrieval from the vacuole to MVBs [16]. By a still unknown mechanism, PtdIns[3,5]P2 levels are, so the basis of the acidification defect is not yet understood.
3. Vacuolar morphology
The vacuole is a dynamic organelle whose morphology is highly responsive to different extracellular and intracellular stimuli. During log phase, actively metabolizing cells have vacuoles that are comprised of multiple medium-sized lobes (Fig. 1). These lobes fuse into one enlarged vacuole during stationary phase or with glucose deprivation. A more striking effect occurs during osmotic stress, where the vacuole fragments into multiple small vesicles. In contrast, exposure to hypo-osmotic conditions causes the vacuole to swell, occupying a large volume of the cell. These changes in size and compartment number correspond to the uptake or release of water and ions from the vacuole. The vacuole must be capable of receiving both lumen and membrane-associated cargo from arriving vesicles via various trafficking pathways (discussed below). Conversely, the vacuole must also be able to send off membrane/lumenal contents destined to other compartments. A highly orchestrated example of this is vacuolar inheritance, where part of the mother vacuole is partitioned and directed to the daughter cell in response to cell cycle cues. All these cellular events require a labile vacuole that is capable of changing size and contents while maintaining sufficient organelle homeostasis to support normal function. In this context, vacuolar membranes must be capable of undergoing fission and fusion at will (reviewed in [26]).
Figure 1. Vacuolar morphology is sensitive to growth conditions.
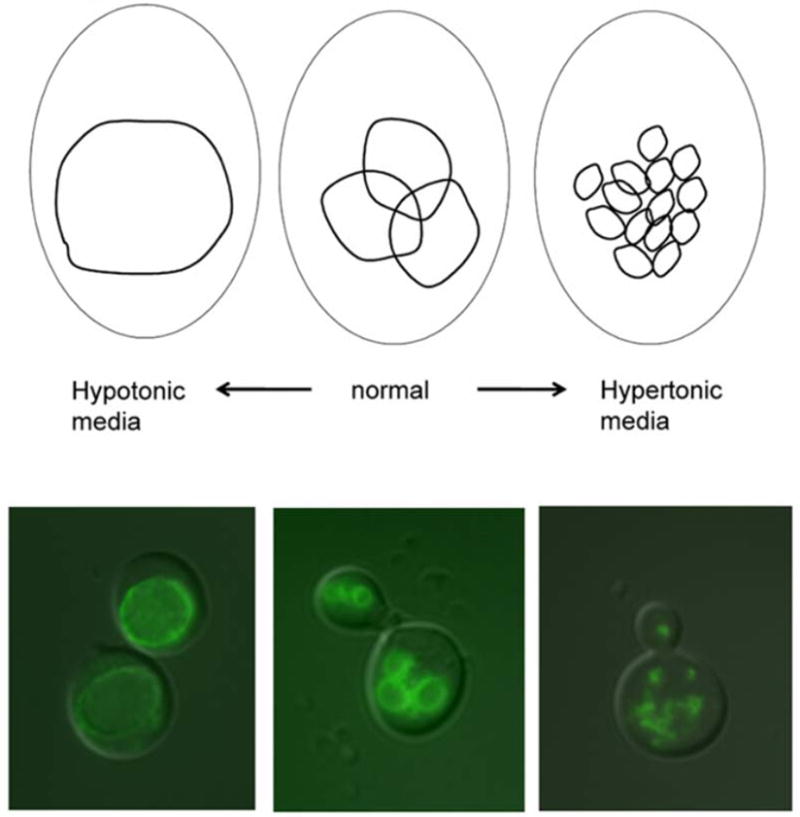
The vacuole can change in volume and vesicle number depending on extracellular condtions. Top: Under normal conditions, the cell has two to three medium-sized vacuoles. In hypo-osmotic media, a single large vacuole takes up most of the cell. Upon hyper-osmotic shock, the vacuole fragments into multiple small lobes. Bottom: Superimposed images of fluorescently stained vacuoles on yeast cells viewed under Nomarski optics. Vacuoles were visualized using a C-terminally GFP-tagged V-ATPase subunit (Vma2p-GFP) during log-phase growth under hypotonic conditions (synthetic complete medium), normal conditions (YEPD, buffered to pH 5 (100 mM added salt)), and hypertonic conditions (YEPD, buffered to pH 5 with 1 M NaCl added).
3.1 The equilibrium between vacuolar fission and fusion dictates vacuolar morphology
The fusion and fission of vacuolar membranes is necessary for delivering cargo in and out of the vacuole, and for regulating vacuolar volume in response to extracellular stresses. Current knowledge about these mechanisms are summarized below.
3.1.1 Vacuolar Fusion
The isolation of vam (vacuolar morphology) mutants [27], led to the discovery of genes that were required for normal vacuolar morphology. These turned out to be players in the vacuolar homotypic fusion process. Vacuolar fusion involves highly concerted interactions between SNAREs their regulators and effectors, SNAREs (Vam3), SNARE chaperones (Sec17p/Sec18p), phosphoinositides, and Sec1/Muncproteins [26, 28]. This process involves several major steps: priming, tethering, docking, and fusion. ATP-dependent priming capacitates vesicles for fusion and activating Rab GTPases. The AAA-ATPase Sec18p and its cofactor Sec17p disassemble cis-SNARE complexes, and the soluble SNARE Vam7p and Vps1p are released. The activation of fusion factors such as Vac8p andVam10p and the Rab GTPase Ypt7p by the HOPS tethering complex also occurs at this stage. Tethering is the reversible initial contact between vacuoles, and requires an assembled HOPS complex and activated Ypt7p Rab GTPase. Docking follows, where SNAREs from opposing vesicles establish more secure trans-SNARE. Trans-SNARE complexes may facilitate membrane fusion by exerting enough physical strain on the lipid bilayer [30, 31], disrupting existing bilayer structures via SNARE transmembrane domains or concentrating membrane destabilizing lipids at sites of fusion [13, 30] [32].
complexes and the Rho1p and Cdc42p GTPases become activated. Vacuolar acidification and PI4,5P2 are required for vesicle docking. Docking leads to calcium release from the vacuole. Calcium activates calmodulin, which binds to the membrane associated domain of the V-ATPase (V0) [29]involvement of V0 in trans-complex formation is still being debated. The assembly of trans complexes is proposed to create enough physical strain on vesicle membranes to facilitate lipid bilayer mixing [30]. Calcium activates calmodulin, which binds to the membrane associated domain of the V-ATPase (V0) [35]. This is proposed to create trans-complex formation between V0 complexes on opposed vesicles [32, 36], though the involvement of V0 in trans-complex formation is still being debated.
3.1.2 Vacuolar Fission
While vesicle fusion requires vesicle and target SNAREs, Rab GTPases, NSF and α-SNAP, vesicle formation/fission during endocytosis requires coat proteins such as COPI, COPII, and clathrin [37]. While much is known about the mechanisms behind vesicle fission during endocytosis, the molecular mechanisms behind the fission of vacuolar vesicles remain elusive.
In yeast, vacuole fission is necessary for proper vacuolar inheritance during mitotic cell division and for the acute response to osmotic shock (see below). Information about this process can be gleaned from mutants that are defective in vacuole inheritance, since mutants in genes required for this process often also show a failure to fragment vacuoles [38]. This includes the mutants of Vac14p, Vac7p. and Fab1p, which are required for PtdIns[3,5]P2 production. As previously mentioned, these mutants have grossly enlarged vacuoles, pointing to a defect in vacuolar fission and membrane recycling. Mutants lacking Atg18p, a Fab1p display a similar defect [23]. VAC14, VAC7 and FAB1 deletion mutants have no detectable PtdIns[3,5]P2, while the ATG18 deletion mutant has high PtdIns[3,5]P2 levels. Fusing Atg18p to a vacuolar trans-membrane protein rescues the morphology defects of a vac14Δ strain. Hence, Atg18p has been proposed levels that mediates vesicle fission and membrane recycling at the vacuole [23, 39].
Vps1p is a dynamin-like protein which has been proposed to be involved in both vacuolar fusion and fission [40, 41]. Like fab1Δ, vps1Δ cells have enlarged vacuoles that are unable to fragment in response to osmotic stress, but unlike fab1Δ cells, vps1Δ cells also have numerous small vacuolar vesicles surrounding the large central vacuole. Further analysis revealed that vps1Δ vacuoles are also fusion-deficient. Vps1p release from the vacuole is dependent on Vam3p and Sec18p, placing this protein at the interface of fission and fusion reactions. Peters et al [42] have suggested that Vps1p sequesters Vam3p during fission and is released just before fusion, though this hypothesis has yet to be confirmed.
Interestingly, the V-ATPase is required for both vacuolar fusion and fission [40]. A recent study has shown that the presence of the V0 membrane sector of the V-ATPase (see below) is required for fusion, while the proton translocation activity of the enzyme is required for fission [40]. In this study, researchers provide compelling evidence that vacuolar morphology is dictated by the equilibrium between fusion and fission reactions. Cells that had no V-ATPase activity due to the deletion of a V-ATPase subunit or inhibition by concanamycin (a V-ATPase specific drug) were defective for fission and had a big vacuole phenotype. In contrast, the deletion of a V0 subunit (vph1Δ) that could still partially support acidification had a more pronounced fusion defect, and had fragmented vacuoles. Interestingly, vph1Δ cells that were treated with concanamycin A, completely abolishing vacuolar acidification, contained big vacuoles. This suggests that fusion-fission equilibrium can be shifted to favor one reaction over another, and this ultimately dictates vacuolar morphology [40].
3.2 Vacuolar Inheritance during mitosis
In response to cell cycle cues, vacuolar compartments must be distributed from the mother cell to the daughter bud. This starts early in the cell cycle and commences just before nuclear migration [43, 44]. For vacuolar inheritance to occur, there must be spatial and temporal control of vacuole movement, along with the coordination of these changes with vacuolar function (reviewed in [38, 45]). In this process, tubular/vesicular “segregation structures” emanate from the side of the vacuole closest to the nascent bud, and move along a track from the mother to the daughter. This actin-dependent process involves the molecular motor, Myo2p and Vac17p and Vac8p as the main mother-daughter vacuole transport complex. The interaction of Vac17p with Myo2p is vacuole specific and is only necessary for vacuolar inheritance, while Vac8p attaches Vac17p to the vacuole. The assembly of this complex draws vacuolar vesicles into the growing bud. It has been hypothesized that Vac17p turnover disrupts vacuole movement, leaving the new vacuole in its appropriate compartment [38].
3.3 Vacuole inheritance during meiosis
During sporulation, a diploid yeast cell undergoes meiotic division to yield four spores enclosed in an ascus (sac). Unlike vacuolar inheritance during mitosis, these spores do not inherit vacuolar compartments from the parent diploid cell [46]. Instead, the vacuole vesicles seem to be excluded from spores and reside in between the spore cell walls and the ascus. The sequestration of the diploid vacuole from haploid spores during meiosis may serve as a mechanism to exclude unnecessary vacuolar cargo that may be deleterious to new spores [46]. In contrast, the exclusion of the diploid vacuole from spores also facilitates the release of vacuolar proteases into the ascus, and this may be important for spore germination [46].
If little or no vacuolar material is inherited from the diploid cell, there must be mechanisms by which vacuoles are re-generated in spores from precursors [46, 47]. This kind of de-novo vacuole formation has also been observed in vac mutants that have defects in vacuolar inheritance during mitosis [43, 48]. Studies from mammalian cells have shed some light on this problem. Various groups have shown that organelles that are connected to the endoplasmic reticulum by membrane traffic can be newly formed if part of the ER is inherited by daughter cells [46, 49, 50]. Accordingly, yeast spores could form vacuoles by using vesicles from pre-vacuolar compartments that arise from the ER-Golgi pathway. Class E vps mutants accumulate vacuolar proteins such as the V-ATPase in structures proximal to the vacuole and recapitulate a number of vacuolar functions in this compartment [51–53]. The existence of Class E compartments suggests that the vacuole can be formed by the maturation of compartments such as these [46].
4. Trafficking to and from the yeast vacuole
The distinctive protein and lipid composition of the vacuole requires machinery for protein targeting. Genetic and biochemical studies of vacuolar transport pathways in yeast have been extremely fruitful, both in defining components required for transport of newly synthesized proteins to the vacuole, and in elucidating general properties of protein sorting and vesicular transport. (Sorting pathways for vacuolar proteins were recently reviewed in more detail by Bowers and Stevens [54]).
4.1 vps mutants define conserved trafficking components
Newly synthesized soluble vacuolar and lysosomal proteases contain signal sequences that target them for insertion into the endoplasmic reticulum, and they transit from ER through the Golgi before they are diverted toward the lysosomes in the late Golgi. The vacuolar protein sorting (vps) mutants emerged from two different genetic screens [55, 56], both directed toward identifying mutants that mislocalize soluble vacuolar proteins, specifically carboxypeptidase Y (CPY), to the secretory pathway rather than targeting them to the vacuole. This set of approximately 70 mutants implicated in vacuolar protein sorting was further classified by vacuolar morphology [51], and many of the mutated proteins have now been biochemically characterized [54]. Yeast soluble vacuolar proteins do not receive mannose-6-phosphate, a canonical targeting signal for soluble lysosomal proteins in mammals. Nevertheless, the vps mutant screen identified a number of highly conserved proteins. These include classical components of the vesicular transport machinery, such as Rab proteins, tethering factors, and SNARE proteins, the ESCRT machinery operative in multivesicular bodies, and the PI3-kinase Vps34p [54, 57, 58]. In addition, a sorting receptor for carboxypeptidase Y (Vps10p), may serve a similar recognition function to the mannose-6-phosphate receptor, was identified [59]. Characterization of the vps mutants suggests that even though lysosomal sorting may use a different sorting determinant in some cases, this determinant operates on the background of highly conserved pathways for transport.
4.2. The many routes to the yeast vacuole
Multiple pathways can direct proteins to the vacuole (Fig. 2; reviewed by [54]). The “CPY pathway” is perhaps the best-studied pathway for newly synthesized vacuolar proteins, and involves vesicular transport from the late Golgi through the multivesicular body (MVB) to the vacuole. A number of membrane proteins, including dipeptidyl aminopeptidase B (DPAP-B) and the V-ATPase also transit this pathway [60]. Carboxypeptidase S (CPS) uses a variation in which it is transported to the MVB as a membrane protein, then inserted into intralumenal vesicles before transport to the vacuole and final processing to remove its membrane domain. The “ALP pathway”, named for its alkaline phosphatase cargo, is characterized by direct vesicular transport from the Golgi apparatus to the vacuole [61–63]. In addition, aminopeptidase I follows an unusual pathway to the vacuole, the Cvt pathway, that is characterized by direct transport from the cytosol to the vacuole; this pathway utilizes a subset of the autophagy machinery [64].
Figure 2. Trafficking Pathways to and from the Vacuole.
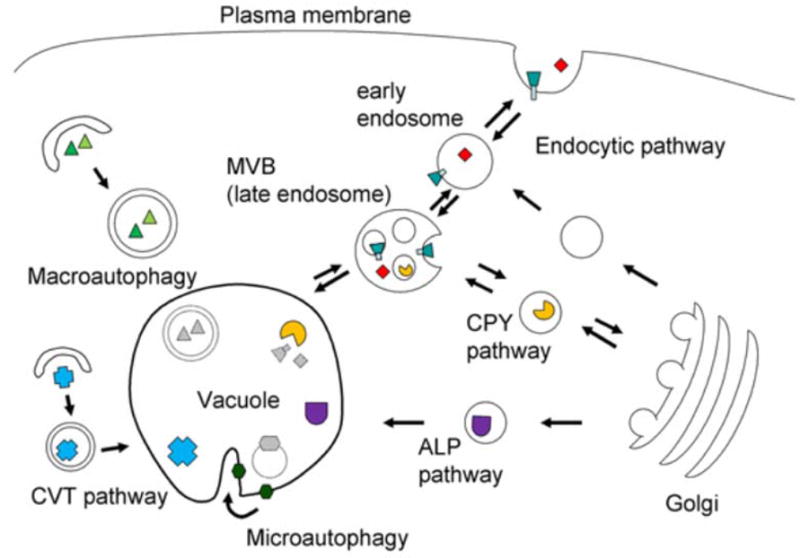
Multiple vesicular pathways deliver proteins to and from the vacuole. Resident vacuolar proteins are sent to the vacuole by several biosynthetic pathways, while proteins targeted for degradaton may be sent via endocytosis or autophagy. Biosynthetic pathways: The ALP pathway delivers cargo from the late Golgi to the vacuole. The CPY pathway also starts from the late Golgi, but traverses multivesicular bodies (MVBs) before reaching the vacuole. The CVT pathway delivers biosynthetic cargo from the cytosol to the vacuole in a process that has common components with autophagy. Trafficking pathways away from the vacuole may recycle membrane and proteins to the endocytic or CPY pathways. Degradative pathways: Endocytosis transports both soluble and membrane-bound cargo from the plasma membrane and extracellular space to the vacuole. Cargo progresses from early endosomes to late endosomes (multivesicular bodies) before reaching the vacuole. In macroautophagy, vesicles called autophagosomes engulf cytosolic material for degradation at the vacuole. In microautophagy, the vacuolar membrane itself forms invaginations which form vesicles that are then degraded at the vacuole.
In addition to these biosynthetic pathways, multiple proteins are sent to the vacuole for degradation (see below). Extracellular or cell surface proteins may enter the cells by endocytosis, first transiting to early endosomes and then intersecting the CPY pathway in multivesicular bodies before transport to the vacuole [60]. The collection of endocytosis (end) mutants [65, 66] shows substantial overlap with the vps mutants because of the interdependence of the biosynthetic and endocytic pathways [55, 60]. Intracellular proteins, both cytosolic and organellar, are also digested in the vacuole under certain conditions. These proteins are generally targeted via the autophagy machinery (see below).
4.3. Membrane transport from the vacuole/lysosome
Pathways involved in targeting proteins and membrane to the vacuole must be balanced by pathways capable of retrieving membrane and targeting machinery from the vacuole, or the capacity for sorting will be depleted and the vacuole will lose its identity. The major retrieval pathway from the vacuole appears to be routed through the pre-vacuolar/late endosome compartment [67]. Lysosomes must also contain retrieval pathways, and situations where lysosomes fuse with earlier compartments may represent a variation of constitutive retrieval pathways.
Another interesting mechanism of directed lysosomal transport has been reported in various metazoan systems. Lysosomes have been found to fuse with the plasma membrane. The exocytosis of conventional lysosomes is triggered by an increase in cytosolic Ca2+. This appears to be necessary for plasma membrane repair in response to injury and also allows the formation of “parasitophorous vacuoles”, which sequester pathogens such as Trypanosoma cruzi away from the cell [68, 69].
Still other cell types have specialized secretory lysosomes that store newly produced secretory cargo. Examples of these are melanosomes, basophil granules, and cytotoxic T lymphocyte granules. Cytotoxic T lymphocyte lysosomes can fuse with the plasma membrane at the junction between the lymphocyte and antigen presenting cell to expel the contents of lytic granules [68]. This happens when the T-cell receptor recognizes antigen on the antigen-presenting cell. Secretory lysosomes are then directed to the plasma membrane along microtubule tracks, and fusion requires the RAB27A GTPase and Munc 13-4 [68].
5. Acidification as a defining feature of vacuoles and lysosomes
Yeast vacuoles and mammalian lysosomes are the most acidic cellular organelles. Almost all vacuolar and lysosomal functions are tied to the acidic pH of the vacuolar lumen and/or the pH gradient across the membrane. Lysosomal pH is generally 4.5–5 [70], and vacuolar pH has been shown to vary from <5–6.5 depending on growth conditions [71–74]. In both mammalian and yeast cells, acidification is achieved through the action of a proton pump, the vacuolar H+-ATPase (V-ATPase), which couples ATP hydrolysis to proton transport from the cytosol to the organelle lumen.
5.1. V-ATPases acidify both vacuoles and lysosomes
V-ATPases are remarkably conserved enzymes that are evolutionarily related to the F1F0-ATP synthase of mitochondria [75, 76]. They are multisubunit enzymes, consisting of a complex of peripheral membrane subunits containing the sites of ATP hydrolysis, which is designated V1, bound to a complex of integral membrane subunits containing the proton pore, which is designated V0. A current model of the yeast V-ATPase is shown in Fig. 3. Mammalian enzymes have a very similar structure overall [75]. Individual subunit genes show sequence identities as high as 80% between yeast and mammalian sequences [77], and even subunits with significantly less sequence identity can be functionally interchangeable [78]. The yeast V-ATPase consists of 14 different subunits. All subunits are encoded by a single gene except for the largest membrane subunit, subunit a, which is present as two different isoforms [79]. The two yeast a subunit isoforms, Vph1p and Stv1p, are localized predominantly to the vacuole and Golgi/endosomes, respectively, where they assemble with other V-ATPase subunits to generate V-ATPases with organelle-specific properties. Specifically, Vph1p-containing V-ATPases at the yeast vacuoles appear to show more efficient coupling of ATP hydrolysis to proton transport, higher levels of assembly of V1 subunits with V0 subunits, and greater responsiveness to extracellular glucose concentration (see below) than Stv1p-containing V-ATPase complexes [80, 81]. Based on their differential localization and chimeric a subunit experiments, the yeast a subunit isoforms are known to contain targeting information[80].
Figure 3. The Vacuolar H+-ATPase.
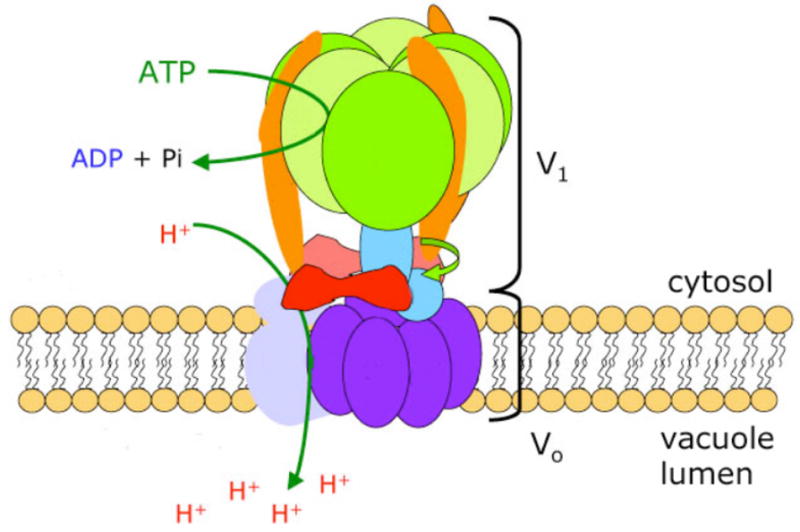
The vacuolar H+-ATPase (V-ATPase) is a proton pump on the vacuolar membrane composed of a membrane-bound V0 sector and a cytosolic V1 sector. ATP hydrolysis in the V1 sector drives the translocation of protons through the V0 sector.
There is a much larger set of V-ATPase isoforms in mammalian cells [75, 82], and this lends tremendous potential for versatility in targeting and function to the mammalian enzymes. In general, at least one isoform for each mammalian subunit is ubiquitously expressed, while the others show tissue-specific expression, often associated with tissues where high-levels of V-ATPases are expressed at the plasma membrane [82–84]. It is also clear, however, that multiple V-ATPase isoforms can exist in a single cell, and that there may be functional isoform substitution [84–86]. This complicates biochemical analysis of mammalian lysosomal V-ATPases and is one of the major reasons why yeast has emerged as the major model system for analysis of V-ATPase activity. Mice and humans have four isoforms of the a subunit, and as in yeast, these isoforms are believed to impart localization information to the V-ATPase, as well as participating in proton transport [87, 88]. The mouse a3 isoform has been localized to late endosomes and lysosomes, but has also been shown to be recruited to the plasma membrane during osteoclast maturation [89]. Homozygous mutations in both the mouse and human a3 isoforms result in osteopetrosis, an overgrowth of bone resulting from loss of osteoclast activity [90, 91]. In addition, the oc/oc mouse has defects in insulin secretion that suggest an involvement of the a3 isoform in other non-lysosomal functions, specifically regulated secretion [92]. The relatively specific effects of these mutations suggests that tissue-specific functions of the a3 isoform are disrupted, but lysosomal acidification may be preserved, perhaps by substitution with another a subunit isoform.
5.2 Consequences of loss of vacuolar acidification
Genetic deletions of ubiquitously expressed V-ATPase subunits appears to be lethal in all organisms except fungi. Mice with homozygous deletions in the c subunit died at the point of implantation [93], while inactivation of a constitutive V-ATPase subunit gene in Drosophila resulted in a larval lethal phenotype [94]. Thus, because they are viable, yeast V-ATPase subunit deletion mutants (vma mutants) provide invaluable insights into the functional importance of vacuolar acidification [76]. Deletion of any of the single-copy V-ATPase subunit genes, or both of the a subunit isoform genes, abolishes uptake of the lysosomotropic base quinacrine into the vacuole, suggesting that organelle acidification has been abolished. These deletions also result in a signature growth phenotype (the Vma− phenotype) that is characterized by sensitivity to elevated extracellular pH (vma mutant cells can grow at an extracellular pH of 5, but not at pH 7.5), sensitivity to elevated extracellular calcium concentrations, inability to grow on non-fermentable carbon sources, sensitivity to heavy metals and oxidants, and multidrug sensitivity [76].
Although the Vma− phenotype reports loss of acidification in all compartments acidified by V-ATPases, not just vacuoles, several aspects can be linked to the storage and detoxification functions of the vacuole described in more detail in subsequent sections. Specifically, the calcium sensitivity of vma mutants results from the central role of the vacuolar Ca2+/H+ exchanger Vcx1p in calcium homeostasis and its dependence on a V-ATPase-established H+ gradient. Heavy metals are also sequestered in the vacuole, and the loss of V-ATPase activity compromises both the activity of some transporters and maintenance of the extensive vacuolar polyphosphate stores that may help bind metal ions in the vacuole [96–98]. vma mutants are also unable to grow on medium containing low iron or calcium concentrations [99, 100], reflecting the importance of the vacuole as a storage compartment, but probably influenced as well by acidification requirements in compartments such as endosomes and Golgi [100, 101]. The pH dependent growth of the vma mutants has been more difficult to explain [74, 102:Plant, 1999 #213]. It may reflect both the importance of the V-ATPase and the vacuole in pH homeostasis and more stringent requirements for iron and copper uptake elevated extracellular pH [103].
One of the most surprising results that emerged from initial experiments on the vma mutants was that some processes expected to be absolutely dependent on vacuolar acidification were only moderately affected. For example, although vacuolar proteases have an acidic pH optimum, there was slow but effective activation of zymogen forms of the vacuolar proteases, suggesting that considerable protease activity remained despite elevated vacuolar pH [104]. Similarly, vma mutants showed defects in sorting of vacuolar proteases to the vacuole, but the rate of secretion of these proteases was much lower than that of most vps and in fact, V-ATPase mutants were not present in the initial set of vps mutants [104–106]. These results suggested that both sorting and maturation of hydrolytic enzymes in the vacuole were less reliant on V-ATPase activity than expected, although compartment acidification is important for the efficiency of these Although mutations that abolish lysosomal V-ATPase activity do not appear to support viability, plecomacrolide antibiotics (the bafilomycins and concanamycins) are highly-specific V-ATPase inhibitors [107] that have been used to determine the effects of acute loss of organelle acidification in cultured mammalian cells [108, 109]. Over longer times or at higher doses, they also induce apoptotic cell death in a number of cell types, although the mechanism of this effect is still under debate [110, 111]. As with the yeast vma mutants, the widely used versions of inhibitors do not distinguish the lysosomal V-ATPase from V-ATPases active in other compartments. Lysosomotropic weak bases such as NH4+ have also been used to alkalinize acidic organelles, but these weak bases cause organelle swelling (vacuolization) as well as neutralizing acidic organelles, and this can complicate interpretation of results [112, 113]. This has made bafilomycins, concanamycins, and related compounds preferable as a means of assessing the contribution of organelle acidification to different processes.
5.3 Regulation of vacuolar acidification
Organelle acidification, and the V-ATPase itself, are regulated by a number of different mechanisms. The yeast V-ATPase is regulated at the level of assembly of the peripheral V1 sectors with the integral membrane V0 sectors [75, 76, 114], and a similar mechanism of regulation has been invoked for the control of lysosomal acidification in maturing dendritic cells [115]. In yeast, reversible disassembly is rapid and post-translational (reviewed in [75, 76]). Disassembly appears to inactivate both ATP hydrolysis in detached V1 sectors and proton transport through free V0 sectors. Reversible disassembly is not an all-or-none response, but rather, may adjust the level of assembled enzyme to the availability of ATP and/or need for proton pump activity. The mechanisms for sensing and responding to glucose are still under investigation. Glucose metabolism to fructose-6-phosphate or beyond is necessary to maintain V-ATPase assembly [116], and glycolytic enzymes, particularly aldolase, associate directly with the V-ATPase and may support its assembly and signal glucose deficiency [117–121]. Other cellular proteins, such as the RAVE complex, are required for reassembly of disassembled V-ATPase complexes, but are not directly involved in glucose sensing [122]. Recent evidence from an insect plasma membrane V-ATPase that also undergoes reversible disassembly implicates phosphorylation of the C subunit, which dissociates from both V1 and V0 upon starvation, in controlling assembly [123]. Protein kinase A is implicated in controlling assembly in this system, but mutations in yeast protein kinase A do not appear to affect assembly or reversible disassembly of the V-ATPase [116], suggesting that an alternative mechanism is available in yeast. There is also evidence that coupling of ATP hydrolysis and proton pumping in V-ATPases can be regulated under conditions where the enzyme remains assembled [124].
In addition to regulatory mechanisms focused on the V-ATPase itself, there are many indirect ways of regulating organelle acidification [125]. V-ATPases are electrogenic pumps, and counterion transport is critical for continued pumping [126]. In addition, a number of exchangers, including Nhx1p, a Na+(K+)/H+ exchanger, transport H+ against the pH gradient created by the V-ATPase, and Nhx1p has been shown to limit acidification in the compartment where it resides, the late endosome/multivesicular body, and indirectly, to control vacuolar pH [127]. The lipid environment in different organelles has also been implicated both in regulating V-ATPase activity and in determining the final pH of acidic compartments by other means [125, 128]. Finally, the buffering systems in the vacuole and the cytosol will influence the final pH of the vacuole and other organelles. The major vacuolar buffering system in yeast is polyphosphate, which is present at extremely high concentrations in the vacuole [97, 129, 130], but recent work has also suggested that vacuolar proteins may have evolved to act as buffers as well [131]. These systems operate simultaneously to determine the final pH of the vacuole and/or lysosome, and each also represents a possible site for regulating the extent of organelle acidification. In yeast, there is clear evidence of alterations in vacuolar pH with different growth conditions, suggesting a high degree of regulation [71, 72, 74]; in mammals, lysosomes appear to exhibit a lower, more consistent pH [132], but there are also conditions where lysosomal pH is regulated [115].
6. Constitutive functions of the yeast vacuole
The physiological context of the vacuole described above supports both constitutive functions, operating more or less constantly in yeast cells (Fig. 4) and stress-related functions, in which the capabilities of the vacuole are recruited to allow adaptation to different stresses. In this section, we will outline the constitutive functions of the vacuole, and in the next section, we will describe how these functions contribute to response to a number of different stresses.
Figure 4. Constitutive Functions of The Yeast Vacuole.
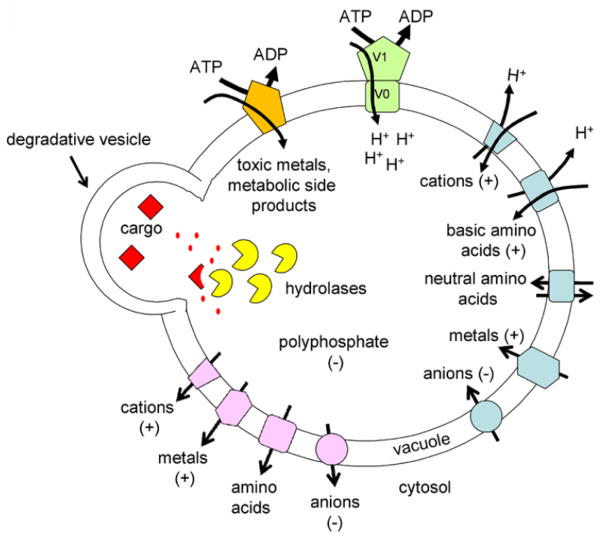
The vacuole is responsible for multiple constitutive processes: 1) degradation, 2) storage, 3) buffering, and 4) detoxification. 1) Degradation: The vacuole is highly enriched for hydrolases (yellow circles) that break down cargo delivered via multiple trafficking pathways to the vacuole. 2) Storage: The vacuolar membrane contains multiple transporters (blue polygons) that import amino acids, ions, and metals. Vacuolar acidification by the vacuolar H+ ATPase (V1 and V0, green) is required for proper maturation of hydrolases and for establishing the proton gradient that drives many transporters. 3) Buffering: The vacuolar membrane also contains transporters that recycle amino acids, ions, and metals to the cytosol. The combined activity of importers and exporters is important for ion homeostasis and amino acid recycling. The vacuole is also the main storage site for polyphosphate which buffers cations and is a source for cellular phosphate, 4) Detoxification: The vacuole also sequesters toxic metals and potentially harmful metabolic bi-products via ABC transporters.
6.1. Vacuoles as degradative organelles
Like lysosomes, vacuoles are degradative organelles, and serve as one of two major cellular degradation systems, with proteosomes constituting the second system. Hydrolytic enzymes are among the best-characterized vacuolar proteins (reviewed in [1, 133–135]), and include soluble vacuolar proteases proteinase A (Pep4p) carboxypeptidase Y (CPY), proteinase B (Prb1p), carboxypeptidase S (CPS), all of which have been used as model vacuolar proteins in trafficking studies. Membrane-bound proteases have also been described, including dipeptidyl aminopeptidase B (DPAP-B). In general, vacuolar proteases are relatively non-specific and are directed toward degradation of a large variety of substrates rather than specific processing of any one protein. Like lysosomal proteases, vacuolar proteases are transported to the vacuole in zymogen form, and activated in a complex cascade [135]. Proteinase A (pep4) mutants accumulate multiple zymogens, indicating that Pep4p initiates processing of multiple different precursors [136]. These processing events are optimal at low pH, but as described above, there is sufficient activity in the absence of V-ATPase activity to give appreciable zymogen activation and vacuolar protease activity, suggesting that low vacuolar pH is not essential.
Consistent with its role as a major degradative organelle, substrates are directed to the vacuole by multiple different routes. Plasma membrane proteins are targeted to the vacuole for degradation in both a constitutive manner, as a means of continually refreshing the population, and in a signal dependent manner, as a means of downregulating signalling or transport functions. Dependence on the activity of vacuolar proteases, such as Pep4p, rather than proteosome activity, is the defining feature of protein degradation by this route. Degradation of the yeast pheromone receptors (Ste2p and Ste3p) is well-studied. Both a slow, constitutive, ligand-independent degradation of Ste2p and ligand-dependent downregulation were shown to occur at the vacuole [137]. Ligand-dependent downregulation involves mono-ubiquitination of the C-terminal tail of the receptor prior to endocytosis [138]. This modification is then engaged by the ESCRT machinery in the late endosome/multivesicular body, culminating in in deubiquitination and targeting of the receptor to intralumenal vesicles before transport to the vacuole and degradation [139, 140]. Cell-surface receptors, such as the EGF receptor, are targeted for lysosomal degradation via a very similar pathway in mammalian cells (reviewed in [138, 139]).
Certain plasma membrane permeases are also selectively targeted to the vacuole for degradation, both as part of their normal life-cycle and in response to an excess of the nutrient that they transport. Fur4p, a plasma membrane uracil permease, shows ubiquitin-dependent targeting to the vacuole for degradation via the same route as Ste2p; Fur4p shows more efficient ubiquitination and turnover in the presence of high levels of extracellular uracil [141, 142]. Targeting of Gap1p, a general amino acid permease, to the vacuole is controlled by amino acid levels, but interestingly, intracellular amino acid levels are recognized during biosynthesis, as well as after transport to the plasma membrane [143, 144]. If cytosolic amino acids are scarce, Gap1p can be diverted to the plasma membrane, but if levels are sufficient, it is targeted to the vacuole for degradation [145]. It is notable that in this and other examples, there is little regulation of degradation at the vacuole, but instead, sorting decisions on the way to the vacuole determine whether a protein will be targeted to degradation.
Cytosolic and organellar proteins can also be degraded by vacuolar proteases under certain conditions. During starvation-induced macroautophagy (discussed below), a large percentage of total cellular protein degraded at the vacuole, but vacuoles also participate constitutively in removing cytosolic and organellar protein. This basal level of autophagy (reviewed in [146]) probably accounts for the degradation of a number of longer-lived cytosolic proteins, and can also allow for degradation of organelles [147]. In yeast, mutations in autophagy machinery only cause obvious phenotypes under conditions of starvation, suggesting that sufficient levels of constitutive protein turnover can be achieved by other means. However, in mammalian cells, there is growing evidence that basal levels of autophagy play a critical role in development and quality control [146]. The overall goals of both basal and induced autophagy appear to be removal of nonessential cytosolic components and recycling of amino acids [146]. Compromising vacuolar protease activity or vacuolar acidification allows initial steps of autophagy to proceed, but results in accumulation of autophagic vesicles in the vacuole [147]. Both yeast and mice deficient in autophagy exhibit lower intracellular and extracellular amino acid levels under starvation conditions [148, 149]. These data suggest that both vacuoles and lysosomes must have mechanisms for release of amino acids for reuse after autophagic protein digestion (discussed below)l.
6.2. Vacuolar involvement in metabolite and ion storage and homeostasis
Yeast cells encounter conditions of deprivation or starvation at multiple points in their normal life cycle and are able to store nutrients for buffering cytosolic levels transient periods of deprivation and for mobilizing stores during revival after prolonged starvation, for example during spore germination. The vacuole is the major storage compartment for amino acids, phosphate, Ca2+, and many metal ions (reviewed in [1, 150].
Basic and neutral amino acids can accumulate to high levels in the yeast vacuole, but there is little vacuolar storage of acidic amino acids [1]. It was known for a number of years that many of these amino acids require the proton gradient for their accumulation. More recently, specific amino acid transporters have been identified and localized to the vacuole. Three basic amino acid transporters (Vba1p, Vba2p, Vba3p) belonging to the major facilitator superfamily (MFS) are responsible for uptake of lysine, arginine, and histidine [151]. The S. cerevisiae genome encodes 7 transporters of the Unc-47/GABA-glycine vesicular amino acid transporter (VGAT) family. One of these (Avt1p) is responsible for uptake of large neutral amino acids (tyrosine, glutamine, aspragine, isoleucine, and leucine) into the vacuole, while three of the other family members (Avt3p, Avt4p, and Avt6p) are implicated in amino acid export from the vacuole [152]. Interestingly, all of the Avt family transporters appear to require the proton gradient across the membrane, even though they transport in opposite directions [152]. Atg22p, originally believed to be part of the autophagy machinery, was more recently shown to act as an amino acid permease, responsible for efflux of leucine and other amino acids derived from autophagic degradation from the vacuole [153]. Yeast cells also contain a functional paralog (Ers1p) of the lysosomal cystine exporter cystinosin; defects in the mammalian cystinosin cause the disease cystinosis, which is characterized by a toxic accumulation of cystine in the lysosome [154]. Bidirectional transport of amino acids across the vacuolar membrane is a critical feature of vacuolar function; influx and efflux must be selectively balanced to allow storage or amino acid recycling depending on cytosolic conditions and needs, but the basis of this balance is not fully understood. Another vacuolar protein that his highly conserved in mammals, Btn1p, appears to help balance cellular arginine levels in yeast [155]. Loss of function of the human Btn1p homolog (CLN3) results in juvenile Batten disease, and understanding the function of Btn1p could help unravel both the basis of this disease and mechanisms that regulate vacuolar/lysosomal amino acid storage.
The vacuole contains very high concentrations of phosphate, which is stored as polyphosphate. The VTC genes (VTC1, VTC2, VTC3, VTC4) are required for vacuolar polyphosphate accumulation [97, 156]. Vacuolar polyphosphate acts as both a phosphate store and as a macromolecular anion that facilitates uptake and retention of positively charged ions and amino acids. Although polyphosphate had long been viewed as a cellular phosphate store, the efficiency of vacuolar polyphosphate as a cytosolic phosphate buffer during transient periods of extracellular phosphate depletion was demonstrated recently [130]. Cells capable of storing polyphosphate were able to maintain sufficient cytosolic phosphate levels to inhibit induction of the Pho5p phosphatase even in the face of a short term extracellular phosphate deprivation. In contrast, mutants unable to accumulate vacuolar polyphosphate induced PHO5 production almost immediately, suggesting that they were immediately vulnerable to a transient decrease in environmental phosphate. This may be viewed as a general paradigm for the buffering function of the vacuole, which applies to storage of other nutrients as well. In this paradigm, nutrients are imported into the vacuole during times of excess and released during times of deficiency, with influx and release usually requiring transporters. This suggests that there must be mechanisms for recognizing cytosolic levels of nutrients and that the buffering mechanisms may compensate for fluctuations arising from apparently diverse cellular systems. The PHO pathway is the signaling pathway that is primarily responsible for regulating phosphate storage and utilization in yeast [157]. However, a recent genomic screen for mutations affecting total polyphosphate levels identified many more mutations, including mutations in many aspects of energy metabolism, that impact the final levels of vacuolar polyphosphate [97]. In some of these mutants, polyphosphate storage may be directly compromised. In others, the vacuole is responding to metabolic perturbation by mobilization or sequestration of phosphate.
The vacuole is also involved in regulation of free ionized calcium levels in yeast and serves as the major intracellular calcium store. Vacuoles contain a H+/Ca2+ antiporter, Vcx1p, which drives Ca2+ uptake at the expense of the proton gradient, and a P-type Ca2+-ATPase (Pmc1p) that operates independently of the H+-gradient [158]. Vcx1p appears to generate high capacity, but low affinity, Ca2+ uptake into the vacuole, while Pmc1p, which is upregulated under conditions of Ca2+ stress, becomes important for vacuolar Ca2+ uptake under stress conditions. Pmc1p is strongly upregulated by calcineurin activation [159]. In the absence of a functional V-ATPase to drive Vcx1p-mediated uptake, calcineurin is constitutively activated, and Pmc1p becomes essential for growth under elevated extracellular Ca2+ concentrations. Calcium can be released from the vacuole via a transient receptor potential (TRP) calcium channel, Yvc1p [160, 161].
Storage and release of other metals follows a similar pattern. The vacuole is the major storage organelle for Zn2+. During times of Zn2+ excess, the Zrc1p and Cot1p zinc transporters are responsible for uptake of Zn2+ into the vacuole; Zn2+ deficiency signals transcription of a vacuolar Zn2+ exporter, Zrt3p [96, 162]. Through the sequential action of this combination of transporters, both total cellular and vacuolar zinc levels can increase by more that ten-fold in the presence of excess extracellular zinc, and this stored zinc can subsequently sustain growth for multiple generations in the absence the extracellular zinc [163].
As it is a redox-active metal as well as a scarce and essential nutrient, control of iron storage is particularly important, and vacuoles again play a central role. Ccc1p is responsible for iron import into the vacuole [164]. Overexpression of the iron importer Ccc1p can suppress oxidative stress arising from excess cytosolic Fe3+ in certain mutants by allowing sequestration of excess Fe into the vacuole [164, 165]. Vacuolar iron is mobilized during times of limited supply by the Fet5p/Fth1p complex and Smf3p metal ion transporter [166]. Many of these transporters are coordinately upregulated in response to iron deprivation [167].
6.3. Detoxification functions of the vacuole
In addition to its storage functions, the vacuole also serves an important detoxification function, through which multiple toxic molecules are sequestered away from the cytosol and other organelles. Some of these “toxins” (like the redox active heavy metals) are nutrients or metabolites that have essential functions at physiological concentrations but become toxic in excess. Two ATP binding cassette (ABC) transporters, Ycf1p and Bpt1p are present on the vacuolar membrane and transport several substrates as glutathione conjugates [168–170]. These two pumps have similar mechanisms and overlapping specificities, but appear to be regulated differently. Among their substrates are toxic metals arsenite and cadmium and accumulated side-products of adenine metabolism present in the ade2 mutant [170, 171].
The importance of vacuolar function in detoxification was emphasized in a recent chemical genetic screen which identified a number of mutants in vacuolar function, including mutants lacking V-ATPase subunit genes, as a prominent class of multi-drug sensitive mutants [172]. One interpretation of this result is that many of the drugs tested can be sequestered in the vacuole, but an alternative explanation is that the stress-response functions of the vacuole are required for tolerance of multiple drugs.
7. Stress-related functions of the yeast vacuole
While the vacuole is mainly known as the endpoint for macromolecular degradation, it also has a central role in responding to various stresses such as nutrient deprivation, osmotic and ionic stress, and calcium stress (Fig. 5).
Figure 5. Stress responses.
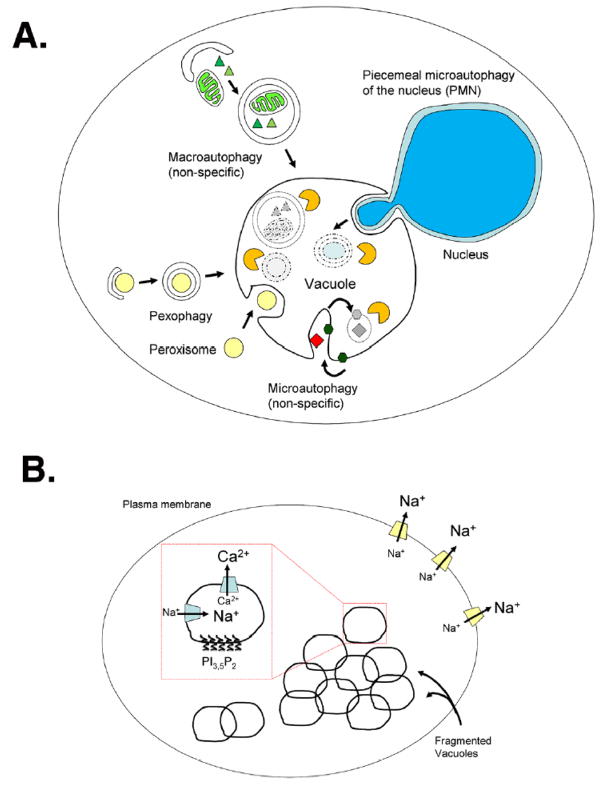
Panel A: Upon nutrient deprivation, multiple autophagic pathways deliver specific and non-specific cargo to the vacuole. Macroautophagy is the non-specific degradation of cytosolic proteins and organelles. Autophagic membranes originate from pre-autosomal structures (PAS) that enclose cytosolic cargo, forming autophagosomes, which are then delivered to the vacuole. In microautophagy, vacuolar membranes invaginate and directly engulf cargo for degradation. Piecemeal microautophagy of the nucleus (PMN) is a type of autophagy that specifically degrades nuclear compartments, while pexophagy degrades peroxisomes via two mechanisms similar to macroautophagy and microautophagy.
Panel B: Upon salt stress, vacuoles fragment into multiple tiny vesicles. This requires increased production of PI3,5P2 by the Fab1p lipid kinase. Calcium is released from vacuolar stores by Yvc1p, activating calcineurin and the Crz1p transcription factor. This leads to the increased production of the Ena1p Na+/Li+ pump at the plasma membrane and the Pho89p Na+/Pi+ symporter at the vacuole.
7.1 Vacuoles in response to nutrient deprivation
Yeast cells are constantly subjected to fluctuations in extracellular nutrients. As such, they have evolved various mechanisms to adapt to different nutritional conditions. Autophagy is the vesicular sequestration of cytosolic cargo for degradation at the vacuole [173]. It is both a housekeeping and a stress-responsive process. As a stress response, autophagy is activated in low-nutrient conditions to provide supplementary reserves for the starving cell. Autophagy can also involve the specific degradation of proteins and organelles [173].
There are different types of autophagy, with macroautophagy considered as the main degradative form (reviewed in [146, 149, 173]). In macroautophagy, a double membrane vesicle called the autophagosome encloses cytosolic cargo. Autophagosome formation takes place at the pre-autophagosomal structure (PAS), which is adjacent to the vacuole. Subsequent fusion of autophagosomes with the vacuole leads to the release of cargo enclosed by the inner membrane into the vacuolar lumen. Both the inner membrane and autophagosomal cargo are digested by vacuolar hydrolases into component macromolecules that are then released back into the cytosol through vacuolar permeases. Microautophagy entails direct engulfment of cytosolic materials by the vacuole via invaginations of its limiting membrane [174], and is implicated in the degradation of specific organelles such as the nucleus [175].
In yeast, autophagy is induced by the absence of high-quality nitrogen and carbon sources [176, 177]. In a nutrient-rich environment, autophagy is inhibited by the Tor kinase, a phosphatidylinositol-3 (PtdIns-[3]) kinase homolog. The TORC1 complex regulates cell proliferation and the transition between growth and quiescence [176, 178]. Upon starvation, Tor becomes inactivated, and autophagy is enhanced. While the nitrogen-sensing mechanisms upstream of Tor have not been elucidated, more is known about Tor effectors. [176, 177]. Atg13p is a substrate of Tor that binds to different proteins depending on its phosphorylation state. During starvation, dephosphorylated Atg13p has a higher affinity for Atg1p and Atg17p, forming a complex which is thought to mediate autophagy induction [177].
How autophagic vesicles are precisely formed still remains a mystery. It is known that the formation of autophagic vesicles requires two ubiquitin-like conjugation systems: Atg8-phosphatidylethanolamine and Atg12p-Atg5p. (reviewed by [177]) These complexes localize at the pre-autophagosomal structure (PAS), and are thought to have a role in vesicle formation [177, 179]. A complex containing PtdIns[3]P, Atg14p, Vps30p, Vps15p, and Vps34p (the PtdIns-[3] kinase) has also been found to recruit various autophagy-related proteins to the PAS [177, 180, 181]. However, the double membrane autophagic vesicles are not derived from existing organelle; they appear to be generated from new membrane [177]. It is known that Atg proteins are necessary for vesicle formation, and that most of these proteins localize at the PAS, but how these proteins are involved in the nucleation of the initial autophagic membrane is unclear [177].
Fusion of autophagic vesicles with the vacuole requires Ccz1p and Mon1p, proteins that are also implicated in homotypic vacuole fusion [177, 182, 183]. The outer membrane of the autophagosome fuses with the vacuole while the inner vesicle (called the autophagic body) is exposed to the lumen and its proteases. Dissolution of the autophagic body requires specific proteases (Pep4p and Prb1p), an acidified vacuole, and Cvt17p (a putative lipase) [147, 177, 184–186]. The cytoplasmic cargo is exposed to the hydrolases of the lumen, resulting in their degradation.
The massive influx of material into the vacuole during autophagy exerts increased pressure on the vacuole’s degradative capacities. To cope with this, the hydrolytic capacity of the vacuole is also increased under starvation conditions [173, 187]. Vacuolar hydrolases such as API are produced at a much higher rate compared to total protein synthesis during nitrogen deprivation [188]. The influx of material into the vacuole can also conceivably lead to the accumulation of both membrane and luminal material [189]. The vacuole has mechanisms for recycling membrane from the vacuole [1, 189], which may be upregulated after autophagy. In line with this, microautophagy, in which vesicles invaginate from the vacuole itself for degradation in the vacuolar lumen, has been observed to occur after macroautophagy. This coupling of microautophagy to macroautophagy may be such a mechanism for vacuolar membrane recycling [189]. The EGO complex, in conjunction with TOR, counterbalances the massive macroautophagy-mediated membrane influx at the vacuolar membrane by positively regulating microautophagy [190].
7.2 Vacuoles in response to ionic and osmotic stress
Yeast cells have two distinct responses to osmotic shock in the presence of high sorbitol or salt media. The long-term response to osmotic challenge is mediated by the HOG (high osmolarity glycerol) pathway, a transcriptional response that leads to increased amounts of a cellular osmoprotectant, glycerol [191, 192]. In contrast, the immediate response involves the formation of small, fragmented vacuoles and a release of calcium from the vacuole.
The first findings that suggested a link between vacuolar function and osmoregulation came from studies of mutants defective in vacuolar protein sorting. Some of these mutants that lacked structurally intact vacuoles were also osmosensitive, [1, 56] reflecting a defect in osmoregulatory capabilities. More concrete evidence came from a study involving a mutant of SSV1, a gene required for vacuole biogenesis and protein sorting. Within 10 seconds of treatment, 1.5 M NaCl proved lethal to ssv1–2 mutant cells, suggesting the presence of a mechanism that facilitates the fast adaptation of cells to osmotic or ionic stress [193]. Stress from growth in 1.5 M NaCl has an charge-dependent ionic component as well as a charge-independent osmotic component. The vacuole is capable of handling charge-dependent stress via numerous ion-specific transporters and charge-independent osmotic shock via vacuolar shrinkage.
As the storage vessel for a wide variety of ions, the vacuole is the main organelle responsible for intracellular ion homeostasis and response to ionic shock. Vacuolar transporters mediate ion homeostasis by regulating ion transport between the vacuole and the cytosol, along with plasma membrane ion transporters that regulate ion influx/efflux between the cytosol and the extracellular environment. The activity of many vacuolar transporters is dependent on the pH gradient across vacuolar membranes generated by the vacuolar H+-ATPase. For instance, the late endosomal transporter Nhx1 confers osmotolerance following acute hypertonic shock [194].
As mentioned above, the vacuole is the major storage site for calcium in yeast. Upon salt shock, the vacuole releases calcium via Yvc1p, resulting in a transient increase in the cytosolic concentration of calcium [160]. Interestingly, Yvc1p is mechanosensitive – it directly responds to mechanical strain. A model by which Yvc1p senses the mechanical strain generated by osmotic shock has been proposed: osmosis draws water from the cytoplasm and the vacuole, and the osmotic pressure across vacuole membranes forces Yvc1p to assume an open conformation [161]. The exact role of transient calcium release from the vacuole in response to osmotic shock remains unclear. Calcium is a major signaling molecule and its release is likely to activate cellular responses that mitigate osmotic shock. Calcium signaling in yeast is facilitated by the Ca2+/calmodulin dependent phosphatase, calcineurin, which activates the Crz1p transcription factor [195]. Crz1p activation leads to increased expression of a battery of 160 or more genes implicated in different processes [196]. These include cell wall synthesis, vesicle transport and lipid/sterol synthesis, showing that Crz1p activates a repertoire of genes that promote cell surface remodeling. Notably, ion transporters such as Ena1p (a plasma membrane Na+/Li+ pump) and Pho89p (a vacuolar Na+/Pi symporter) are also upregulated. Cell-wall remodeling and the increased production of ion transporters may be possible mechanisms for dealing with osmotic stress. Indeed, calcineurin is essential for salt tolerance in yeast, and activated calcineurin confers high tolerance to ion stress by promoting ENA1 expression. This suggests that adaptation to high salt requires the Ca2+/calmodulin/calcineurin signaling pathway [197, 198].
The acute vacuolar response to osmotic shock involves a rapid and transient 20-fold increase in PI3,5P2 levels, and requires the activation of the Fab1 lipid kinase by Vac7p [17] and the Vac14p-Fig4p complex [19, 199]. Interestingly, PtdIns[3,5]P2 levels are only readily detectable after subjecting cells to an even though an inability to produce PI3,5P2 leads to constitutive defects in vacuolar morphology. This rise in cellular PI3,5P2 is a transient response that is necessary for the decrease in vacuolar volume in response to osmotic shock. Normal vacuole volume and morphology are restored as levels of PI3,5P2 drop back to basal levels, and both the rise and fall of PI3,5P2 levels are dependent on the PI3,5P2-phosphatase, Fig4p [200]. The rise in PtdIns[3,5]P2 may also regulate channels that extrude ions, osmolytes and water from the vacuole [18, 201]. These results suggest that presence of size and for changes in vacuolar morphology in response to stress.
Several proteins acting downstream of PI3,5P2 have been identified. Among these, only ATG18 deletion mutants have high levels of PI3,5P2 and grossly enlarged vacuoles. Furthermore, subjecting atg18Δ cells to hypertonic shock does not result in a change in vacuolar size even though PI3,5P2 levels rise to 60 times that of wildtype [22, 39]. This suggests that Atg18p is the PtdIns[3,5]P2 effector responsible for vacuolar fission in response to osmotic stress.
Vacuolar fragmentation as a response to osmotic shock is well-documented. From an osmotic context, vacuolar fragmentation may be a result of the release of water from the vacuole to maintain proper osmotic pressure in the cytosol. Interestingly, chronically high levels of calcium in the cytosol also induce vacuolar fragmentation [202]. Vacuolar fragmentation may function as a normal response to calcium stress that increases the surface/volume ratio of the vacuole, to enable maximal sequestration of calcium at the vacuole [202]. This effect is common to both calcium and sodium stress (see above). From an ionic context, vacuolar fragmentation may be a general mechanism for increasing the efficiency of ion uptake by vacuolar transporters.
8. Conclusion
As an acidic, digestive organelle, the yeast vacuole is analogous to the lysosome; both compartments are endpoints for degradation of proteins delivered via endocytosis and autophagy. Both compartments also recycle hydrolytic products to replenish cytosolic stores. Unlike lysosomes, the vacuole actively sequesters nutrients such as amino acids, metal ions, and metabolic biproducts. In this light, the yeast vacuole has storage and detoxification roles that diverge from lysosome function.
While certain processes, such as autophagy, are inexorably tied to the vacuole’s digestive capacities, other stress mechanisms such as osmotic recovery and the calcium response rely on the vacuole’s ability to store and transport water and different ions. Under osmotic and ionic stress, vacuoles must be able to fuse and fragment at will. Still other mechanisms such as vacuolar inheritance involve the transport of vacuolar compartments across the cell in response to cell cycle-derived cues. In all these scenarios, vacuoles must be capable of sensing fluctuations in both extracellular and intracellular stimuli, and react accordingly. The temporal nature of these processes suggest that the vacuole is more than just a proteolytic reservoir; it is indeed a highly responsive structure that adjusts to different cellular challenges.
Acknowledgments
Work in the Kane lab is supported by NIH grant GM50322.
Footnotes
Yeast proteins are indicated by the gene name followed by “p” for protein, for example, Vac7p.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990;54:266–92. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 4.Sarry JE, Chen S, Collum RP, Liang S, Peng M, Lang A, Naumann B, Dzierszinski F, Yuan CX, Hippler M, Rea PA. Analysis of the vacuolar luminal proteome of Saccharomyces cerevisiae. Febs J. 2007;274:4287–305. doi: 10.1111/j.1742-4658.2007.05959.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt UG, Endler A, Schelbert S, Brunner A, Schnell M, Neuhaus HE, Marty-Mazars D, Marty F, Baginsky S, Martinoia E. Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds. Plant Physiol. 2007;145:216–29. doi: 10.1104/pp.107.096917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, Bourguignon J. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol Cell Proteomics. 2007;6:394–412. doi: 10.1074/mcp.M600250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–8. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneiter R. Brave little yeast, please guide us to thebes: sphingolipid function in S. cerevisiae. Bioessays. 1999;21:1004–10. doi: 10.1002/(SICI)1521-1878(199912)22:1<1004::AID-BIES4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991;173:2026–34. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagnat M, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A. 2000;97:3254–9. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauwers E, Andre B. Association of yeast transporters with detergent-resistant membranes correlates with their cell-surface location. Traffic. 2006;7:1045–59. doi: 10.1111/j.1600-0854.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 12.Kato M, Wickner W. Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion. Embo J. 2001;20:4035–40. doi: 10.1093/emboj/20.15.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167:1087–98. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nebauer R, Rosenberger S, Daum G. Phosphatidylethanolamine, a limiting factor of autophagy in yeast strains bearing a defect in the carboxypeptidase Y pathway of vacuolar targeting. J Biol Chem. 2007;282:16736–43. doi: 10.1074/jbc.M611345200. [DOI] [PubMed] [Google Scholar]
- 15.Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–92. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- 16.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonangelino CJ, Catlett NL, Weisman LS. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17:6847–58. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duex JE, Tang F, Weisman LS. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol. 2006;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudge SA, Anderson DM, Emr SD. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol Biol Cell. 2004;15:24–36. doi: 10.1091/mbc.E03-05-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dove SK, McEwen RK, Mayes A, Hughes DC, Beggs JD, Michell RH. Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr Biol. 2002;12:885–93. doi: 10.1016/s0960-9822(02)00891-6. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995;6:525–39. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dove SK, Piper RC, McEwen RK, Yu JW, King MC, Hughes DC, Thuring J, Holmes AB, Cooke FT, Michell RH, Parker PJ, Lemmon MA. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. Embo J. 2004;23:1922–33. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efe JA, Botelho RJ, Emr SD. The Fab1 phosphatidylinositol kinase pathway in the regulation of vacuole morphology. Curr Opin Cell Biol. 2005;17:402–8. doi: 10.1016/j.ceb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollapour M, Phelan JP, Millson SH, Piper PW, Cooke FT. Weak acid and alkali stress regulate phosphatidylinositol bisphosphate synthesis in Saccharomyces cerevisiae. Biochem J. 2006;395:73–80. doi: 10.1042/BJ20051765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickner W. Yeast vacuoles and membrane fusion pathways. Embo J. 2002;21:1241–7. doi: 10.1093/emboj/21.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada Y, Ohsumi Y, Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J Biol Chem. 1992;267:18665–70. [PubMed] [Google Scholar]
- 28.Ostrowicz CW, Meiringer CT, Ungermann C. Yeast vacuole fusion: a model system for eukaryotic endomembrane dynamics. Autophagy. 2008;4:5–19. doi: 10.4161/auto.5054. [DOI] [PubMed] [Google Scholar]
- 29.Starai VJ, Hickey CM, Wickner W. HOPS Proofreads the trans-SNARE Complex for Yeast Vacuole Fusion. Mol Biol Cell. 2008;19:2500–8. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci U S A. 2007;104:8755–60. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–72. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 32.Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–8. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- 33.Merz AJ, Wickner WT. Trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol. 2004;164:195–206. doi: 10.1083/jcb.200310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starai VJ, Thorngren N, Fratti RA, Wickner W. Ion regulation of homotypic vacuole fusion in Saccharomyces cerevisiae. J Biol Chem. 2005;280:16754–62. doi: 10.1074/jbc.M500421200. [DOI] [PubMed] [Google Scholar]
- 35.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–80. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 36.Bayer MJ, Reese C, Buhler S, Peters C, Mayer A. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J Cell Biol. 2003;162:211–22. doi: 10.1083/jcb.200212004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peplowska K, Ungermann C. Expanding dynamin: from fission to fusion. Nat Cell Biol. 2005;7:103–4. doi: 10.1038/ncb0205-103. [DOI] [PubMed] [Google Scholar]
- 38.Weisman LS. Organelles on the move: insights from yeast vacuole inheritance. Nat Rev Mol Cell Biol. 2006;7:243–52. doi: 10.1038/nrm1892. [DOI] [PubMed] [Google Scholar]
- 39.Efe JA, Botelho RJ, Emr SD. Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate. Mol Biol Cell. 2007;18:4232–44. doi: 10.1091/mbc.E07-04-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baars TL, Petri S, Peters C, Mayer A. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol Biol Cell. 2007;18:3873–82. doi: 10.1091/mbc.E07-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–86. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters C, Baars TL, Buhler S, Mayer A. Mutual control of membrane fission and fusion proteins. Cell. 2004;119:667–78. doi: 10.1016/j.cell.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Weisman LS, Emr SD, Wickner WT. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad Sci U S A. 1990;87:1076–80. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond CK, O’Hara PJ, Eichinger G, Rothman JH, Stevens TH. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J Cell Biol. 1990;111:877–92. doi: 10.1083/jcb.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisman LS. Yeast vacuole inheritance and dynamics. Annu Rev Genet. 2003;37:435–60. doi: 10.1146/annurev.genet.37.050203.103207. [DOI] [PubMed] [Google Scholar]
- 46.Roeder AD, Shaw JM. Vacuole partitioning during meiotic division in yeast. Genetics. 1996;144:445–58. doi: 10.1093/genetics/144.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suda Y, Nakanishi H, Mathieson EM, Neiman AM. Alternative modes of organellar segregation during sporulation in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:2009–17. doi: 10.1128/EC.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw JM, Wickner WT. vac2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. Embo J. 1991;10:1741–8. doi: 10.1002/j.1460-2075.1991.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maniotis A, Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991;67:495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- 50.Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- 51.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vida TA, Huyer G, Emr SD. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J Cell Biol. 1993;121:1245–56. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cereghino JL, Marcusson EG, Emr SD. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol Biol Cell. 1995;6:1089–102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowers K, Stevens TH. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2005;1744:438–54. doi: 10.1016/j.bbamcr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Rothman JH, Howald I, Stevens TH. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. Embo J. 1989;8:2057–65. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banta LM, Robinson JS, Klionsky DJ, Emr SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988;107:1369–83. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–98. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odorizzi G, Babst M, Emr SD. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem Sci. 2000;25:229–35. doi: 10.1016/s0968-0004(00)01543-7. [DOI] [PubMed] [Google Scholar]
- 59.Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–86. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 60.Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–17. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. Embo J. 1997;16:2769–82. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–74. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–45. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J Biol Chem. 1996;271:17621–4. doi: 10.1074/jbc.271.30.17621. [DOI] [PubMed] [Google Scholar]
- 65.D’Hondt K, Heese-Peck A, Riezman H. Protein and lipid requirements for endocytosis. Annu Rev Genet. 2000;34:255–295. doi: 10.1146/annurev.genet.34.1.255. [DOI] [PubMed] [Google Scholar]
- 66.Dulic V, Riezman H. Characterization of the END1 gene required for vacuole biogenesis and gluconeogenic growth of budding yeast. Embo J. 1989;8:1349–59. doi: 10.1002/j.1460-2075.1989.tb03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bryant NJ, Piper RC, Weisman LS, Stevens TH. Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol. 1998;142:651–63. doi: 10.1083/jcb.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–32. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 69.Andrews NW. Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol. 2002;158:389–94. doi: 10.1083/jcb.200205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 71.Brett CL, Tukaye DN, Mukherjee S, Rao R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005;16:1396–405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padilla-Lopez S, Pearce DA. Saccharomyces cerevisiae lacking Btn1p modulate vacuolar ATPase activity to regulate pH imbalance in the vacuole. J Biol Chem. 2006;281:10273–80. doi: 10.1074/jbc.M510625200. [DOI] [PubMed] [Google Scholar]
- 73.Plant PJ, Manolson MF, Grinstein S, Demaurex N. Alternative mechanisms of vacuolar acidification in H(+)-ATPase-deficient yeast. J Biol Chem. 1999;274:37270–37279. doi: 10.1074/jbc.274.52.37270. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Munoz GA, Kane PM. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem. 2008 doi: 10.1074/jbc.M710470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–29. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 76.Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev. 2006;70:177–91. doi: 10.1128/MMBR.70.1.177-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernasconi P, Rausch T, Struve I, Morgan L, Taiz L. An mRNA from human brain encodes an isoform of the B subunit of the vacuolar H(+)-ATPase. J Biol Chem. 1990;265:17428–31. [PubMed] [Google Scholar]
- 78.Lu X, Yu H, Liu SH, Brodsky FM, Peterlin BM. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–56. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 79.Manolson MF, Wu B, Proteau D, Taillon BE, Roberts BT, Hoyt MA, Jones EW. STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H(+)-ATPase subunit Vph1p. J Biol Chem. 1994;269:14064–14074. [PubMed] [Google Scholar]
- 80.Kawasaki-Nishi S, Bowers K, Nishi T, Forgac M, Stevens TH. The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J Biol Chem. 2001;276:47411–20. doi: 10.1074/jbc.M108310200. [DOI] [PubMed] [Google Scholar]
- 81.Kawasaki-Nishi S, Nishi T, Forgac M. Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J Biol Chem. 2001;276:17941–8. doi: 10.1074/jbc.M010790200. [DOI] [PubMed] [Google Scholar]
- 82.Karet FE. Physiological and metabolic implications of V-ATPase isoforms in the kidney. J Bioenerg Biomembr. 2005;37:425–9. doi: 10.1007/s10863-005-9484-x. [DOI] [PubMed] [Google Scholar]
- 83.Jouret F, Auzanneau C, Debai H, Wada GH, Pretto C, Marbai E, Karet FE, Courtoy PJ, Devuyst O. Ubiquitous and Kidney-Specific Subunits of Vacuolar H+-ATPase Are Differentially Expressed during Nephrogenesis. J Am Soc Nephrol. 2005 doi: 10.1681/ASN.2004110935. [DOI] [PubMed] [Google Scholar]
- 84.Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod. 2006;74:185–94. doi: 10.1095/biolreprod.105.043752. [DOI] [PubMed] [Google Scholar]
- 85.Da Silva N, Shum WW, El-Annan J, Paunescu TG, McKee M, Smith PJ, Brown D, Breton S. Relocalization of the V-ATPase B2 subunit to the apical membrane of epididymal clear cells of mice deficient in the B1 subunit. Am J Physiol Cell Physiol. 2007;293:C199–210. doi: 10.1152/ajpcell.00596.2006. [DOI] [PubMed] [Google Scholar]
- 86.Paunescu TG, Russo LM, Da Silva N, Kovacikova J, Mohebbi N, Van Hoek AN, McKee M, Wagner CA, Breton S, Brown D. Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Am J Physiol Renal Physiol. 2007;293:F1915–26. doi: 10.1152/ajprenal.00160.2007. [DOI] [PubMed] [Google Scholar]
- 87.Oka T, Murata Y, Namba M, Yoshimizu T, Toyomura T, Yamamoto A, Sun-Wada GH, Hamasaki N, Wada Y, Futai M. a4, a unique kidney-specific isoform of mouse vacuolar H+-ATPase subunit a. J Biol Chem. 2001;276:40050–4. doi: 10.1074/jbc.M106488200. [DOI] [PubMed] [Google Scholar]
- 88.Toyomura T, Oka T, Yamaguchi C, Wada Y, Futai M. Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem. 2000;275:8760–5. doi: 10.1074/jbc.275.12.8760. [DOI] [PubMed] [Google Scholar]
- 89.Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023–30. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 90.Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet. 2000;9:2059–63. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- 91.Borthwick KJ, Karet FE. Inherited disorders of the H+-ATPase. Curr Opin Nephrol Hypertens. 2002;11:563–8. doi: 10.1097/00041552-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 92.Sun-Wada GH, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci. 2006;119:4531–40. doi: 10.1242/jcs.03234. [DOI] [PubMed] [Google Scholar]
- 93.Sun-Wada G, Murata Y, Yamamoto A, Kanazawa H, Wada Y, Futai M. Acidic endomembrane organelles are required for mouse postimplantation development. Dev Biol. 2000;228:315–25. doi: 10.1006/dbio.2000.9963. [DOI] [PubMed] [Google Scholar]
- 94.Davies SA, Goodwin SF, Kelly DC, Wang Z, Sozen MA, Kaiser K, Dow JA. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem. 1996;271:30677–84. doi: 10.1074/jbc.271.48.30677. [DOI] [PubMed] [Google Scholar]
- 95.Forster C, Kane PM. Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J Biol Chem. 2000;275:38245–53. doi: 10.1074/jbc.M006650200. [DOI] [PubMed] [Google Scholar]
- 96.MacDiarmid CW, Milanick MA, Eide DJ. Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J Biol Chem. 2002;277:39187–94. doi: 10.1074/jbc.M205052200. [DOI] [PubMed] [Google Scholar]
- 97.Freimoser FM, Hurlimann HC, Jakob CA, Werner TP, Amrhein N. Systematic screening of polyphosphate (poly P) levels in yeast mutant cells reveals strong interdependence with primary metabolism. Genome Biol. 2006;7:R109. doi: 10.1186/gb-2006-7-11-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eide DJ, Bridgham JT, Zhao Z, Mattoon JR. The vacuolar H(+)-ATPase of Saccharomyces cerevisiae is required for efficient copper detoxification, mitochondrial function, and iron metabolism. Mol Gen Genet. 1993;241:447–56. doi: 10.1007/BF00284699. [DOI] [PubMed] [Google Scholar]
- 99.Davis-Kaplan SR, Ward DM, Shiflett SL, Kaplan J. Genome-wide analysis of iron-dependent growth reveals a novel yeast gene required for vacuolar acidification. J Biol Chem. 2004;279:4322–9. doi: 10.1074/jbc.M310680200. Epub 2003 Nov 21. [DOI] [PubMed] [Google Scholar]
- 100.Yadav J, Muend S, Zhang Y, Rao R. A phenomics approach in yeast links proton and calcium pump function in the Golgi. Mol Biol Cell. 2007;18:1480–9. doi: 10.1091/mbc.E06-11-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis-Kaplan SR, Askwith CC, Bengtzen AC, Radisky D, Kaplan J. Chloride is an allosteric effector of copper assembly for the yeast multicopper oxidase Fet3p: an unexpected role for intracellular chloride channels. Proc Natl Acad Sci U S A. 1998;95:13641–5. doi: 10.1073/pnas.95.23.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nelson H, Nelson N. Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci U S A. 1990;87:3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Serrano R, Bernal D, Simon E, Arino J. Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J Biol Chem. 2004;279:19698–704. doi: 10.1074/jbc.M313746200. Epub 2004 Mar 01. [DOI] [PubMed] [Google Scholar]
- 104.Yamashiro CT, Kane PM, Wolczyk DF, Preston RA, Stevens TH. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol. 1990;10:3737–3749. doi: 10.1128/mcb.10.7.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klionsky DJ, Nelson H, Nelson N. Compartment acidification is required for efficient sorting of proteins to the vacuole in Saccharomyces cerevisiae. J Biol Chem. 1992;267:3416–22. [PubMed] [Google Scholar]
- 106.Yaver DS, Nelson H, Nelson N, Klionsky DJ. Vacuolar ATPase mutants accumulate precursor proteins in a pre-vacuolar compartment. J Biol Chem. 1993;268:10564–72. [PubMed] [Google Scholar]
- 107.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–6. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Umata T, Moriyama Y, Futai M, Mekada E. The cytotoxic action of diphtheria toxin and its degradation in intact Vero cells are inhibited by bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase. J Biol Chem. 1990;265:21940–21945. [PubMed] [Google Scholar]
- 109.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–12. [PubMed] [Google Scholar]
- 110.Nishihara T, Akifusa S, Koseki T, Kato S, Muro M, Hanada N. Specific inhibitors of vacuolar type H(+)-ATPases induce apoptotic cell death. Biochem Biophys Res Commun. 1995;212:255–62. doi: 10.1006/bbrc.1995.1964. [DOI] [PubMed] [Google Scholar]
- 111.Okahashi N, Nakamura I, Jimi E, Koide M, Suda T, Nishihara T. Specific inhibitors of vacuolar H(+)-ATPase trigger apoptotic cell death of osteoclasts. J Bone Miner Res. 1997;12:1116–1123. doi: 10.1359/jbmr.1997.12.7.1116. [DOI] [PubMed] [Google Scholar]
- 112.Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981;90:656–64. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90:665–9. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kane PM. Regulation of V-ATPases by reversible disassembly. FEBS Lett. 2000;469:137–141. doi: 10.1016/s0014-5793(00)01265-5. [DOI] [PubMed] [Google Scholar]
- 115.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–3. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 116.Parra KJ, Kane PM. Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol Cell Biol. 1998;18:7064–7074. doi: 10.1128/mcb.18.12.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 118.Lu M, Holliday LS, Zhang L, Dunn WA, Jr, Gluck SL. Interaction between aldolase and vacuolar H+-ATPase: evidence for direct coupling of glycolysis to the ATP-hydrolyzing proton pump. J Biol Chem. 2001;276:30407–13. doi: 10.1074/jbc.M008768200. [DOI] [PubMed] [Google Scholar]
- 119.Lu M, Sautin YY, Holliday LS, Gluck SL. The glycolytic enzyme aldolase mediates assembly, expression and activity of V-ATPase. J Biol Chem. 2003:M303871200. doi: 10.1074/jbc.M303871200. [DOI] [PubMed] [Google Scholar]
- 120.Su Y, Zhou A, Al-Lamki RS, Karet FE. The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans. J Biol Chem. 2003;278:20013–8. doi: 10.1074/jbc.M210077200. [DOI] [PubMed] [Google Scholar]
- 121.Lu M, Ammar D, Ives H, Albrecht F, Gluck SL. Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J Biol Chem. 2007;282:24495–503. doi: 10.1074/jbc.M702598200. [DOI] [PubMed] [Google Scholar]
- 122.Smardon AM, Tarsio M, Kane PM. The RAVE complex is essential for stable assembly of the yeast V-ATPase. J Biol Chem. 2002;13:13. doi: 10.1074/jbc.M200682200. [DOI] [PubMed] [Google Scholar]
- 123.Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H(+)-ATPase by protein kinase A. J Biol Chem. 2007;282:33735–42. doi: 10.1074/jbc.M703368200. [DOI] [PubMed] [Google Scholar]
- 124.Shao E, Nishi T, Kawasaki-Nishi S, Forgac M. Mutational analysis of the non-homologous region of subunit A of the yeast V-ATPase. J Biol Chem. 2003;278:12985–91. doi: 10.1074/jbc.M212096200. [DOI] [PubMed] [Google Scholar]
- 125.Paroutis P, Touret N, Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 2004;19:207–15. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- 126.Arai H, Pink S, Forgac M. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry. 1989;28:3075–3082. doi: 10.1021/bi00433a051. [DOI] [PubMed] [Google Scholar]
- 127.Ali R, Brett CL, Mukherjee S, Rao R. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J Biol Chem. 2004;279:4498–506. doi: 10.1074/jbc.M307446200. [DOI] [PubMed] [Google Scholar]
- 128.Qi J, Forgac M. Cellular environment is important in controlling V-ATPase dissociation and its dependence on activity. J Biol Chem. 2007;282:24743–51. doi: 10.1074/jbc.M700663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beauvoit B, Rigoulet M, Raffard G, Canioni P, Guerin B. Differential sensitivity of the cellular compartments of Saccharomyces cerevisiae to protonophoric uncoupler under fermentative and respiratory energy supply. Biochemistry. 1991;30:11212–20. doi: 10.1021/bi00111a004. [DOI] [PubMed] [Google Scholar]
- 130.Thomas MR, O’Shea EK. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc Natl Acad Sci U S A. 2005;102:9565–70. doi: 10.1073/pnas.0501122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brett CL, Donowitz M, Rao R. Does the proteome encode organellar pH? FEBS Lett. 2006;580:717–9. doi: 10.1016/j.febslet.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 132.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75:3327–31. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knop M, Schiffer HH, Rupp S, Wolf DH. Vacuolar/lysosomal proteolysis: proteases, substrates, mechanisms. Curr Opin Cell Biol. 1993;5:990–6. doi: 10.1016/0955-0674(93)90082-2. [DOI] [PubMed] [Google Scholar]
- 134.Jones EW. Three proteolytic systems in the yeast saccharomyces cerevisiae. J Biol Chem. 1991;266:7963–6. [PubMed] [Google Scholar]
- 135.Van Den Hazel HB, Kielland-Brandt MC, Winther JR. Review: biosynthesis and function of yeast vacuolar proteases. Yeast. 1996;12:1–16. doi: 10.1002/(sici)1097-0061(199601)12:1<1::aid-yea902>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 136.Zubenko GS, Park FJ, Jones EW. Mutations in PEP4 locus of Saccharomyces cerevisiae block final step in maturation of two vacuolar hydrolases. Proc Natl Acad Sci U S A. 1983;80:510–4. doi: 10.1073/pnas.80.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Davis NG, Horecka JL, Sprague GF., Jr Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–87. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 139.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 140.Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 141.Galan JM, Moreau V, Andre B, Volland C, Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J Biol Chem. 1996;271:10946–52. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- 142.Seron K, Blondel MO, Haguenauer-Tsapis R, Volland C. Uracil-induced down-regulation of the yeast uracil permease. J Bacteriol. 1999;181:1793–800. doi: 10.1128/jb.181.6.1793-1800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Risinger AL, Cain NE, Chen EJ, Kaiser CA. Activity-dependent reversible inactivation of the general amino acid permease. Mol Biol Cell. 2006;17:4411–9. doi: 10.1091/mbc.E06-06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Risinger AL, Kaiser CA. Different Ubiquitin Signals Act at the Golgi and Plasma Membrane to Direct GAP1 Trafficking. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-06-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Helliwell SB, Losko S, Kaiser CA. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J Cell Biol. 2001;153:649–62. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 147.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–11. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J Biol Chem. 2005;280:31582–6. doi: 10.1074/jbc.M506736200. [DOI] [PubMed] [Google Scholar]
- 149.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 150.Van Ho A, Ward DM, Kaplan J. Transition metal transport in yeast. Annu Rev Microbiol. 2002;56:237–61. doi: 10.1146/annurev.micro.56.012302.160847. [DOI] [PubMed] [Google Scholar]
- 151.Shimazu M, Sekito T, Akiyama K, Ohsumi Y, Kakinuma Y. A family of basic amino acid transporters of the vacuolar membrane from Saccharomyces cerevisiae. J Biol Chem. 2005;280:4851–7. doi: 10.1074/jbc.M412617200. [DOI] [PubMed] [Google Scholar]
- 152.Russnak R, Konczal D, McIntire SL. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J Biol Chem. 2001;276:23849–57. doi: 10.1074/jbc.M008028200. [DOI] [PubMed] [Google Scholar]
- 153.Yang Z, Huang J, Geng J, Nair U, Klionsky DJ. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol Biol Cell. 2006;17:5094–104. doi: 10.1091/mbc.E06-06-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao XD, Wang J, Keppler-Ross S, Dean N. ERS1 encodes a functional homologue of the human lysosomal cystine transporter. Febs J. 2005;272:2497–511. doi: 10.1111/j.1742-4658.2005.04670.x. [DOI] [PubMed] [Google Scholar]
- 155.Vitiello SP, Wolfe DM, Pearce DA. Absence of Btn1p in the yeast model for juvenile Batten disease may cause arginine to become toxic to yeast cells. Hum Mol Genet. 2007;16:1007–16. doi: 10.1093/hmg/ddm046. [DOI] [PubMed] [Google Scholar]
- 156.Ogawa N, DeRisi J, Brown PO. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell. 2000;11:4309–21. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carroll AS, O’Shea EK. Pho85 and signaling environmental conditions. Trends Biochem Sci. 2002;27:87–93. doi: 10.1016/s0968-0004(01)02040-0. [DOI] [PubMed] [Google Scholar]
- 158.Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–6. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- 159.Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou XL, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc Natl Acad Sci U S A. 2003;100:7105–10. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.MacDiarmid CW, Gaither LA, Eide D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. Embo J. 2000;19:2845–55. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Simm C, Lahner B, Salt D, LeFurgey A, Ingram P, Yandell B, Eide DJ. Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryot Cell. 2007;6:1166–77. doi: 10.1128/EC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li L, Chen OS, McVey Ward D, Kaplan J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J Biol Chem. 2001;276:29515–9. doi: 10.1074/jbc.M103944200. [DOI] [PubMed] [Google Scholar]
- 165.Li L, Kaplan J. A mitochondrial-vacuolar signaling pathway in yeast that affects iron and copper metabolism. J Biol Chem. 2004;279:33653–61. doi: 10.1074/jbc.M403146200. [DOI] [PubMed] [Google Scholar]
- 166.Singh A, Kaur N, Kosman DJ. The metalloreductase Fre6p in Fe-efflux from the yeast vacuole. J Biol Chem. 2007;282:28619–26. doi: 10.1074/jbc.M703398200. [DOI] [PubMed] [Google Scholar]
- 167.Rutherford JC, Bird AJ. Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells. Eukaryot Cell. 2004;3:1–13. doi: 10.1128/EC.3.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Klein M, Mamnun YM, Eggmann T, Schuller C, Wolfger H, Martinoia E, Kuchler K. The ATP-binding cassette (ABC) transporter Bpt1p mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett. 2002;520:63–7. doi: 10.1016/s0014-5793(02)02767-9. [DOI] [PubMed] [Google Scholar]
- 169.Rebbeor JF, Connolly GC, Dumont ME, Ballatori N. ATP-dependent transport of reduced glutathione on YCF1, the yeast orthologue of mammalian multidrug resistance associated proteins. J Biol Chem. 1998;273:33449–54. doi: 10.1074/jbc.273.50.33449. [DOI] [PubMed] [Google Scholar]
- 170.Sharma KG, Mason DL, Liu G, Rea PA, Bachhawat AK, Michaelis S. Localization, regulation, and substrate transport properties of Bpt1p, a Saccharomyces cerevisiae MRP-type ABC transporter. Eukaryot Cell. 2002;1:391–400. doi: 10.1128/EC.1.3.391-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ghosh M, Shen J, Rosen BP. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1999;96:5001–6. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parsons AB, Brost RL, Ding H, Li Z, Zhang C, Sheikh B, Brown GW, Kane PM, Hughes TR, Boone C. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol. 2004;22:62–9. doi: 10.1038/nbt919. [DOI] [PubMed] [Google Scholar]
- 173.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000;69:303–42. doi: 10.1146/annurev.biochem.69.1.303. [DOI] [PubMed] [Google Scholar]
- 174.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roberts P, Moshitch-Moshkovitz S, Kvam E, O’Toole E, Winey M, Goldfarb DS. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:129–41. doi: 10.1091/mbc.E02-08-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zaman S, Lippman SI, Zhao X, Broach JR. How Saccharomyces Responds to Nutrients. Annu Rev Genet. 2008 doi: 10.1146/annurev.genet.41.110306.130206. [DOI] [PubMed] [Google Scholar]
- 177.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.De Virgilio C, Loewith R. Cell growth control: little eukaryotes make big contributions. Oncogene. 2006;25:6392–415. doi: 10.1038/sj.onc.1209884. [DOI] [PubMed] [Google Scholar]
- 179.Kim J, Huang WP, Stromhaug PE, Klionsky DJ. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J Biol Chem. 2002;277:763–73. doi: 10.1074/jbc.M109134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wurmser AE, Emr SD. Novel PtdIns(3)P-binding protein Etf1 functions as an effector of the Vps34 PtdIns 3-kinase in autophagy. J Cell Biol. 2002;158:761–72. doi: 10.1083/jcb.200112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stromhaug PE, Reggiori F, Guan J, Wang CW, Klionsky DJ. Atg21 is a phosphoinositide binding protein required for efficient lipidation and localization of Atg8 during uptake of aminopeptidase I by selective autophagy. Mol Biol Cell. 2004;15:3553–66. doi: 10.1091/mbc.E04-02-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang CW, Stromhaug PE, Kauffman EJ, Weisman LS, Klionsky DJ. Yeast homotypic vacuole fusion requires the Ccz1-Mon1 complex during the tethering/docking stage. J Cell Biol. 2003;163:973–85. doi: 10.1083/jcb.200308071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–69. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 184.Nakamura N, Matsuura A, Wada Y, Ohsumi Y. Acidification of vacuoles is required for autophagic degradation in the yeast, Saccharomyces cerevisiae. J Biochem (Tokyo) 1997;121:338–44. doi: 10.1093/oxfordjournals.jbchem.a021592. [DOI] [PubMed] [Google Scholar]
- 185.Teter SA, Eggerton KP, Scott SV, Kim J, Fischer AM, Klionsky DJ. Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase. J Biol Chem. 2001;276:2083–7. doi: 10.1074/jbc.C000739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Epple UD, Suriapranata I, Eskelinen EL, Thumm M. Aut5/Cvt17p, a putative lipase essential for disintegration of autophagic bodies inside the vacuole. J Bacteriol. 2001;183:5942–55. doi: 10.1128/JB.183.20.5942-5955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Teichert U, Mechler B, Muller H, Wolf DH. Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J Biol Chem. 1989;264:16037–45. [PubMed] [Google Scholar]
- 188.Scott SV, Hefner-Gravink A, Morano KA, Noda T, Ohsumi Y, Klionsky DJ. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc Natl Acad Sci U S A. 1996;93:12304–8. doi: 10.1073/pnas.93.22.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mijaljica D, Prescott M, Klionsky DJ, Devenish RJ. Autophagy and vacuole homeostasis: a case for self-degradation? Autophagy. 2007;3:417–21. doi: 10.4161/auto.4441. [DOI] [PubMed] [Google Scholar]
- 190.Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell. 2005;19:15–26. doi: 10.1016/j.molcel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 191.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–72. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hohmann S, Krantz M, Nordlander B. In: Osmosensing and Osmosignaling. Sies DHuaH., editor. Academic Press; 2007. pp. 29–45. [Google Scholar]
- 193.Latterich M, Watson MD. Evidence for a Dual Osmoregulatory Mechanism in the Yeast Saccharomyces cerevisiae. Biochemical and Biophysical Research Communications. 1993;191:1111–1117. doi: 10.1006/bbrc.1993.1331. [DOI] [PubMed] [Google Scholar]
- 194.Nass R, Rao R. The yeast endosomal Na+/H+ exchanger, Nhx1, confers osmotolerance following acute hypertonic shock. Microbiology. 1999;145( Pt 11):3221–8. doi: 10.1099/00221287-145-11-3221. [DOI] [PubMed] [Google Scholar]
- 195.Cyert MS. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun. 2003;311:1143–50. doi: 10.1016/s0006-291x(03)01552-3. [DOI] [PubMed] [Google Scholar]
- 196.Yoshimoto H, Saltsman K, Gasch AP, Li HX, Ogawa N, Botstein D, Brown PO, Cyert MS. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:31079–88. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- 197.Mendoza I, Quintero FJ, Bressan RA, Hasegawa PM, Pardo JM. Activated calcineurin confers high tolerance to ion stress and alters the budding pattern and cell morphology of yeast cells. J Biol Chem. 1996;271:23061–7. doi: 10.1074/jbc.271.38.23061. [DOI] [PubMed] [Google Scholar]
- 198.Mendoza I, Rubio F, Rodriguez-Navarro A, Pardo JM. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem. 1994;269:8792–6. [PubMed] [Google Scholar]
- 199.Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol. 2002;156:1015–28. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell. 2006;5:723–31. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thumm M. Structure and function of the yeast vacuole and its role in autophagy. Microsc Res Tech. 2000;51:563–72. doi: 10.1002/1097-0029(20001215)51:6<563::AID-JEMT6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 202.Kellermayer R, Aiello DP, Miseta A, Bedwell DM. Extracellular Ca(2+) sensing contributes to excess Ca(2+) accumulation and vacuolar fragmentation in a pmr1Delta mutant of S. cerevisiae. J Cell Sci. 2003;116:1637–46. doi: 10.1242/jcs.00372. [DOI] [PubMed] [Google Scholar]